Abstract
Plant microRNAs (miRNAs) are important regulators of development and stress responses and are oftentimes under transcriptional regulation by stresses and plant hormones. We recently showed that polycistronic MIR842 and MIR846 are expressed from the same primary transcript which is subject to alternative splicing. ABA treatment affects the alternative splicing of the primary cistronic transcript which results in differential expression of the two miRNAs that are predicted to target the same family of jacalin lectin genes. One variant of miR846 in roots can direct the cleavage of AT5G28520, which is also highly upregulated by ABA in roots. In this addendum, we present additional results further supporting the regulation of AT5G28520 by MIR846 using a T-DNA insertion line mapping upstream of MIR842 and MIR846. We also show that AT5G28520 is transcriptionally induced by ABA and this induction is subject to ABA signaling effectors in seedlings. Based on previous results and data presented in this paper, we propose an interaction loop between MIR846, AT5G28520 and ABA in roots.
Keywords: alternative splicing, microRNA, root, abscisic acid, plant development, pri-miRNA
microRNAs (miRNAs) are ~21 nucleotide (nt)-long, single-stranded small RNAs that play important regulatory roles in eukaryotes. miRNAs bind to complementary target mRNAs to promote their cleavage post-transcriptionally or to interfere with translation.1 Plant miRNAs are important regulators of development and stress responses and are oftentimes regulated by stresses and plant hormones such as auxin and abscisic acid (ABA).2-4 It is remarkable that miRNA and RNAi effectors AGO1, AGO2 and DCL1 are targeted by miR168, miR403 and miR162, respectively,5-7 whereas DCL2 and SGS3 (in legumes and soybean, respectively) are targeted by miR1507/1515 and miR2118,8-10 and DCL1 encodes miR838 in its intron,11 underscoring the evolutionary importance of recruitment of RNAi into homeostatic feedback circuits. Recently we and others have shown that besides transcriptional regulation, some MIRNA genes (e.g., MIR400, MIR842 and MIR846) are also regulated post-transcriptionally by alternative splicing.12,13 These miRNAs are found only in Arabidopsis and closely related species,14,15 raising broad questions about MIRNA evolution and its functional significance for the targets and their regulation by RNAi crosstalk networks.16 Recent work on tomato17 shows unexpected positive correlations between miRNAs and their predicted targets, supporting the notion that there are other processes (e.g., feedback loops) that can impact miRNAs as canonical negative regulators.
We found that polycistronic MIR842 and MIR846 are transcribed from the same primary transcript that produces three different splicing isoforms. ABA affects the alternative splicing of the pri-miRNA and thereby represses the expression of the miRNAs.13 We also showed that AT5G28520, a gene encoding a predicted target jacalin lectin10,18 is cleaved, possibly by a variant “isoMIR” of miR846 in roots. RNA gel blots showed several-fold upregulation of AT5G28520 by ABA, but real-time PCR experiments demonstrate AT5G28520 is highly (~50-fold) upregulated within a few hours (Fig. 1A). Real-time PCR also showed that ABA treatment repressed pre-miR846 with the strongest (60% reduction) effect observed after three hr exposure to 10 μM ABA (Fig. 1B). Furthermore, in the absence of exogenous ABA, pre-miR846 levels decreased after 24 h (Fig. 1B) when the expression of AT5G28520 was elevated (Fig. 1A), which is consistent with a causal relationship. However, a 50-fold upregulation of AT5G28520 by ABA within hours raises questions about whether this induction is by miRNA action alone or if some other type of regulation, for example a positive feedback on transcription initiation,19 might be involved.
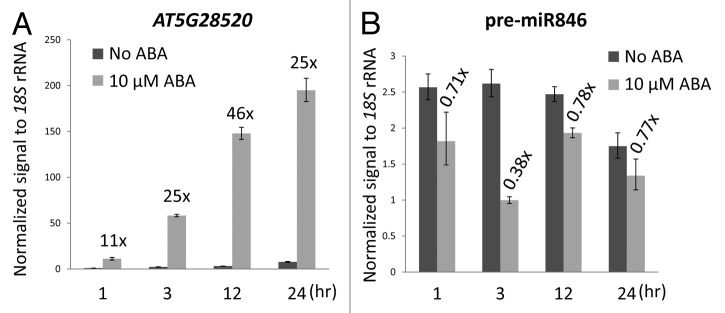
Figure 1. Real-time PCR results showing the ABA-regulated expression of (A) AT5G28520 and (B) pre-miR846 in 10-d-old seedlings over 24 h. Numbers above the bars indicate fold changes (comparing to no ABA at the same time point). Error bars are ± s.d., n = 3.
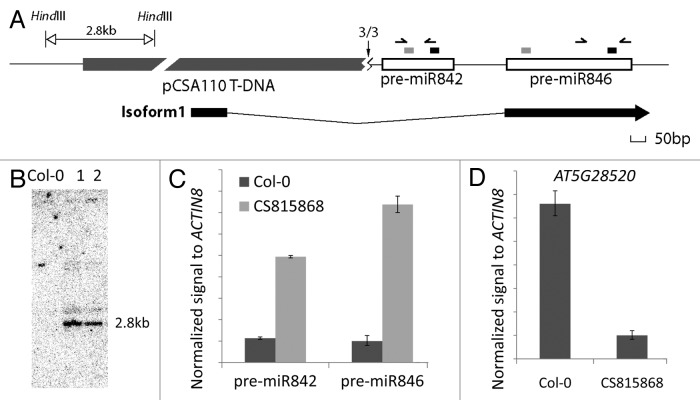
To further study the function of miR842 and miR846, we searched on TAIR (www.arabidopsis.org) and identified a T-DNA insertion line20 CS815868 which carries an insertion upstream of MIR842 and MIR846, suggesting it may function as an activation-tagged allele. Analysis of the flanking sequence and a genomic Southern blot showed that this T-DNA is inserted near the transcription start site (TSS) of MIR842 and MIR846 (Fig. 2A and B). 5' RACE showed that the TSS is altered (a 40 nt rDNA-like insertion fragment; data not shown) but pre-miR842 is otherwise intact in CS815868 (Fig. 2A). Real-time PCR showed enhanced expression of pre-miR842 and pre-miR846 in roots of CS815868 (Fig. 2C) while AT5G28520 mRNA was reduced (Fig. 2D), supporting a causal effect of elevated miR842 and/or miR846 expression on downregulation of AT5G28520. This supports our claim13 that miR846 can direct the cleavage of AT5G28520, which is subsequently degraded.
Figure 2.AT5G28520 is downregulated in CS815868 T-DNA insertion plants, which overexpress pre-miR842 and pre-miR846. (A) Genomic structure around MIR842 and MIR846 in T-DNA insertion line CS815868. TSS revealed by 5′ RACE is shown with a vertical black arrow (numbers are sequenced clones mapping to site out of the total clones sequenced). The zigzag below the arrow represents a 40 bp sequence found that does not map to the T-DNA and has homology to rDNA. Open boxes represent pre-miR842 and pre-miR846. The short gray rectangles above the open boxes represent miRNA* and short black rectangles represent mature miRNA sequences. Isoform 1, which is the most abundant splice variant expressed from this locus in wild type is shown below. Black boxes represent exons. Horizontal arrows represent primers used for real-time PCR of pre-miRNAs in (C). (B) Genomic Southern blot showing single insert in CS815868. Lanes 1 and 2 are HindIII-digested genomic DNAs from two individual CS815868 plants. Blot was probed with BASTA selectable marker gene fragment from the vector. (C and D) Real-time PCR results showing the different expression levels of AT5G28520 (C) and pre-miR842 and -miR846 (D) in roots of control Col-0 and CS815868 line. Error bar, ± s.d., n = 2.
To study the transcriptional regulation of AT5G28520, transgenic plants were engineered21 that express the reporter gene GUS under control of the AT5G28520 promoter. GUS staining of 10-d-old Pro-AT5G28520:GUS seedlings had no detectable GUS activity in the absence of exogenous ABA (Fig. 3A). When treated with ABA (30 μM, 4 hr), strong GUS activity was observed in roots, especially in lateral root primordia, but not in leaves (Fig. 3B and C). RNA gel blot analysis further showed that this induction was strongly suppressed by ABA-insensitive signal transduction mutants such as PP2Cs abi1-1 (75% reduction), abi2-122 (84% reduction), APETALA2 domain abi4-123 (35% reduction) and bZIP abi5-124 (32% reduction), whereas B3 domain abi3-125 mutant had a relatively weak effect (12% reduction) (Fig. 2D), consistent with previous results showing that ABI3 is expressed in seeds and ABI5 expression decreases progressively after germination.26
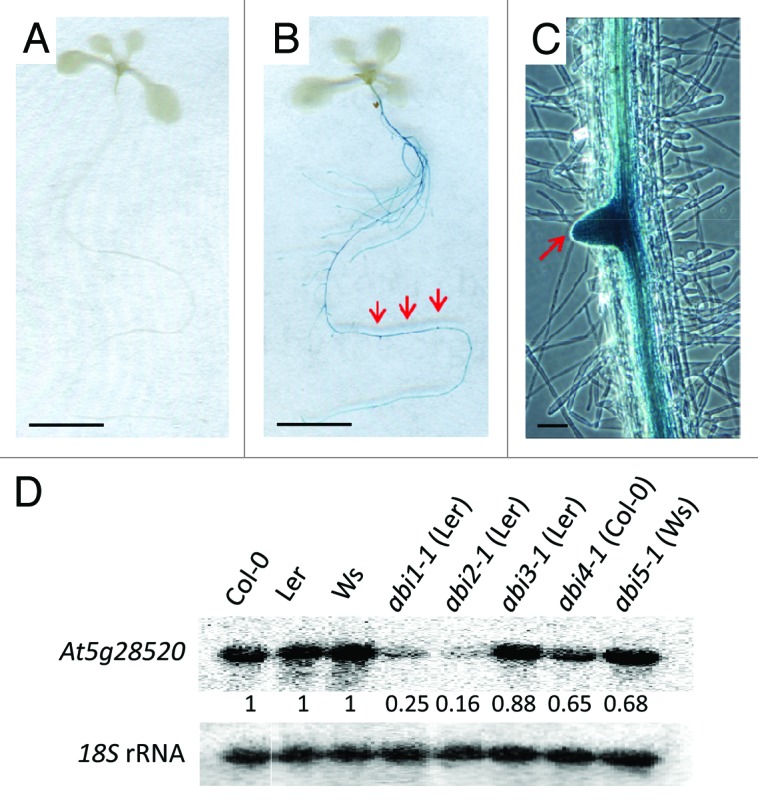
Figure 3.AT5G28520 is upregulated transcriptionally by ABA. (A–C) GUS staining of Pro-AT5G28520:GUS transgenic seedlings after 4 h treatment in the absence (A) or presence (B) of exogenous 30 μM ABA. A lateral root primordium in (B) is imaged under higher magnification light microscope in (C). Red arrows point to lateral root primordia. Scale bars in (Aand B) represent 5 mm. Scale bar in (C) represents 1 mm. (D) Blot of total RNA from root samples from different ABA signaling mutants and their corresponding wild type controls treated with 30 μM ABA for 3 hr. Numbers below the panels are normalized band intensities compared with 18S rRNA signal.
Together with the results presented in our recent paper,13 we have shown that: (1) miR846 and AT5G28520 are co-expressed in roots; (2) a possible isomer variant (5' shifted) of miR846 directs the cleavage of AT5G28520 in the absence of ABA; (3) AT5G28520 is downregulated in an MIR842 and MIR846 T-DNA activation-tagged overexpression allele; (4) ABA upregulates AT5G28520 transcriptionally and this regulation requires the functions of ABI1,-2, -4 and -5; and (5) ABA downregulates miR846 post-transcriptionally by alternative splicing. Based on these results, we propose a model in which ABA, MIR846 and AT5G28520 form a regulatory network in roots (Fig. 4). In the absence of exogenous ABA (and presumably low concentrations of endogenous ABA), expression of AT5G28520 is low and the expression of miR846 would direct the jacalin mRNA to be endonucleolytically cleaved or possibly subject to translational repression. In the presence of exogenous ABA (or high endogenous ABA concentrations, e.g., in response to stress), transcription of AT5G28520 is elevated, and at the same time miR846 biogenesis is repressed by alternate splicing, which results in AT5G28520 mRNA accumulation. This synergistic effect could account for the rapid ~50-fold induction of AT5G28520 by exogenous ABA at as low as 1 μM, which is within the physiological range found in plants.27 We speculate that biogenesis of pre-miR842/846 by complexes containing effectors of ABA sensitivity and splicing such as ABA hypersensitive1/cap-binding protein80,28 hyponastic leaves,29 serrate,30 argonaute31 and others may be important for miR846 function. In addition, ABA may also repress the expression of the MIR842-MIR846 cistron transcriptionally. The function of jacalin lectins is poorly understood and recent studies link their activities to virus resistance and biotic stress.32,33 Further work is needed to understand the function of AT5G28520 and the importance of RNAi regulatory networks to ABA signaling in root development and environmental stress responses.
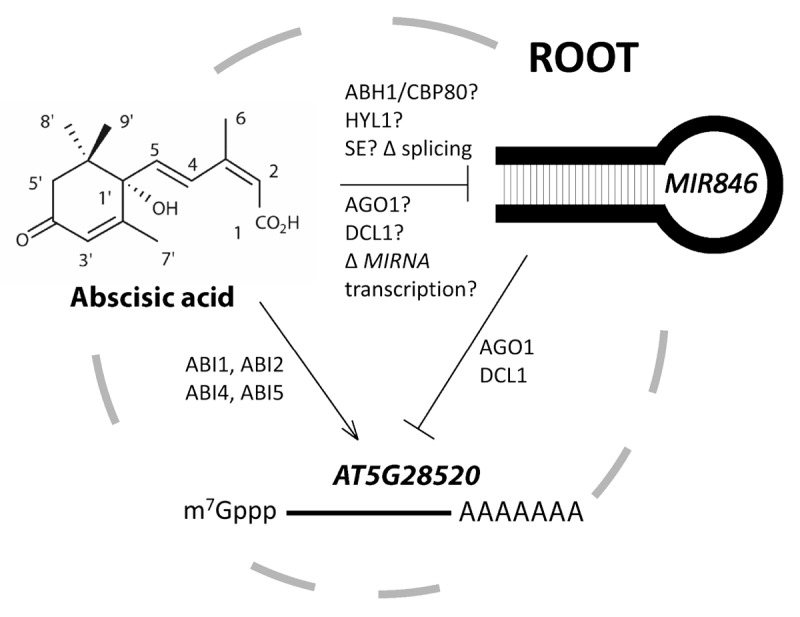
Figure 4. A model showing the possible interactions between RNAi pathways and MIR846, AT5G28520 and ABA in roots. A variant of miR846 is normally expressed that directs the post-transcriptional cleavage/repression of AT5G28520 in the absence of ABA; ABA induces the expression of AT5G28520 transcriptionally and simultaneously represses miR846 by directing alternative pri-miR846 splicing (Δ = change) and possibly modulating biogenesis through RNAi effectors known to impact ABA sensitivity by unknown mechanisms. AGO1 and DCL1 are known to act downstream of pre-miR846 hairpin biogenesis. Transcriptional repression (Δ) of MIR846 by ABA may also be involved.
Materials and Methods
Plasmid construction
For the promoter analysis of AT5G28520, a 1.5 kb sequence upstream of the start codon was PCR-amplified using PrimeSTAR HS DNA polymerase (Takara) and inserted between the Hind III and BamHI sites in pBI121, replacing the 35S promoter which drives uidA GUS reporter gene expression. Oligonucleotide primers were designed using “Perlprimer” (perlprimer.sourceforge.net) and synthesized commercially (Sigma). A list of primers used in the study is provided in Table 1.
Table 1. Primers used in this study.
|
promoter cloning, At5g28520 |
Pro5g28520_F | ACCAAGCTTAGAGCTTCAAATCATT |
|---|---|---|
| Pro5g28520_R | AACGGATCCCACGAACTGTTTTGA | |
| Real-time PCR | Pre846_F | ACGATTGGAAGCTGAATGGTTGCG |
| Pre846_R | CGAATTTACCGGGATACACGAGCA | |
| Pre842_F | TAAGCGGGATGATGAATCCGACCA | |
| Pre842_R | TGATGGTGTTTCGATCCCTGGACA | |
| At5g28520_QRT_F | GTCGTGAAGGCATTCAGTACGTCA | |
| At5g28520_QRT_R | TTGTGTGAATCCTCCTCTACCCGA | |
| 18S_F | AAATACCGCGGCTGCTGGCA | |
| 18S_R | CGGCTACCACATCCAAGGAA | |
| ACTIN8_F | GGAATGGTTAAGGCTGGATTCG | |
| ACTIN8_R | ACGCATCTTTCTGATTCATCCC | |
| 5′ RACE | 5RACE1 | CGAATTTACCGGGATACACGAGCA |
| 5RACE2 | CCTTGAATGAAACCGGATGTGGCA | |
| 5RACE3 | CGAGGCGGTTAATCTCAAACTGCT | |
| 5RACE4 | TGATGGTGTTTCGATCCCTGGACA | |
| Northern | At5g28520CDS_F | ATGGCTCAGAAGTTAGAAGCAATAG |
| At5g28520CDS_R | TCACTCGCGCGTGATGGGC | |
| 18S primer pair | same as 18S_F and 18S_R pair used in real-time PCR | |
| Southern | BASTA_F | GGTCTGCACCATCGTCAACC |
| BASTA_R | CCAGAAACCCACGTCATGCC |
Plant material and growth conditions
The accessions used in this study are listed as follows: Col-0 [CS60,000], Ler-0 [CS20], Ws-2 [CS2360], abi1-1 [CS22], abi2-1 [CS23], abi3-1 [CS24], abi4-1 [CS8104], abi5-1 [CS8105], CS815868 and were obtained from the Arabidopsis Biological Resource Center (abrc.osu.edu). Arabidopsis thaliana seeds were soaked in water and kept in the dark at 4°C for 3 d before transferring to soil. The growth condition was 21°C with a 16 h light and 8 h dark cycle. For promoter analysis, plants were transformed with Pro-AT5G28520:GUS constructs first electroporated into Agrobacterium tumefaciens strain GV3101 and then transferred to the plants using the floral dip method.21
For ABA treatments, seeds were sown on plates containing 0.5× Murashige and Skoog salts (Research Products International) 1% sucrose and 0.5% phytagel (Sigma). Plates were kept in the dark at 4°C for 3 d before transferring into a growth chamber. The growth condition was 21°C with continuous light. Plates were kept in the chamber for 10 d before spraying with ABA solutions. ABA [(±)-Abscisic acid, 98%; ACROS Organics] solutions were diluted with water from a 100 µM stock in 70% ethanol. The same amount of 70% ethanol was added to water to spray the control group.
β-Glucuronidase GUS assays
Plant samples were developed at 37°C for 12 h with a 1 mM solution of the indigogenic GUS substrate 5-bromo-4-chloro-3-indolyl b-D-glucu-ronide (X-Gluc; Rose Scientific) in 50 mM KH2PO4 (pH 7.0), 0.1 mM EDTA, 0.5 mM ferricyanide, 0.5 mM ferrocyanide, 0.05% sodium azide, 0.1% Triton X-100. After staining overnight, samples were immersed in 70% ethanol overnight. Samples were then mounted in 30% glycerol on microscope slides for imaging with a dissecting microscope or with an Olympus BX41 light microscope for high magnification and documented using QCapture software (v2.68.6, Silicon Graphics).
Real-time PCR
Total RNA from roots of 10-d-old seedlings was extracted with Iso-RNA Lysis Reagent (5 PRIME). Four μg total RNA was digested with RQ1 RNase-free DNase (Promega) and reverse transcribed by MMLV reverse transcriptase (Promega) with random primers (Promega). Real-time PCR was performed using FastStart Universal SYBR Green Master (Rox) (Roche) according to the supplier’s protocol. PCR reactions were run on an ABI7000 (Applied Biosystems) instrument with default settings and results were analyzed with affiliated software. cDNA preparation was the same as for reverse transcriptase-PCR except that random primers (Promega) was used in reverse transcription.
5′ RACE
RACE experiments were performed with GeneRacer Kit (Invitrogen) according to the manufacturer’s specification.
Southern hybridization
Genomic DNA was isolated from Col-0 plants and two individual CS815868 plants using CTAB (Hexadecyl trimethyl-ammonium bromide, ACROS Organics). 15 μg genomic DNA was used for each HindIII digestion. The samples were resolved on a 0.7% agarose gel and blotted to a Hybond-N+ membrane (GE Healthcare) according to the supplier’s protocol. BASTA template was amplified using primers “BASTA_F” and “BASTA_R” from CS815868 genomic DNA and was eluted after excision of the band from an agarose gel. Probes were synthesized using Random Primer DNA Labeling Kit (Takara) with [α-32P]-dCTP (PerkinElmer). Hybridization was performed with the PerfectHyb Plus hybridization buffer (Sigma) according to the manufacturer’s instructions. A storage phosphor screen (GE Healthcare) was used for autoradiography and it was scanned using a Storm 860 PhosphorImager (GE Healthcare).
Northern hybridization
Samples of 10 μg total RNA were resolved on a 1.2% denaturing agarose gel and blotted to a Hybond-N+ membrane (GE Healthcare) according to the supplier’s protocol. AT5G28520 template was amplified using primers “At5g28520CDS_F” and “At5g28520CDS_R” from a cDNA library and was eluted after excision of the band from an agarose gel. Probes were synthesized and blots processed as described for genomic DNA gel blots.
Acknowledgments
This work was supported by the National Institutes of Health grant GM077245 to C.D.R. and a Texas Tech University AT&T Chancellor’s Fellowship to F.J. The funders had no role in the study design, in the collection, analysis or interpretation of data or in the writing of the manuscript or decision to submit the manuscript for publication.
Glossary
Abbreviations:
- ABA
abscisic acid
- AGO
Argonaute
- bZIP
basic leucine zipper transcription factor
- DCL
Dicer-Like
- GUS
Escherichia coli β- glucuronidase gene uidA
- PP2C
protein phosphatase type 2C
- pri-miRNA
primary MIRNA transcript
- Pro-
promoter
- RACE
Rapid Amplification of cDNA Ends
- RNAi
RNA interference
- SGS3
Suppressor of Gene Silencing3
- TSS
transcription start site
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24563
References
- 1.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–87. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita N, Wang H, Kasahara H, Liu J, Macpherson C, Machida Y, et al. IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell. 2012;24:3590–602. doi: 10.1105/tpc.112.097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyes JL, Chua NH. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007;49:592–606. doi: 10.1111/j.1365-313X.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS. Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res. 2010;38:1382–91. doi: 10.1093/nar/gkp1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–21. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Kasschau KD, Carrington JC. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr Biol. 2003;13:784–9. doi: 10.1016/S0960-9822(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 7.Vaucheret H. AGO1 homeostasis involves differential production of 21-nt and 22-nt miR168 species by MIR168a and MIR168b. PLoS ONE. 2009;4:e6442. doi: 10.1371/journal.pone.0006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song QX, Liu YF, Hu XY, Zhang WK, Ma B, Chen SY, et al. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol. 2011;11:5. doi: 10.1186/1471-2229-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Deng Y, Wu T, Subramanian S, Yu O. Misexpression of miR482, miR1512, and miR1515 increases soybean nodulation. Plant Physiol. 2010;153:1759–70. doi: 10.1104/pp.110.156950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, Li Y, et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011;25:2540–53. doi: 10.1101/gad.177527.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopalan R, Vaucheret H, Trejo J, Bartel DP. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006;20:3407–25. doi: 10.1101/gad.1476406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan K, Liu P, Wu CA, Yang GD, Xu R, Guo QH, et al. Stress-induced alternative splicing provides a mechanism for the regulation of microRNA processing in Arabidopsis thaliana. Mol Cell. 2012;48:521–31. doi: 10.1016/j.molcel.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Jia F, Rock CD. MIR846 and MIR842 comprise a cistronic MIRNA pair that is regulated by abscisic acid by alternative splicing in roots of Arabidopsis. Plant Mol Biol. 2013;81:447–60. doi: 10.1007/s11103-013-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, Coruh C, Axtell MJ. Arabidopsis lyrata small RNAs: transient MIRNA and small interfering RNA loci within the Arabidopsis genus. Plant Cell. 2010;22:1090–103. doi: 10.1105/tpc.110.073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahlgren N, Jogdeo S, Kasschau KD, Sullivan CM, Chapman EJ, Laubinger S, et al. MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana. Plant Cell. 2010;22:1074–89. doi: 10.1105/tpc.110.073999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLean D, Elina N, Havecker ER, Heimstaedt SB, Studholme DJ, Baulcombe DC. Evidence for large complex networks of plant short silencing RNAs. PLoS ONE. 2010;5:e9901. doi: 10.1371/journal.pone.0009901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Gomollon S, Mohorianu I, Szittya G, Moulton V, Dalmay T. Diverse correlation patterns between microRNAs and their targets during tomato fruit development indicates different modes of microRNA actions. Planta. 2012;236:1875–87. doi: 10.1007/s00425-012-1734-7. [DOI] [PubMed] [Google Scholar]
- 18.Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE. 2007;2:e219. doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo QJ, Mittal A, Jia F, Rock CD. An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Mol Biol. 2012;80:117–29. doi: 10.1007/s11103-011-9778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–7. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1:641–6. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- 22.Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–71. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–54. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–61. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein R, Gampala SS, Lynch TJ, Thomas TL, Rock CD. Redundant and distinct functions of the ABA response loci ABA-INSENSITIVE(ABI)5 and ABRE-BINDING FACTOR (ABF)3. Plant Mol Biol. 2005;59:253–67. doi: 10.1007/s11103-005-8767-2. [DOI] [PubMed] [Google Scholar]
- 27.Zeevaart JA. Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol. 1980;66:672–8. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–87. doi: 10.1016/S0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 29.Lu C, Fedoroff N. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell. 2000;12:2351–66. doi: 10.1105/tpc.12.12.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Z, Han MH, Fedoroff N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc Natl Acad Sci USA. 2008;105:9970–5. doi: 10.1073/pnas.0803356105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Cui X, Meng Z, Huang X, Xie Q, Wu H, et al. Transcriptional regulation of Arabidopsis MIR168a and argonaute1 homeostasis in abscisic acid and abiotic stress responses. Plant Physiol. 2012;158:1279–92. doi: 10.1104/pp.111.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagano AJ, Fukao Y, Fujiwara M, Nishimura M, Hara-Nishimura I. Antagonistic jacalin-related lectins regulate the size of ER body-type beta-glucosidase complexes in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:969–80. doi: 10.1093/pcp/pcn075. [DOI] [PubMed] [Google Scholar]
- 33.Yamaji Y, Maejima K, Ozeki J, Komatsu K, Shiraishi T, Okano Y, et al. Lectin-mediated resistance impairs plant virus infection at the cellular level. Plant Cell. 2012;24:778–93. doi: 10.1105/tpc.111.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]