Abstract
Purpose
To determine the impact of elevated serum estradiol levels (EE2-defined as levels > 90th percentile) on the day of hCG administration during IVF on oocyte fertilization, embryo development, implantation, clinical pregnancy and miscarriage rates.
Methods
A total of 2,995 consecutive IVF cycles in 1,889 patients with non-donor oocyte retrieval resulting in fresh embryo transfer between 1/1/2005 and 12/31/2011 were analyzed. Cycles were stratified by serum E2 level on the day of hCG administration into those with levels >90th percentile and ≤ 90th percentile. Rates of normal fertilization, embryo development, positive pregnancy test, implantation, clinical pregnancy and spontaneous miscarriage were compared.
Results
Serum estradiol above the 90th percentile on the day of hCG administration was associated with a significantly lower rate of normal fertilization (68.6 ± 20 vs. 71.6 ± 21, p = 0.02) when compared with patients with a lower serum estradiol threshold. The proportion of embryos that progressed from 2PN to 6–8 cell on day 3 was not different between the two groups. Although rates of positive pregnancy test (55.2 % vs. 57 %), implantation (26.4 % vs. 28.5 %) and clinical pregnancy (45.5 % vs. 49.4 %) were lower in patients with a higher estradiol threshold, these differences were not statistically significant. Similarly, there was no difference in the spontaneous miscarriage rates between the two groups (8.4 % vs. 7.1 %).
Conclusions
Serum estradiol levels above the 90th percentile on the day of hCG administration is associated with lower oocyte fertilization rate; however, such levels do not impact embryo development, implantation, clinical pregnancy or spontaneous miscarriage rates.
Keywords: Fertilization, Implantation, Hyperstimulation, Oocyte quality, Estradiol, Pregnancy outcome
Introduction
Assisted reproductive technology (ART) has proven effective in helping infertile patients achieve pregnancy. While ART is an effective means of building a family in those for whom other treatments are unsuccessful, research geared towards defining those aspects of the treatment protocols associated with adverse outcomes continues. Some of the well-described adverse outcomes include multiple gestation, chromosomal abnormalities, birth defects, preterm delivery, small for gestational age/low birth weight infants, preeclampsia, placenta previa, cesarean delivery and perinatal morbidity [1–10].
Recent studies have suggested that the supraphysiologic hormonal milieu unique to controlled ovarian hyperstimulation during ART may be responsible for adverse outcomes such as preeclampsia and delivery of small fetuses [11–13]. In 1998, Barker became the first to link maternal malnutrition during the pre-implantation period to low birth weight and showed that affected individuals are at greater risk of developing coronary heart disease, hypertension, and diabetes [14]. This phenomenon, later termed the fetal origin of adult disease, was named the “Barker’s Hypothesis” by the British Medical Journal. Thus, an intriguing question worthy of investigation is whether downstream effects of elevated peri-implantation estradiol levels in ART such as preeclampsia and low birth weight are associated with upstream adverse effects like impaired oocyte/embryo quality and implantation, decreased clinical pregnancy and increased spontaneous miscarriage rates. Although potential negative effects of relatively high estradiol levels in the pre-ovulatory and early luteal phases have been documented [15–18], its effects during the peri-implantation and early pregnancy period are not well understood [16, 19]. Additionally, these reported negative impacts have not been consistently seen [20–23]. The variability in several of these prior studies may be due to varying estradiol thresholds at which clinically significant differences in implantation and overall pregnancy outcomes were defined. The inconsistent estradiol threshold, in addition to small sample sizes and differences in the study populations, has proved challenging in making adequate comparisons of outcomes and maintaining external validity [16, 24, 25].
It is theorized that if aberrant trophoblastic invasion due to elevated serum estradiol (EE2) mediates reported adverse obstetrical outcomes such as preeclampsia and small for gestational age infants following in vitro fertilization (IVF) conception [12, 13], such aberrant invasion may also be associated with decreased implantation and higher miscarriage rates in these patients. In addition, oocyte quality and fertilization rates, as well as embryo quality and development rates may also be affected. Therefore, the objective of this study was to investigate the relationship between EE2 levels and oocyte/embryo quality, embryo development and implantation, clinical pregnancy and spontaneous miscarriage rates.
Materials and methods
A retrospective cohort study was conducted after obtaining approval from the Partners Healthcare institutional review board. A total of 4,469 consecutive IVF cycles in our institution from January 1, 2005 to December 31, 2011 were reviewed. The 3,911 cycles resulting in embryo transfer were analyzed and of these, 2,995 concurrent non-donor oocyte retrieval cycles resulting in fresh embryo transfer in 1,889 patients constituted our final study cohort. The detailed flow chart of the inclusion and exclusion criteria for the study is shown in Fig. 1. The distribution of the 2,995 cycles during the 7-year study period was 265 (8.8 %) in 2005, 317 (10.6 %) in 2006, 371 (12.4 %) in 2007, 393 (13.1 %) in 2008, 499 (16.7 %) in 2009, 566 (18.9 %) in 2010 and 584 (19.5 %) in 2011. The cycles were divided into two groups based on the serum estradiol measurement on the day of hCG administration. Cycles with elevated estradiol levels greater than the 90th percentile comprised the study group, while cycles with levels less than the 90th percentile served as the control. The 90th percentile threshold was chosen based on published data that suggested that patients with extremes of serum estradiol levels measured on the day of hCG trigger were more likely to develop preeclampsia and deliver small for gestational age infants [12, 13]. To test the hypothesis whether high estradiol levels were associated with worse oocyte, fertilization and peri-implantation parameters and early pregnancy outcomes in IVF, we decided to use similar methodology to establish our cut off to discriminate between the study (high estradiol) and control (lower estradiol) groups. The study outcomes included percentage of normal fertilization {defined as number of 2 pronuclei embryos/number of matured oocytes (MII); MII oocytes were determined on the day of oocyte retrieval prior to injection for ICSI cycles, and ~16–18 h following retrieval for conventional IVF cycles [26]}, proportion of embryos that developed to 6–8 cell stage on day 3 (number of 6–8 cell embryos/total number of 2 pronuclei embryos), positive pregnancy test (positive hCG 12–14 days following embryo transfer), biochemical pregnancy [defined as positive hCG without a gestational sac], implantation rate [defined as number of gestational sacs/number of embryos transferred], clinical pregnancy rate (documented intrauterine pregnancy with fetal heart activity), and first trimester spontaneous miscarriage [defined as pregnancy failure after visualization of intrauterine gestation sac].
Fig. 1.
Study design and flow chart with the inclusion and exclusion criteria
All patients underwent IVF (with conventional insemination or ICSI) using standard oral contraceptive pills pretreatment with luteal phase GnRH-agonist pituitary downregulation, follicular phase GnRH-agonist flare or GnRH-antagonist with variable start and exogenous gonadotropins (recombinant FSH and human menopausal gonadotropins) as previously described [27]. Criteria for hCG trigger, oocyte retrieval, type of immunoassay utilized to measure serum estradiol (Roche cobas immunoassay-Roche Diagnostics), embryo transfer guidelines and luteal support have previously been described [11].
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS, Version 21 for Windows; SPSS, Inc., Chicago, IL, USA). All tests were conducted using a p-value ≤ 0.05 defining statistical significance. The data were expressed as mean ± standard deviation for continuous variables and number of cases (n) and percentage of occurrence (%) for categorical variables. Differences between groups were analyzed with chi-square and Fisher’s exact tests for categorical data, and Student’s t-test and Mann Whitney test for continuous variables, as appropriate. The univariate analysis was followed by Forward Stepwise Logistic regression with potential confounders included in the model if considered risk factors for the IVF outcome of interest based on p < 0.10. Covariates included antral follicle count (AFC), body mass index (BMI), total gonadotropin dose required for controlled ovarian stimulation (COH), type of stimulation protocol, total number of retrieved and matured oocytes.
Results
During the 7-year study period, there were a total of 2,995 IVF cycles in 1,889 patients with non-donor oocyte retrieval resulting in fresh embryo transfer. The mean ± SD serum estradiol level on the day of hCG trigger was 1,995 ± 798 pg/mL, while the 90th percentile was 2,991 pg/mL. There were a total of 299 cycles with EE2 levels (> the 90th percentile). The mean (±SD) normal fertilization rate was 71 ± 21 % and the proportion of embryos that developed appropriately on day 3 was 58 ± 31 % [95 % confidence interval (57–59)]. Overall, 57 % and 49 % of the cycles resulted in a positive pregnancy test and clinical pregnancy, respectively. The implantation, biochemical pregnancy and spontaneous miscarriage rates (95 % confidence interval) were 28 ± 38 % (27–30), 7 % (6–8), and 7 % (6–8), respectively.
Comparison of the demographic characteristics among cycles with lower and higher serum estradiol thresholds on the day of hCG administration did not reveal statistically significant differences in maternal age, baseline ovarian reserve measures (AFC, day 3 FSH and E2) or utilization of ICSI for oocyte insemination (Table 1). Compared to the control group, the patients with EE2 levels had a lower average BMI (23 ± 3 vs. 24 ± 4, p = 0.02), required lower doses of gonadotropins for stimulation (2,284 ± 1043 vs. 2,916 ± 1824, p = 0.001) and were more likely to use a luteal phase GnRH-agonist pituitary downregulation stimulation protocol [79 % vs. 60 %, p = 0.001, (Table 1)].
Table 1.
Demographics characteristics among patients with estradiol levels ≤ 90th and > 90th percentile (2,991 pg/mL) on the day of hCG administration during an IVF cycle
| Variables | Serum Estradiol Level on the day of hCG trigger | P value | ||
|---|---|---|---|---|
| ≤ 90th Percentile (n = 2696) | > 90th Percentile (n = 299) | |||
| Age (years) | 36 ± 4 | 35 ± 4 | 0.15 | |
| Antral follicle count | 13 ± 6 | 15 ± 6 | 0.06 | |
| Body mass index (kg/m2) | 24 ± 4 | 23 ± 3 | 0.02 | |
| Basal FSH (mIU/mL) | 7.4 ± 3.4 | 6.9 ± 1.8 | 0.21 | |
| Basal Estradiol (pg/mL) | 44.1 ± 21.8 | 42.1 ± 15.2 | 0.41 | |
| Intracytoplasmic sperm injection (ICSI) | 1353 (50.2 %) | 160 (53.5 %) | 0.30 | |
| Day of hCG trigger | 11.6 ± 1.7 | 11.7 ± 1.4 | 0.36 | |
| Dose of gonadotropin (IU) | 2916 ± 1824 | 2284 ± 1043 | 0.001 | |
| Stimulation Protocol | Luteal phase GnRH agonist | 1613 (59.9 %) | 237 (79.3 %) | 0.001 |
| Follicular phase GnRH agonist | 684 (25.4 %) | 39 (13 %) | ||
| Follicular phase GnRH antagonist | 398 (14.8 %) | 23 (7.7 %) | ||
The patients with EE2 thresholds had more oocytes retrieved (15 ± 5 vs. 10 ± 5, p = 0.001) and more matured oocytes (12 ± 5 vs. 8 ± 4, p = 0.001) than those in the control group; however, their fertilization rate was significantly lower (69 ± 20 vs. 72 ± 21, p = 0.02). This difference remained significant after controlling for covariates such as AFC, BMI, total gonadotropin dose required for COH, type of stimulation protocol, total number of retrieved and matured oocytes using multivariable logistic regression. The proportion of embryos that developed to 6–8 cells on day 3, positive pregnancy test, implantation, clinical pregnancy and spontaneous miscarriage were not different between groups (Table 2).
Table 2.
IVF, pregnancy and obstetrical outcomes among patients with estradiol levels ≤ 90th and > 90th percentile (2,991 pg/mL) on the day of hCG administration during an IVF cycle
| Variables | Serum Estradiol Level on the day of hCG trigger | P value (OR) | ||
|---|---|---|---|---|
| ≤ 90th Percentile (n = 2696) | > 90th Percentile (n = 299) | |||
| Number of oocytes retrieved | 10.0 ± 5.0 | 15.0 ± 5.0 | 0.001 | |
| Number of matured oocytes | 8.0 ± 4.0 | 12.0 ± 5.0 | 0.001 | |
| Normal oocyte fertilization [2PN/matured oocytes (%)] | 71.6 ± 20.8 | 68.6 ± 20.0 | 0.02 | |
| Stage specific embryo development [# of 6-8cell/2PN (%)] | 57.5 ± 31.1 | 58.4 ± 27.3 | 0.60 | |
| Number of embryo transferred | 2.2 ± 0.8 | 2.3 ± 0.9 | 0.60 | |
| Stage of embryo transferred | Cleavage | 2029 (75.3 %) | 183 (61.4 %) | 0.001(1.49 – 2.46) |
| Blastocyst | 666 (24.7 %) | 115 (38.6 %) | ||
| Positive pregnancy test | 1537 (57 %) | 165 (55.2 %) | 0.54 (0.73 – 1.18) | |
| Biochemical pregnancy rate | 185 (6.9 %) | 24 (8.0 %) | 0.47 (0.76 – 1.84) | |
| Implantation rate | 28.5 ± 37.9 | 26.4 ± 37.9 | 0.38 | |
| Clinical pregnancy rate | 1331 (49.4 %) | 136 (45.5 %) | 0.20 (0.67 – 1.09) | |
| Spontaneous miscarriage rate | 192 (7.1 %) | 25 (8.4 %) | 0.41 (0.77 – 1.84) | |
| Ectopic pregnancy | 31 (1.2 %) | 3 (1.0 %) | 1.00 (0.26 – 2.87) | |
Stratification of all cycles into quartiles of serum estradiol levels revealed a decline in normal oocyte fertilization rate after the 1st quartile until the 3rd quartile with a further dramatic decline noted in the 4th quartile. Cycles with estradiol >2,465 pg/mL (4th quartile) were associated with significantly lower normal oocyte fertilization rate (Fig. 2a). The rates of embryo development to 6–8 cells on day 3 and positive pregnancy test were lowest in cycles with an estradiol level in the 1st quartile (≤1,403 pg/mL). These parameters gradually increased in the 2nd and 3rd quartiles, but started to decline again in the 4th quartile [>2,465 pg/mL, (Fig. 2b–c)]. Similar but non-significant trends were seen with embryo implantation, the proportion of patients with clinical pregnancy, spontaneous miscarriage, and singleton live birth following a positive pregnancy test (Fig. 2d–g).
Fig. 2.
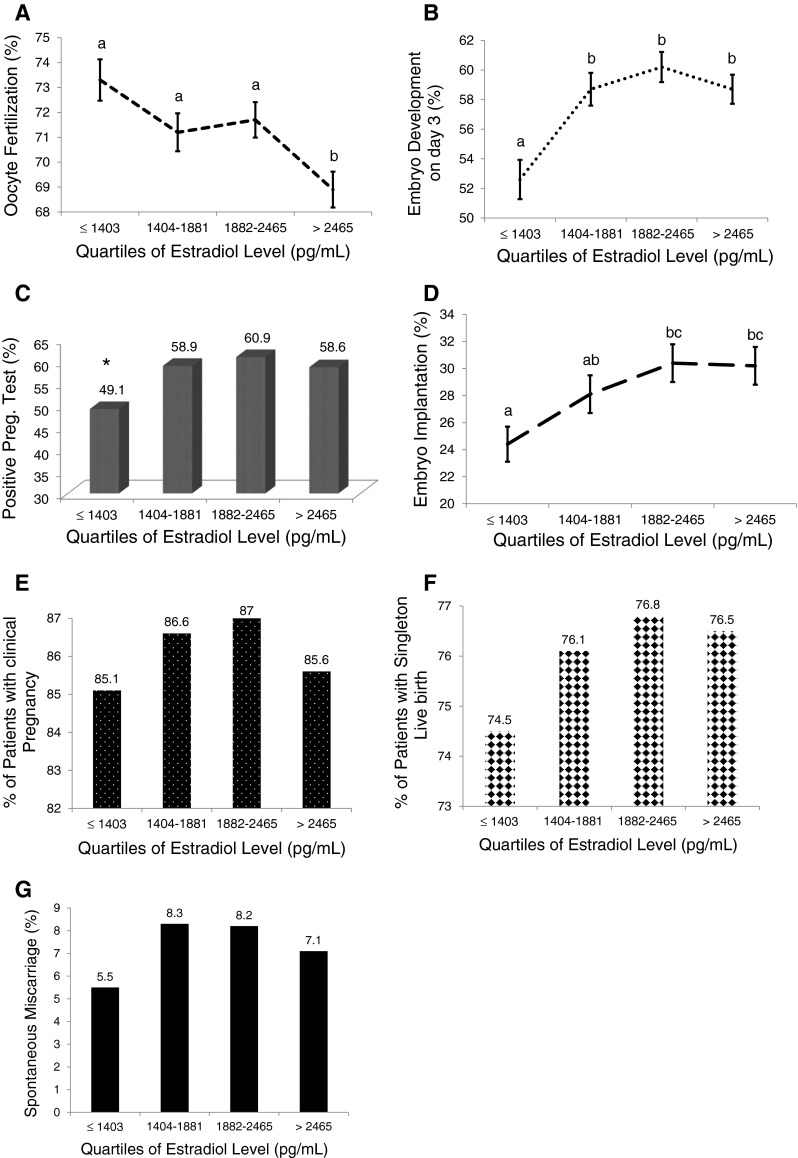
Relationship between IVF and pregnancy outcomes with increasing serum estradiol levels represented in quartiles. The rate of oocyte fertilization (a), stage specific embryo development (b), positive pregnancy test (c), embryo implantation (d), proportion of patients with clinical pregnancy after a positive pregnancy test (e), singleton live birth (f) and spontaneous miscarriage (g) increased with increasing serum estradiol level until 3rd quartile, with subsequent non-significant downward trend. Groups with different letters are significantly different. *p < 0.05 compared to other groups
Discussion
In this study, the peri-implantation supraphysiologic hormonal milieu unique to IVF was found to negatively impact the rate of normal oocyte fertilization, but not the rates of embryo development to 6–8 cells stage on day 3, positive pregnancy test, embryo implantation or spontaneous miscarriage. Cycles in the 3rd quartile of estradiol level (1,882–2,465 pg/mL) measured on the day of hCG administration consistently appear to be associated with optimal IVF and early pregnancy outcomes when compared with cycles in other quartiles of estradiol level. This finding is in agreement with other studies that have shown a negative correlation between elevated estradiol and normal oocyte fertilization during IVF, [20, 28–31]. However, unlike previous studies, we also show that these parameters displayed an inverted U-shaped dose response, confirming a typical biphasic response. Such inverted U-shaped curves are not uncommon and have recently been described in pharmacological [32], metabolic [33] and human [34] studies. The lack of correlation between patients with higher estradiol thresholds during IVF and embryo development on day 3, clinical pregnancy and miscarriage rates has also been shown in other studies [15, 35]. In the study by Gelety and colleagues [20], the IVF outcomes of patients with estradiol > 5,000 pg/mL were compared with those with estradiol < 3,500 pg/mL and found that patients with extremely high estradiol levels had lower normal fertilization rates compared with the control group. However, there was no difference in oocyte morphology, embryo implantation, rates of clinical pregnancy or miscarriage between the two groups. A possible critique of this study was that it only included 25 cycles in each group and fertilization rates in cycles with estradiol levels between >3,500 and 5,000 pg/mL were not included in the study. Additionally, none of these studies have shown any potential dose-effect relationship between estradiol and early reproductive outcomes during IVF.
This current study attempted to better understand the relationship between peri-implantation estradiol levels and IVF outcomes by stratifying the cycles into quartiles of estradiol to determine if a dose response effect exists. Normal oocyte fertilization, embryo development and positive pregnancy test rates each exhibited an overall negative trend with increasing estradiol level. This trend was less apparent with embryo implantation, clinical pregnancy, singleton live birth and spontaneous miscarriage rates. All outcomes, with the exception of oocyte fertilization rate, were worse in cycles in the lowest quartile of estradiol level (≤1,403 pg/mL). These outcomes improved and peaked in the 2nd and 3rd quartiles of estradiol level with decline in the measured parameters in the highest quartile in an inverted U-shaped manner as mentioned previously. There was no clear association between increasing estradiol levels and endometrial receptivity as measured by positive pregnancy test and implantation rate, suggesting that other factors intrinsic or extrinsic to IVF might additionally be operational.
Recent reports suggest that EE2 levels have a negative downstream effect on obstetrical outcomes (preeclampsia and small for gestational age) following IVF conception [12, 13]. However, the upstream effects on early reproductive outcomes have not been clearly established. Based on these reports, one would expect that elevated peri-implantation estradiol would lead to impaired endometrial receptivity with a higher propensity for abnormal placentation, leading to increased early pregnancy loss. The findings of this study, like those of others, did not support these assertions [15, 35, 36]. One reason that implantation and miscarriage rates seem grossly unaffected by the estradiol level might be due to the fact that the majority of early pregnancy failures occur as a result of genetic abnormalities, common in early pregnancy losses. This observation may additionally be due to type II error in these different studies.
The strengths of this work include the relatively large number of cycles studied and the analyses to determine whether a dose–response effect to estradiol exists. The findings of this study shed more light on the peri-implantation effect of EE2; however, we acknowledge the retrospective design of the study, with its inherent limitations. Additionally, this is a study from a single institution, and given the known variability in the estradiol immunoassay among different centers, it may be challenging to compare these results to those from other centers. Despite these limitations, the results of this study suggest that optimal peri-implantation outcome is associated with moderately elevated estradiol levels which in current study ranged from 1,900–2,500 pg/mL. We suggest that IVF units should examine their own data to determine the range of estradiol levels that maximize treatment outcome, and endeavor to keep stimulation at these levels.
Footnotes
Capsule Elevated estradiol levels on the day of hCG trigger is associated with lower oocyte fertilization during IVF
References
- 1.Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006;28:220–50. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 2.Bohlmann MK, Fritzsching B, Luedders DW, Hornemann A, Gopel W, Poschl J, et al. Impact of assisted reproduction on obstetrics and neonatology. Z Geburtshilfe Neonatol. 2009;213:221–7. doi: 10.1055/s-0029-1238275. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. New Engl J Med. 2012;366:1803–13. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 4.Gelbaya TA. Short and long-term risks to women who conceive through in vitro fertilization. Hum Fertil (Camb) 2010;13:19–27. doi: 10.3109/14647270903437923. [DOI] [PubMed] [Google Scholar]
- 5.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 6.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–48. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Perri T, Chen R, Yoeli R, Merlob P, Orvieto R, Shalev Y, et al. Are singleton assisted reproductive technology pregnancies at risk of prematurity? J Assist Reprod Genet. 2001;18:245–9. doi: 10.1023/A:1016614217411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retzloff MG, Hornstein MD. Is intracytoplasmic sperm injection safe? Fertil Steril. 2003;80:851–9. doi: 10.1016/S0015-0282(03)01014-8. [DOI] [PubMed] [Google Scholar]
- 9.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. New Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 10.Tan SL, Doyle P, Campbell S, Beral V, Rizk B, Brinsden P, et al. Obstetric outcome of in vitro fertilization pregnancies compared with normally conceived pregnancies. Am J Obstet Gynecol. 1992;167:778–84. doi: 10.1016/S0002-9378(11)91589-0. [DOI] [PubMed] [Google Scholar]
- 11.Calhoun KC, Barnhart KT, Elovitz MA, Srinivas SK. Evaluating the association between assisted conception and the severity of preeclampsia. ISRN Obstet Gynecol. 2011;2011:928592. doi: 10.5402/2011/928592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online. 2010;21:331–7. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–9. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–28. doi: 10.1042/CS19980019. [DOI] [PubMed] [Google Scholar]
- 15.Chen QJ, Sun XX, Li L, Gao XH, Wu Y, Gemzell-Danielsson K, et al. Effects of ovarian high response on implantation and pregnancy outcome during controlled ovarian hyperstimulation (with GnRH agonist and rFSH) Acta Obstet Gynecol Scand. 2007;86:849–54. doi: 10.1080/00016340701415152. [DOI] [PubMed] [Google Scholar]
- 16.Forman R, Fries N, Testart J, Belaisch-Allart J, Hazout A, Frydman R. Evidence for an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertil Steril. 1988;49:118–22. doi: 10.1016/s0015-0282(16)59661-7. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill C, Ferrier AJ, Vaughan J, Sinosich MJ, Saunders DM. Causes of implantation failure after in-vitro fertilisation and embryo transfer. Lancet. 1985;2:615. doi: 10.1016/S0140-6736(85)90615-4. [DOI] [PubMed] [Google Scholar]
- 18.Pellicer A, Valbuena D, Cano F, Remohi J, Simon C. Lower implantation rates in high responders: evidence for an altered endocrine milieu during the preimplantation period. Fertil Steril. 1996;65:1190–5. doi: 10.1016/s0015-0282(16)58337-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen QJ, Sun XX, Li L, Gao XH, Gemzell-Danielsson K, Cheng LN. Effects of ovarian stimulation on endometrial integrin beta3 and leukemia inhibitory factor expression in the peri-implantation phase. Fertil Steril. 2008;89:1357–63. doi: 10.1016/j.fertnstert.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Gelety TJ, Buyalos RP. The influence of supraphysiologic estradiol levels on human nidation. J Assist Reprod Genet. 1995;12:406–12. doi: 10.1007/BF02211139. [DOI] [PubMed] [Google Scholar]
- 21.Gratton RJ, Nisker JA, Daniel S, Toth S, Gunter J, Kaplan BR, et al. An aggressive philosophy in controlled ovarian stimulation cycles increases pregnancy rates. Hum Reprod. 1993;8:528–31. doi: 10.1093/oxfordjournals.humrep.a138089. [DOI] [PubMed] [Google Scholar]
- 22.Levi AJ, Drews MR, Bergh PA, Miller BT, Scott RT., Jr Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76:670–4. doi: 10.1016/S0015-0282(01)01988-4. [DOI] [PubMed] [Google Scholar]
- 23.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–7. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 24.Sharara FI, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. 1999;72:401–5. doi: 10.1016/S0015-0282(99)00293-9. [DOI] [PubMed] [Google Scholar]
- 25.Tarin JJ, Sampaio MC, Calatayud C, Castellvi RM, Bonilla-Musoles F, Pellicer A. Relativity of the concept ‘high responder to gonadotrophins’. Hum Reprod. 1992;7:19–22. doi: 10.1093/humrep/7.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- 26.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27:3583–92. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89:1702–8. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 28.Pellicer A, Ruiz A, Castellvi RM, Calatayud C, Ruiz M, Tarin JJ, et al. Is the retrieval of high numbers of oocytes desirable in patients treated with gonadotrophin-releasing hormone analogues (GnRHa) and gonadotrophins? Hum Reprod. 1989;4:536–40. doi: 10.1093/oxfordjournals.humrep.a136940. [DOI] [PubMed] [Google Scholar]
- 29.Tarin JJ, Pellicer A. Consequences of high ovarian response to gonadotropins: a cytogenetic analysis of unfertilized human oocytes. Fertil Steril. 1990;54:665–70. [PubMed] [Google Scholar]
- 30.Testart J, Belaisch-Allart J, Forman R, Gazengel A, Strubb N, Hazout A, et al. Influence of different stimulation treatments on oocyte characteristics and in-vitro fertilizing ability. Hum Reprod. 1989;4:192–7. doi: 10.1093/oxfordjournals.humrep.a136870. [DOI] [PubMed] [Google Scholar]
- 31.Testart J, Forman R, Belaisch-Allart J, Volante M, Hazout A, Strubb N, et al. Embryo quality and uterine receptivity in in-vitro fertilization cycles with or without agonists of gonadotrophin-releasing hormone. Hum Reprod. 1989;4:198–201. doi: 10.1093/oxfordjournals.humrep.a136871. [DOI] [PubMed] [Google Scholar]
- 32.Harrod SB, Lacy RT, Morgan AJ. Offspring of prenatal IV nicotine exposure exhibit increased sensitivity to the reinforcing effects of methamphetamine. Front Pharmacol. 2012;3:116. doi: 10.3389/fphar.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, et al. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15997. doi: 10.1371/journal.pone.0015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turer AT, Mahaffey KW, Honeycutt E, Tuttle RH, Shaw LK, et al. Influence of body mass index on the efficacy of revascularization in patients with coronary artery disease. J Thorac Cardiovasc Surg. 2009;137:1468–74. doi: 10.1016/j.jtcvs.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Chenette PE, Sauer MV, Paulson RJ. Very high serum estradiol levels are not detrimental to clinical outcome of in vitro fertilization. Fertil Steril. 1990;54:858–63. doi: 10.1016/s0015-0282(16)53946-6. [DOI] [PubMed] [Google Scholar]
- 36.Chen CH, Zhang X, Barnes R, Confino E, Milad M, Puscheck E, et al. Relationship between peak serum estradiol levels and treatment outcome in in vitro fertilization cycles after embryo transfer on day 3 or day 5. Fertil Steril. 2003;80:75–9. doi: 10.1016/S0015-0282(03)00504-1. [DOI] [PubMed] [Google Scholar]