Abstract
Purpose
To evaluate the efficacy of luteal phase support with vaginal progesterone in women undergoing intrauterine insemination (IUI).
Methods
Systematic review and meta-analysis. Randomized controlled trials (RCT) comparing supplementation of luteal phase with vaginal progesterone among women undergoing IUI versus a control group were included. The main outcome assessed was live birth rate.
Results
Five RCT met the inclusion criteria. In all 1,271 patients were included (951 IUI cycles in the progesterone group, 935 in the control group). Women treated with vaginal progesterone achieved significantly higher live birth rate (risk ratio [RR] 1.94, 95 % confidence interval [CI] 1.36 to 2.77,), and clinical pregnancy rate (RR 1.41, 95 % CI 1.14 to 1.76) as compared with controls. In the subgroup analysis per stimulation protocol, this beneficial effect of receiving progesterone was only observed in the group stimulated with gonadotropins (RR 2.28, 95 % CI 1.49 to 3.51), compared to the group stimulated with clomiphene citrate (CC) (RR 1.30, 95 % CI 0.68 to 2.50). No differences were observed in the miscarriage and multiple pregnancy rates.
Conclusions
The supplementation of luteal phase with vaginal progesterone significantly increases live birth among women undergoing IUI when receiving gonadotropins for ovulation induction. Women receiving CC to induce ovulation do not seem to benefit from this treatment.
Keywords: Progesterone, Luteal phase support, Intrauterine insemination, Randomized controlled trial
Introduction
Intrauterine Insemination (IUI) is a fertility treatment that employs different fertility drugs to achieve ovarian stimulation, and triggers ovulation with human chorionic gonadotropin (hCG), followed by the introduction sperm into the uterine cavity.
Despite being one of the oldest assisted reproductive technologies (ART), IUI continues to be widely used. Latest reports from European data provided by the European Society of Human Reproduction and Embryology (ESHRE), show a consistent increase throughout the years, reaching a total of 162.843 IUI cycles by husband (IUI-H) and 29.235 IUI cycles by donor (IUI-D) in 2009 [1].
Implantation and maintenance of an early pregnancy is known to be strongly dependent on the production of progesterone, the major product of the corpus luteum, by inducing a secretory transformation of the endometrium during luteal phase [2]. However, an adequate estrogen priming is necessary to achieve an adequate expression of progesterone receptors in the endometrium, and thus improve endometrial receptivity to progesterone [2].
With reference to the ART, various mechanisms have been proposed to explain the luteal deficiency observed particularly in IVF cycles, although there is some controversy in the published literature [2–6]. Indeed, one can assume that multifollicular development and consequently the supraphysiological estradiol levels secreted by the multiple corpora lutea during the early luteal phase exert a negative feedback of the hypothalamic-pituitary axis, inhibiting the LH release necessary for the corpus luteum function [7].
Certainly, the effect of progesterone supplementation during luteal phase in IVF cycles has been addressed in several studies, and there is more consensus with regard to its beneficial role on reproductive outcomes [8]. However, this effect seems less consistent in IUI cycles.
The aim of this meta-analysis is to assess the effect of supplementing luteal phase with vaginal progesterone on live birth among patients undergoing IUI.
Materials and methods
The study was exempt from Institutional Review Board approval because this was a systematic review and meta-analysis.
We endorsed the preferred reporting items to systematic review and meta-analysis (PRISMA statement) to report the results of this systematic review [9].
Search strategy
We performed an exhaustive electronic search in the following databases: MEDLINE, EMBASE (from their inception until January 2013), and The Cochrane Central Register of Controlled Trials (CENTRAL) (issue 1, 2013). The search combined terms and descriptors related to progesterone, luteal phase and intrauterine insemination. The search strategy was modified to comply with the requirements of each database consulted. We added validated filters to that strategy to retrieve clinical trials [10, 11]. Moreover, we searched for ongoing trials at the main clinical trials registers, including www.controlled-trials.com, www.clinicaltrials.gov and the WHO International Clinical Trials Registry Platform (www.who.int/trialsearch). No language limits were used. Reference lists of all identified articles and overviews, and a Science Citation Index Search (SciSearch, ISI Web of Knowledge) of relevant articles, provided additional sources of potentially eligible clinical trials.
Eligibility criteria
The review included randomized controlled clinical trials (RCTs) of women undergoing IUI of whether the cause of infertility or subfertility was due to male or female factors. The type of intervention evaluated was the administration of vaginal progesterone during luteal phase support compared without treatment or the administration of placebo during luteal phase (Table 1).
Table 1.
Study eligibility criteria
| Target population | Infertile patients undergoing intrauterine insemination |
|---|---|
| Intervention | Luteal phase support with progesterone versus a control group |
| Outcome measure | One or more of the following (per patient or per cycle) |
| • Live birth (per cycle analysed) | |
| • Ongoing pregnancy (per cycle analysed) | |
| • Clinical pregnancy (per cycle analysed) | |
| • Preclinical pregnancy or biochemical pregnancy (per cycle analysed) | |
| • Biochemical loss (per cycle analysed) | |
| • Miscarriage (per cycle analysed) | |
| • Multiple pregnancy (per cycle analysed) | |
| Design | Randomized controlled trial |
Outcome measures
The main outcome of interest for the review was live birth rate. Secondary outcomes were clinical pregnancy rate, biochemical pregnancy rate and adverse events, which include multiple pregnancy and miscarriage rates. All the outcomes of interest were considered per cycle analyzed.
The outcomes were defined according to the terminology recommended in the International Committee Monitoring Assisted Reproductive Technologies (ICMART) [12]. According to this, live birth is defined as the expulsion of its mother of a product of fertilization, irrespective of the duration of the pregnancy, which after such separation shows any evidence of life. Clínical pregnancy is defined as a pregnancy which is diagnosed by ultrsonographic visualization of one or more gestational sacs or definitive clinical signs of pregnancy, while biochemical pregnancy refers to a pregnancy diagnosed only by the detection of hCG in serum or urine. Regarding adverse events, multipe pregnancy (or mltiple gestation) is defined by a pregnancy with more than one fetus, while miscarrage refers to the spontaneous loss of a pregnancy before 20 completed weeks of gestational age.
Data extraction
Data were collected using standard forms in which characteristics of the study design, participants, interventions, comparisons, and main results were recorded. Two independent authors (E.M.R., and P.N.B) judged study eligibility, assessed quality and extracted data solving discrepancies by agreement, and if needed, reaching consensus with a third author (M.A.C). The agreement between reviewers was analyzed using the weighted kappa for each inclusion criterion [13] and kappa with quadratic weighting for the quality components [14].
Assessment of risk of bias
We assessed the risk of bias of the studies included in the review according to the Cochrane Collaboration’s recommended tool [15], which is a two-part tool, addressing six specific domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and ‘other issues’) and answering a pre-specified question about the adequacy of the study in relation to the entry. A judgment of “yes” for all domains indicates a low risk of bias, a judgement of “no” for one or more domains indicates a high risk of bias. We interpreted the risk of bias in the specific domains as “unclear” when the information was not available. The risk of bias for the included trials is detailed in Table 2.
Table 2.
Methodological data of the clinical trials included in the review
| Criterion | Author, year (reference) | ||||
|---|---|---|---|---|---|
| Agha-Hosseini, 2012 [18] | Maher, 2011 [19] | Kyrou, 2010 [20] | Ebrahimi, 2010 [21] | Erdem, 2009 [22] | |
| Explicit eligibility criteria | Yes | Yes | Yes | Yes | Yes |
| Sequence generation | Yes (computer-generated random allocation) | Yes (computer-generated random allocation in each patient for the first cycle and subsequently alternated from then onward) | Yes (computer-generated random allocation) | No (patients were sequentially allocated in each patient for the first cycle, remaining in the same intervention group from then onward) | Yes (computer-generated random allocation in each patient for the first cycle, remaining in the same intervention group from then onward) |
| Allocation concealed | Yes (nurses gave treatment according to allocation charts derived from randomized sequence) | Not described | No (the computer generated list was not concealed to investigators) | No (a nurse sequentially allocated patients) | Yes (an investigator held the random sequence, and two additional investigators performed the insemination procedure) |
| Patient blinding | Open trial | Open trial | Open trial | Open trial | Open trial |
| Outcome assessor blinding | Not described | Not described | Not described | Open trial | Not described |
| Patients lost to follow-up (%) | No patient lost to follow-up. Drop outs: 10 out of 300 patients (3.3 %): 5 patients (2 in the progesterone group, 3 cycles in the control group) did not start the IUI cycle, and 5 additional patients in the control group were non-responders. |
No patient lost to follow-up. Drop outs: - 1 out of 71 patients (1.43 %) after the fourth cycle because an IVF cycle was requested. - 25 of the 283 cycles initiated were abandoned (19 due to hyperstimulation, and 6 due to poor response). |
No patient lost to follow-up. Drop outs: 68 out of 468 patients (14,53 %): - 4 patients left due to personal reasons after randomization - 12 patients (7 in the progesterone group and 5 in the control group) did not start the IUI cycle due to abnormal steroid levels. - 52 patients (38 in the progesterone group, 14 in the control group) after stimulation abandoned due to poor response. |
No patient lost to follow-up. Drop outs: 21 out of 200 patients (10.5 %, 11 in the progesterone group, 10 in the control group) dropped out after the 2nd cycle due to unspecified reasons. |
No patient lost to follow-up. Drop outs: 65 out of 214 patients (30.4 %, 30 in the progesterone group, 35 in the control group): - 21 abandoned because an IVF cycle was requested - 5 cycles were cancelled due to hyperstimulation. |
| Risk of bias | Low risk of bias | Low risk of bias | High risk of bias (due to an incorrect allocation concealment) | High risk of bias (due to an incorrect randomisation) | Low risk of bias |
As we did not expect to identify blinded trials, we centered our assessment in the accuracy of the random sequence generation and its concealment. Those studies with limitations in this design were considered biased, with a limitation in the confidence in their results. We performed a sensitivity analysis excluding studies with a high risk of bias from the pooled effect estimates.
Besides, in order to assess the risk of publication bias, a funnel plot followed by Egger’s test to evaluate a possible asymmetry would be performed if the number of trials included in this metaanalysis reaches 10 or higher, since the power of this test would be too low to distinguish chance from real asymmetry [16].
Analysis
To determine the pooled effect of each variable, a random effects model was used. The relative risk (RR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes accompanied by the 95 % confidence intervals (CIs) were calculated. Statistical significance was set at a P value <.01. We evaluated the degree of variation across studies attributable to heterogeneity with the I2 statistic [17]. We conducted the meta-analysis using Review Manager software (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Results
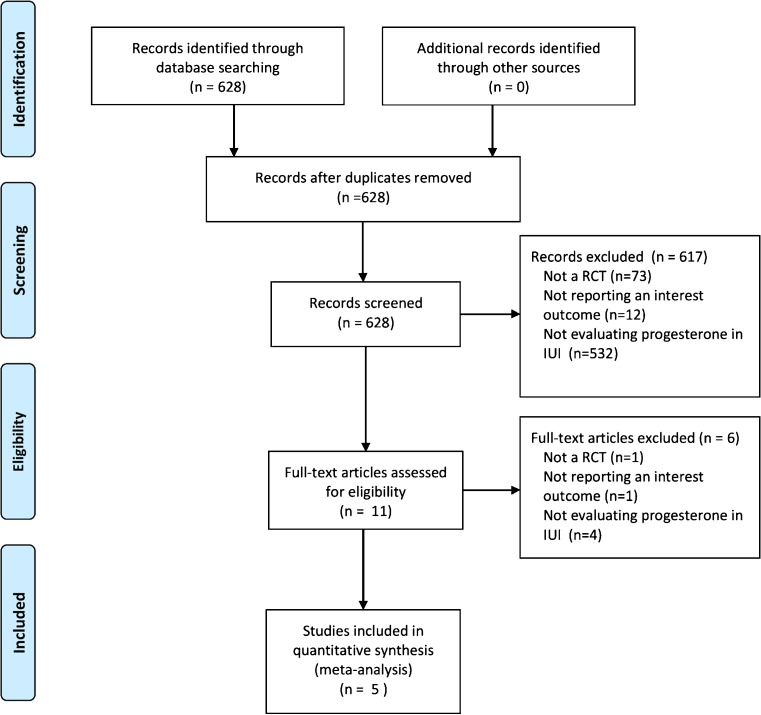
A total of 684 studies were retrieved in the initial electronic search but 617 were excluded by title and/or abstract screening. The remaining 11 studies were considered eligible by one or both reviewers. During the second phase of the inclusion process, out of the 11 trials, 1 was excluded because of non-randomized comparisons [18] and 5 because they did not evaluate the intervention nor the outcomes of interest. Finally, 5 RCT met the inclusion criteria and were included [19–23]. The flow chart of the included in the meta-analysis is shown in Fig. 1. The two reviewers achieved good agreement in the selection of trials for inclusion (98 % agreement, weighted kappa 0.56, 95 % CI 0.28–0.83).
Fig. 1.
Flow chart for the trial identification and selection process
Description of included studies
A total of 1,271 women were randomized to an intervention group receiving vaginal progesterone during luteal phase (n = 951) and a control group without treatment (n = 935), and 1,886 cycles were analyzed.
Two trials considered for inclusion only one cycle per woman randomized [19, 21]. Meanwhile, in the remaining three trials [20, 22, 23], several cycles per patient were analyzed, as shown in Table 2. All studies performed the randomization process per woman. Trials that included more than one cycle, from the second cycle onward, the patient either remained in the same group [22, 23] or alternated groups subsequently [20]. Hence, results from this meta-analysis are expressed per cycle analyzed, since an intention-to-treat analysis, methodologically, cannot be performed.
The mean age reported across the studies was comparable and each study reported and in both intervention and control group.
The inclusion criteria were relatively heterogeneous among the studies, especially regarding the distribution of women undergoing IUI. Unexplained primary aetiology was considered in all trials. However, male factor was only included in the trials by Maher et al. and Kyrou et al., while lesbian and single mother were only considered by Kyrou et al. [20, 21].
With regard to the ovarian stimulation protocol, there are also notable differences across the five studies. Maher et al. [20] and Erdem et al. [23] used 75 IU recombinant follicle stimulating hormone (rFSH), while Ebrahimi et al. [22], Kyrou et al. [21] and Agha-Hosseini et al. [19] used 50 mg of clomiphene citrate (CC) in different regimens. This later trial also administered 5 mg of letrozole daily in one of their study groups.
Ovulation was triggered in all studies with hCG when at least one to three dominant follicles were bigger than 17 mm. Kyrou et al. and Ebrahimi et al. used 5,000 IU of hCG [21, 22], while Agha-Hosseini et al., Maher et al. and Erdem et al. used 10,000 IU [19, 20, 23].
With reference to luteal phase support within the intervention group, there was also certain heterogeneity across the trials regarding dosage and duration of the treatment. Ebrahimi et al. and Agha-Hosseini et al. used 400 mg of vaginal progesterone suppositories daily until the 10th and12th week, respectively [19, 22], while Kyrou et al. used 200 mg of vaginal progesterone suppositories every 8 h until the 7th week [21]. In contrast, Erdem et al. used 90 mg of vaginal progesterone once daily until the 12th week [23], while Maher et al. used the same posology but only for 14 days [20].
Characteristics of the studies included in the meta-analysis are shown in Table 3.
Table 3.
Characteristics of the clinical trials included in the review
| Author, year | Total patients | Total cycles | Age years | Cause infertility | Stimulation of ovulation | Triggering treatment | Luteal phase support | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Treatment | No. intervention/control | Treatment | |||||||
| Agha-Hosseini, 2012 [18] | 300 | 290 | 27 | Unexplained infertility | 38 | Clomiphene citrate 50 mg/12 h for 5 days | 10.000 hCG | 148/142 | 400 mg vaginal progesterone suppositories (Cyclogest®) once daily until the 12th week | CPb, BPc, MPd, Miscarriage |
| 96 | Letrozole 5 mg/24 h for 5 days | |||||||||
| 66 | Clomiphene citrate 5 days + hMGa 75 IU 5 days | |||||||||
| 90 | Letrozole 5 days + hMG 75 IU 5 days | |||||||||
| Maher, 2011 [19] | 71 | 258 | 30 | Female (ovarian, tubal), male, combined, unexplained | 258 | rFSH 75 IU | 10.000 hCG | 132/126 | 90 mg vaginal progesterone gel (Crinone® 8 %) once daily for 14 days | LBe, CP, BP, MP, Miscarriage |
| Kyrou, 2010 [20] | 468 | 400 | 32 | Male, lesbian, single mother, unexplained. | 400 | Clomiphene citrate 50 mg | 5.000 hCG | 196/204 | 200 mg/8 h vaginal progesterone suppositories (Utrogestan®) until the 7th week | CP, BP, MP, Miscarriage |
| Ebrahimi, 2010 [21] | 200 | 511 | 28 | Unexplained infertility | 511 | Clomiphene citrate 50 mg/12 h for 5 days + hMG 75 IU until hCG | 5.000 hCG | 252/259 | 400 mg vaginal progesterone suppositories (Cyclogest®) once daily until the 10th week | LB, CP, BP, MP, Miscarriage |
| Erdem, 2009 [22] | 214 | 427 | 30 | Unexplained infertility | 427 | rFSH 75 IU | 10.000 hCG | 223/204 | 90 mg vaginal progesterone gel (Crinone® 8 %) once daily until the 12th week | LB, CP, BP, MP, Miscarriage |
a hMG human menopausal gonadotropin
b CP clinical pregnancy
c BP biochemical pregnancy
d MP multiple pregnancy
e LB live birth
Internal validity of included studies
In general, trials did not provide complete data regarding methodological aspects of the RCT included in this meta-analysis. Four trials performed randomization through computer-generated sequences [19–21, 23], except for Ebrahimi et al., who performed sequential allocation [22].
In addition, outcome assessment blinding remains unclear in all studies since they do not provide with this information. However, since none of the trials was placebo-controlled, patients were not blinded to the intervention. The methodological data regarding the included trials are shown in Table 2.
Main outcomes
Live birth
Only three studies reported this outcome in their published data [20, 22, 23].
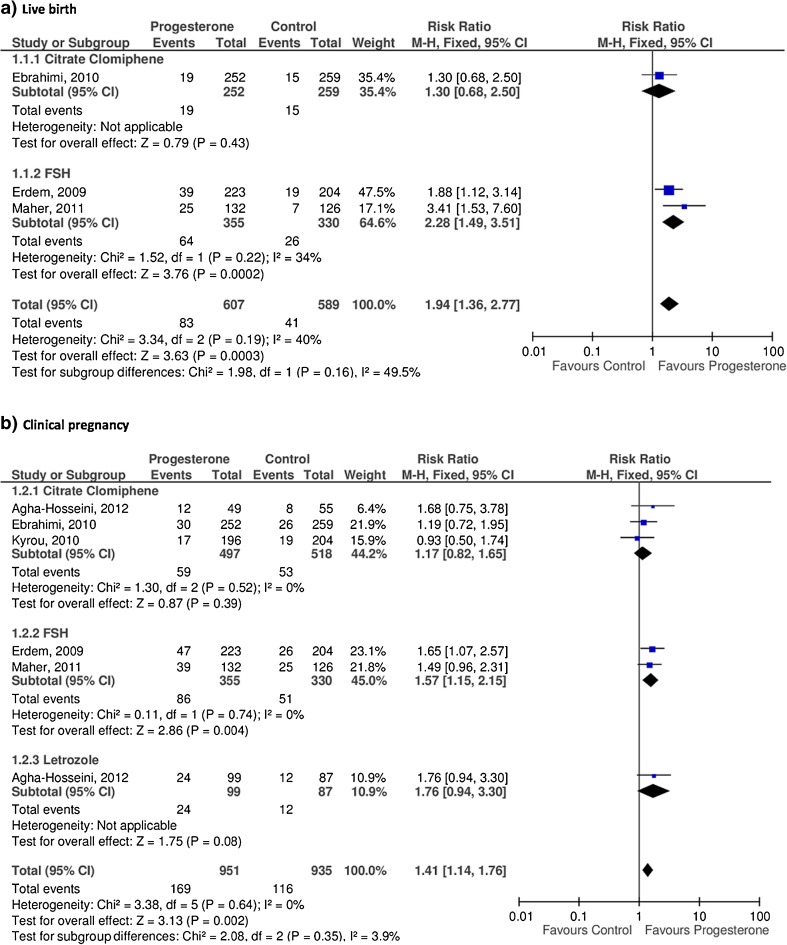
The three studies achieved a higher number of live births among women receiving vaginal progesterone during luteal phase (83 events out of 607 cycles), compared with the control group (41 events out of 589 cycles). After performing a subgroup analysis according to the ovarian stimulation protocol, the group that received rFSH [20, 23] showed higher live birth rate among women receiving progesterone, results that were statistical significant (RR 2.28, 95 % CI 1.49 to 3.51, p < 0.000). However, the group stimulated with CC [22] did not show differences among women receiving progesterone compared to the control group (RR 1.30, 95 % CI 0.68 to 2.50, p = 0.43).
The pooled analysis of the data from the three trials found that women receiving vaginal progesterone achieved significantly higher number of live births as compared with women in the control group (RR 1.94, 95 % CI 1.36 to 2.77), although these results showed moderate inconsistency (p < 0.000, I2 = 40 %) (Fig. 2a).
Fig. 2.
Efficacy of progesterone supplementation
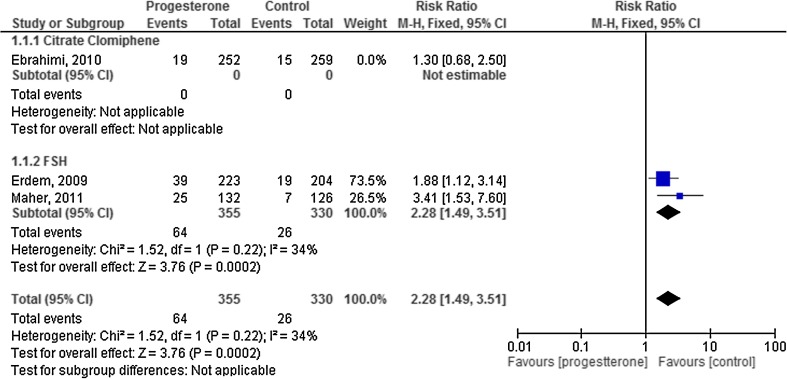
When we considered only the studies at low risk of bias in a sensitivity analysis, the difference between groups were larger (RR 2.28, 95 % CI 1.49 to 3.51), and reslts appeared to be slightly more consistent (I2 = 34 %) (Fig. 3.)
Fig. 3.
Sensitivity analysis for live birth
Clinical pregnancy
The five studies provided data for the outcome of clinical pregnancy, achieving a total of 169 events out of 951 cycles in the progesterone group, and 116 events out of 935 cycles in the control group [19–23].
In the subgroup analysis according to the ovarian stimulation protocol, the group stimulated with rFSH [20, 23] achieved higher number of clinical pregnancies among women in the progesterone group as compared to the control group (RR 1.57, 95 % CI 1.15 to 2.15, p = 0.004). Neither the group stimulated with letrozole [19] nor the CC group [19, 21, 22] showed any differences with regard to clinical pregnancy rate between the intervention and the control group (RR 1.76, 95 % CI 0.94 to 3.30, p = 0.08, and RR 1.17, 95 % CI 0.82 to 1.65, p = 0.39, respectively).
The pooled analysis showed a significantly higher number of clinical pregnancies among women receiving progesterone as compared with the control group (RR 1.41, 95 % CI 1.14 to 1.76, p = 0.002, I2 = 0 %) (Fig. 2b).
Biochemical pregnancy
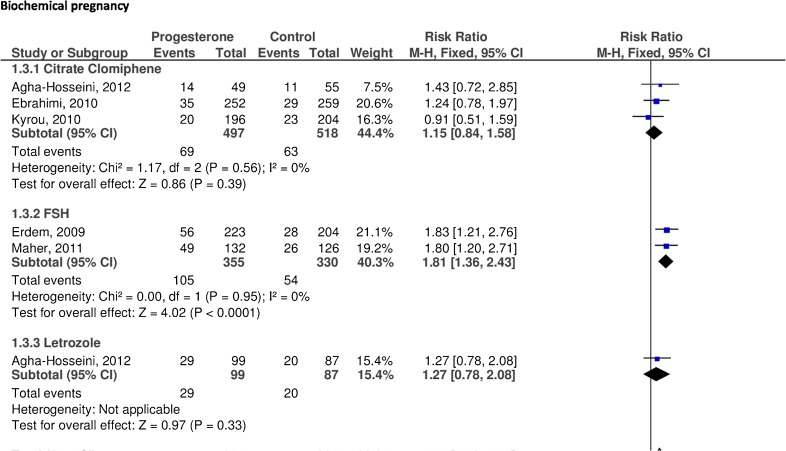
The five studies reported data regarding this outcome, achieving 203 events out of 951 cycles in the progesterone group, and 137 events out of 935 cycles in the control group.
In a subgroup analysis, the group stimulated with rFSH achieved significantly higher biochemical pregnancies in the progesterone group as compared to the control group (RR 1.81, 95 % CI 1.36 to 2.43, p < 0.000). However, in the other two stimulation protocols (letrozole and CC) did not find statistically significant differences (RR 1.27, CI 95 % 0.78 to 2.08, p = 0.33, and RR 1.15, CI 95 % 0.84 to 1.58, p = 0.39, respectively).
The pooled analysis showed a significantly higher number of biochemical pregnancies among women in the progesterone group as compared with the control group (RR 1.44, 95 % CI 1.18 to 1.75, p < 0.000, I2 = 12 %) (Fig. 4).
Fig. 4.
Efficacy of progesterone supplementation
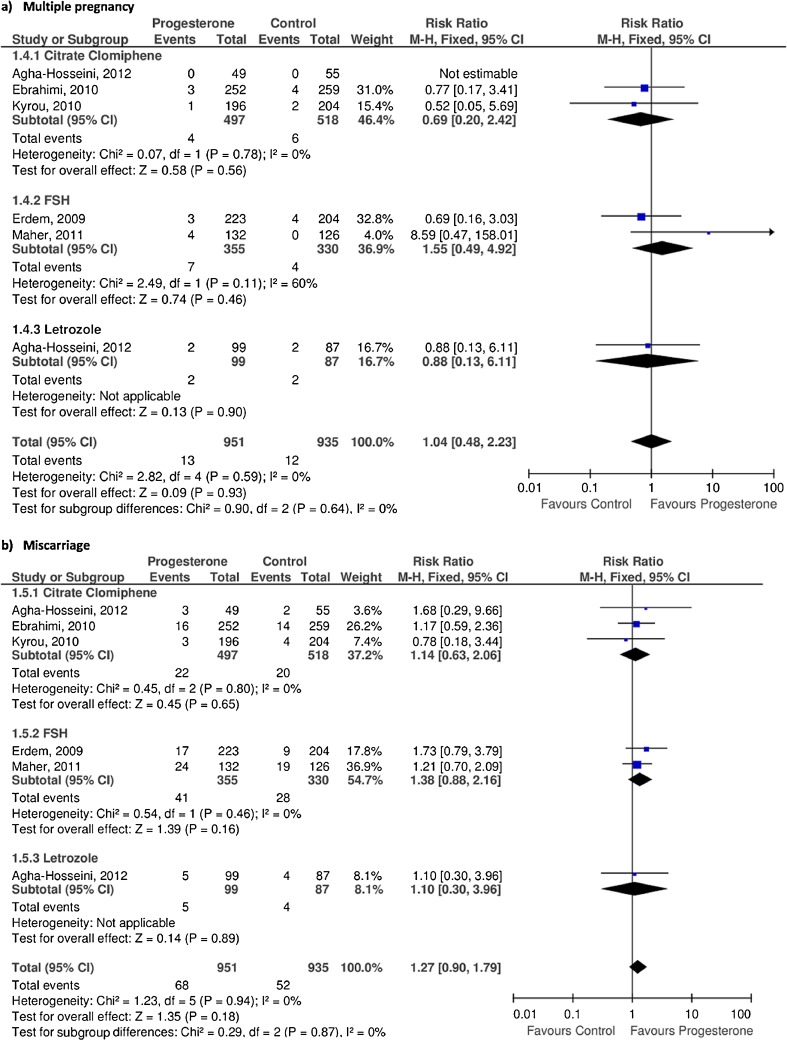
Adverse events
Multiple pregnancy
The five trials provided data regarding multiple pregnancy, with 13 events out of 951 cycles in the progesterone group, and 12 events out of 935 cycles in the control group.
The subgroup analysis did not show significant differences in any of the stimulation protocols (rFSH, letrozole, CC) when comparing the progesterone and the control group.
The pooled analysis did not detect significant differences between the two study group (RR 1.04, 95 % CI 0.48 to 2.23, p = 0.93, I2 = 0 %) (Fig. 5a).
Fig. 5.
Results of progesterone supplementation in terms of adverse events
Miscarriage
Four of the studies provided [19–21, 23] data regarding miscarriage rate. However, Ebrahimi et al. [22] did not report this outcome, although it was calculated indirectly through outcomes of live birth and biochemical pregnancy.
Out of 1,886 cycles, in the progesterone group 68 miscarriages were observed out of 951 cycles, and 52 out of 935 in the control group.
In the subgroup analysis, no differences were observed regarding miscarriage rate between the two study groups in any of the stimulation protocols (rFSH, letrozole, CC).
In the pooled analysis, no statistically significant differences were found between the progesterone group as compared to the control group (RR 1.27, 95 % CI 0.90 to 1.79, p = 0.18, I2 = 0 %) (Fig. 5b).
Discussion
This systematic review and meta-analysis gathered previously published evidence and data obtained from the original authors to provide pooled estimates regarding the use of vaginal progesterone as luteal phase support among women undergoing IUI, compared to a control group. The analysis showed a statistically significant increase in live birth in women receiving vaginal progesterone, without increasing miscarriage and multiple pregnancy rates. However, these differences were observed only in the subgroup of women who received rFSH to induce ovulation.
This is the first systematic review and meta-analysis that evaluates the efficacy of the vaginal progesterone during luteal phase in patients undergoing IUI, in which only randomized controlled trials were included.
One of the strengths is the large number of IUI cycles included, with a total of 1,886 cycles. In addition, the present study used live birth as the primary outcome, since it should be the most relevant outcome in reproductive disorders. On the other hand, data recorded from all the included trials showed that vaginal progesterone supplementation does not increase the risk of adverse events, such as multiple pregnancy and miscarriage rates (Fig. 5).
Despite the strengths mentioned above, the current study has several limitations that need to be considered. First, there is clinical heterogeneity in terms of inclusion criteria, as mentioned previously in the ‘description of the included studies’, particularly the study by Kyrou [21], whose trial included a subgroup of non-subfertile women (lesbian and single mothers), unlike the other RCTs (Table 3).
There is also marked heterogeneity with regard to the ovarian stimulation protocol used throughout the trials, as well as, the dose and duration of treatment with vaginal progesterone. While four of the studies used either CC or rFSH as a single protocol of stimulation [20–23], the trial by Agha-Hosseini et al. [19] established four different regimens using CC and letrozole (Table 3). With reference to the progesterone supplementation, there is limited evidence regarding the dose required and the duration of the treatment, albeit there is homogeneity throughout the trials at using natural micronized progesterone. There is also heterogeneity in the hCG dose to induce ovulation (5.000 or 10.000 UI). This is a significant event for its long half-life and its impact along the luteal phase.
In addition, results for clinical and biochemical pregnancy, as well as multiple pregnancy and miscarriage rates, show high consistency across the studies (defined by I2) [22]. However, it is necessary to highlight the moderate inconsistency of the results in the pooled analysis for live birth, albeit live birth rate is significantly higher in the progesterone group (Fig. 2a). To a certain extent, this could be explained by the fact that only three trials reported this outcome, since the pooled analysis of the remaining outcomes (clinical and biochemical pregnancies, and multiple pregnancy and miscarriage) showed consistency (Figs. 2b, 4 and 5). Besides, when performing a subgroup analysis on live birth per stimulation protocol, the trial using CC [23] did not show significant differences, while the two studies using rFSH [20, 24] achieved significantly higher live births.
When performing a sensitivity analysis by excluding studies at high risk of bias, in terms of live birth, the difference between groups were larger (RR 2.28, 95 % CI 1.49 to 3.51). Interestingly, in this analysis that considered only trials that performed ovarian stimulation with rFSH, the results showed higher consistency (I2 = 34 %).
Furthermore, regarding methodological aspects, it is necessary to point out that the present meta-analysis evaluates results per cycle analysed, and does not perform an intention-to-treat analysis. In this regard, three of the trials included more than one cycle per woman proceeding to randomization per woman instead of per cycle. Thus, from the second cycle onward, depending on the trial’s design, the patient either remained in the same group [23, 24] or alternated groups subsequently [20], impeding the analysis per woman randomized.
Despite all these limitations, a significant increase in live birth was found when supplementing luteal phase with vaginal progesterone. When performing a subgroup analysis, these differences observed in live birth and clinical pregnancy rates were only found in the group receiving rFSH to induce ovulation. Conversely, when using either CC or letrozole for ovarian stimulation, progesterone administration did not improve these reproductive outcomes (Fig. 2), results that could be explained by the biological role of these two drugs.
As a selective aromatase inhibitor, letrozole prevents the conversion from androgens to estrogen, releasing the hypothalamic-pituitary axis from the negative feedback exerted by estrogens. Its role as an ovulation inductor is based on this hypoestrogenic state, which increases GnRH release and thus FSH levels [25].
Likewise, CC exerts comparable effects as an ovulation inductor [26]. Its biological role is based on its capability to block estrogenic hypothalamic receptors, thanks to its structural similarity with estrogen [27]. Thus, similarly to letrozole, the hypoestrogenic environment promotes the GnRH pulsatility, increasing levels of FSH and LH. Clomiphene citrate has a prolonged effect on the hypothalamic receptors and, interestingly, it has been documented that women using CC for ovarian stimulation achieved higher serum progesterone levels during the mid-luteal phase, as compared to spontaneous cycles [28].
With regard to the exogenous administration of rFSH, follicular development is achieved by direct stimulation of granulosa cells in the ovary. The aspiration of granulosa cells of multiple follicles and the use of GnRH agonists in IVF cycles can cause a deficient luteal phase. However, one can assume that the main cause of deficient luteal phase in IVF cycles is probably the multifollicular growth and the supraphysiological estrogen levels, that interfere with LH secretion via negative feedback [7]. Hence, since more than one dominant follicle is often obtained in IUI cycles, it seems reasonable to conceive that the hormonal levels achieved in IUI when stimulating with rFSH could potentially mimic the effect observed in IVF cycles, although at a lower level. In fact, a shortened luteal phase has been reported in up to 20 % of stimulated cycles with rFSH [29].
Therefore, it seems biologically plausible that particularly IUI cycles stimulated with rFSH would benefit from progesterone supplementation during luteal phase, which is in agreement with the results presented herein.
In conclusion, there is evidence to suggest that luteal phase support with vaginal progesterone significantly improves live birth rate among women undergoing IUI, particularly when using rFSH to induce ovulation. However, these results should be interpreted with caution since vaginal progesterone showed positive results only in one subgroup of analysis. In addition, since this meta-analysis includes a low number of events in terms of live birth, the inclusion of studies with a larger sample size could change the direction of these results. Thus, further studies are needed to confirm these findings.
Acknowledgments
We thank Pau Nicolau Batalla, for his assistance when performing the systematic review.
Declaration
The authors report no financial nor commercial conflict of interest.
Footnotes
Capsule Luteal phase support with vaginal progesterone in women undergoing intrauterine insemination significantly improves live birth rate.
Contributor Information
Ester Miralpeix, Email: emiralpeix@parcdesalutmar.cat.
Mireia González-Comadran, Email: mgonzalezcomadran@parcdesalutmar.cat.
Ivan Solà, Email: tsc@cochrane.es.
Dolors Manau, Email: dmanau@clinic.ub.es.
Ramon Carreras, Email: rcarreras@parcdesalutmar.cat.
Miguel A. Checa, Phone: +34-93-2483129, FAX: +34-93-2483254, Email: macheca@hospitaldelmar.cat
References
- 1.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–31. [DOI] [PubMed]
- 2.Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13:581–590. doi: 10.1093/humupd/dmm021. [DOI] [PubMed] [Google Scholar]
- 3.Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, et al. Pituitary gonadotropin secretory capacity during the luteal phase in superovulation using GnRH-agonists and HMG in a desensitization or flare-up protocol. Hum Reprod. 1992;7:1225–1229. doi: 10.1093/humrep/7.suppl_1.49. [DOI] [PubMed] [Google Scholar]
- 4.Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186–4192. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- 5.Tavaniotou A, Albano C, Smitz J, Devroey P. Comparison of LH concentrations in the early and mid-luteal phase in IVF cycles after treatment with HMG alone or in association with the GnRH antagonist Cetrorelix. Hum Reprod. 2001;16:663–667. doi: 10.1093/humrep/16.4.663. [DOI] [PubMed] [Google Scholar]
- 6.Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12:333–340. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 7.Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–242. doi: 10.1016/S1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 8.Van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD009154. doi:10.1002/14651858.CD009154. [DOI] [PubMed]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc. 2006;94:130–136. [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe G, Twaddle S. The Scottish Intercollegiate Guidelines Network (SIGN): an update. Scott Med J. 2005;50:51–52. doi: 10.1177/003693300505000202. [DOI] [PubMed] [Google Scholar]
- 12.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 14.Donner A, Klar N. The statistical analysis of kappa statistics in multiple samples. J Clin Epidemiol. 1996;49:1053–1058. doi: 10.1016/0895-4356(96)00057-1. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Gren S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org of subordinate document. Accessed 22 Jul 2013.
- 16.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;22:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 17.Beretsos P, Partsinevelos GA, Arabatzi E, Drakakis P, Mavrogianni D, Anagnostou E, et al. “hCG priming” effect in controlled ovarian stimulation through a long protocol. Reprod Biol Endocrinol. 2009;7:91. doi: 10.1186/1477-7827-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montville CP, Khabbaz M, Aubuchon M, Williams DB, Thomas MA. Luteal support with intravaginal progesterone increases clinical pregnancy rates in women with polycystic ovary syndrome using letrozole for ovulation induction. Fertil Steril. 2010;94:678–683. doi: 10.1016/j.fertnstert.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 19.Agha-Hosseini M, Rahmani M, Alleyassin A, Safdarian L, Sarvi F. The effect of progesterone supplementation on pregnancy rates in controlled ovarian stimulation and intrauterine insemination cycles: a randomized prospective trial. Eur J Obstet Gynecol Reprod Biol. 2012;165:249–253. doi: 10.1016/j.ejogrb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Maher MA. Luteal phase support may improve pregnancy outcomes during intrauterine insemination cycles. Eur J Obstet Gynecol Reprod Biol. 2011;157:57–62. doi: 10.1016/j.ejogrb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Kyrou D, Fatemi HM, Tournaye H, Devroey P. Luteal phase support in normo-ovulatory women stimulated with clomiphene citrate for intrauterine insemination: need or habit? Hum Reprod. 2010;25:2501–2506. doi: 10.1093/humrep/deq223. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebrahimi M, Asbagh FA, Darvish S. The effect of luteal phase support on pregnancy rates of the stimulated intrauterine insemination cycles in couples with unexplained infertility. Int J Fertil Steril. 2010;4:51–56. [Google Scholar]
- 24.Erdem A, Erdem M, Atmaca S, Guler I. Impact of luteal phase support on pregnancy rates in intrauterine insemination cycles: a prospective randomized study. Fertil Steril. 2009;91:2508–2513. doi: 10.1016/j.fertnstert.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Requena A, Herrero J, Landeras J, Navarro E, Neyro JL, Salvador C, et al. Use of letrozole in assisted reproduction: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:571–582. doi: 10.1093/humupd/dmn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badawy A, Abdel Aal I, Abulatta M. Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2009;92:849–852. doi: 10.1016/j.fertnstert.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 27.Geier A, Lunenfeld B, Pariente C, Kotev-Emeth S, Shadmi A, Kokia E, et al. Estrogen receptor binding material in blood of patients after clomiphene citrate administration: determination by a radioreceptor assay. Fertil Steril. 1987;47:778–784. [PubMed] [Google Scholar]
- 28.Dickey RP. Evaluation and management of threatened and habitual first trimester abortion. In: Osofsky H, editor. Advances in clinical obstetrics and gynecology. Chicago: Yearbook Medical Publishers; 1984. pp. 329–388. [Google Scholar]
- 29.Olson JL, Rebar RW, Schreiber JR, Vaitukaitis JL. Shortened luteal phase after ovulation induction with human menopausal gonadotropin and human chorionic gonadotropin. Fertil Steril. 1983;39:284–291. [PubMed] [Google Scholar]