Abstract
Purpose
The present study was designed to test the hypothesis that long-term treatment with hydrogen-rich saline abated testicular oxidative stress induced by nicotine in mice.
Methods
The effects of hydrogen-rich saline (6 ml/kg, i.p.), vitamin C (60 mg/kg, i.p.) and vitamin E (100 mg/kg, i.p.) on reproductive system and testicular oxidative levels in nicotine-treated (4.5 mg/kg, s.b.) mice were investigated.
Results
It was found that vitamin C and vitamin E attenuated serum oxidative level, but did not lower testicular oxidative levels in mice subjected to chronic nicotine treatment, and did not improve the male reproductive damage and apoptosis induced by nicotine. Different from normal antioxidants, vitamin C and vitamin E, hydrogen-rich saline abated oxidative stress in testis, and protected against nicotine-induced male reproductive damages.
Conclusion
Our results first demonstrated that long-term treatment with hydrogen-rich saline attenuated testicular oxidative level and improved male reproductive function in nicotine-treated mice.
Keywords: Hydrogen-rich saline, Vitamin C, Vitamin E, Male reproductive system, Oxidative stress, Apoptosis
Introduction
An increasing number of studies have demonstrated that there is a detrimental effect of cigarette smoking on spermatogenesis and steroidogenesis [1–4]. Ultrastructural analysis revealed that in nicotine-treated rats, germ cells and junctional specialization between Sertoli cells were degenerated; collagen fibres under the irregular basal lamina increased; acrosomes were irregular and abnormally configured [5]. Cigarette smoke is a complex mixture of over 3,000 chemicals [6], among which nicotine is regarded as a major agent mediating the detrimental effects of smoking on male fertility [7–9]. Nicotine exposure reduces the weight of the testis, the number of spermatocytes and spermatids, and the testosterone levels, probably through its influence on pituitary gonadotropins and testicular antioxidant status, as nicotine is a central nervous system (CNS) depressor that can inhibit the neural stimulus essential for the release of pituitary gonadotropins. Besides, nicotine also contributes to reactive oxygen species (ROS) generation in testis [9–11]. Therefore, those 2 aspects can be regarded as 2 targets for treatment of male reproductive damages induced by nicotine. Human chorionic gonadoriophin (hCG) supplementation with nicotine has been demonstrated to be effective to prevent degeneration of germs cells to some extent and increase testosterone level significantly. However, whether treatment with antioxidants is protective against male reproductive injuries induced by nicotine is less known.
Recently, hydrogen, a highly flammable gas, exhibits wonderful effect of inhibition of oxidative stress, and protects various organs including brain [12, 13], intestine [14], liver [15] and heart [16] against ischemia and reperfusion injury. Different from hydrogen gas, hydrogen-rich saline is safe, economical and easily available, which is also proved to be cardioprotective through the anti-oxidative stress and apoptotic pathways [17].
Here, we sought to examine the effects of hydrogen-rich saline on the male reproductive dysfunction induced by nicotine. Vitamin C and vitamin E, which were well accepted as natural most effective antioxidants, were used for comparison.
Materials and methods
Animals and study design
Male C57BL/6J mice (4 weeks old) were purchased from the Sino-British SIPPR/BK Lab Animal Ltd. All the animals were entrained to controlled temperature (23–25 °C), 12-h light and 12-h dark cycles (light, 08:00–20:00 h; darkness, 20:00–08:00 h), and free access to food and tap water. All the animals used in present study received humane care in compliance with institutional animal care guidelines, and were approved by the Local Institutional Committee. All the surgical and experimental procedures were in accordance with institutional animal care guidelines.
Mice were randomly divided into five groups of 12 animals each and treated as follows: (1) control group (physiological saline, subcutaneous injection volume: 0.5 ml/kg; intraperitoneal injection volume: 6 ml/kg; injected daily in the morning for 3 months); (2) nicotine-treated group (nicotine, 4.5 mg/kg, injection volume: 0.5 ml/kg, subcutaneously injected daily in the morning for 3 months; physiological saline, injection volume: 6 ml/kg, intraperitoneally injected daily in the morning for 3 months); (3) nicotine-treated group with treatment by the Hydrogen-Rich Saline (6 ml/kg, intraperitoneally injected daily in the morning for 3 months); (4) nicotine-treated group with treatment by vitamin C (60 mg/kg, intraperitoneally injected daily in morning for 3 months, injection volume: 6 ml/kg); (5) nicotine-treated group with treatment by vitamin E (100 mg/kg, intraperitoneally injected daily in morning for 3 months, injection volume: 6 ml/kg).
Hydrogen-rich saline production
Hydrogen was dissolved in physiological saline for 6 h under high pressure of 0.4 MPa. The saturated hydrogen saline was regarded as hydrogen-rich saline and stored under atmospheric pressure in an aluminum bag with no dead volume at 4 °C. Hydrogen-rich saline was freshly prepared every week to ensure that its concentration was maintained and sterilized by gamma radiation. Gas chromatography was used to confirm the concentration of hydrogen in physiological saline as described by Ohsawa [12].
Histopathological evaluation
The testicular tissue was fixed in Bouin’s solution, post fixed in 70 % alcohol, and embedded in a paraffin block. A 5-μm section was obtained, deparaffinized, and stained with Hematoxylin and Eosin. The operator was blinded to the experimental group during the analysis.
Analysis of epididymal spermatozoa
Each cauda epididymis of male mice was severed at the vassal end and at the border to the corpus epididymis. After weighing, the caudae were transferred into prewarmed PBS and minced into small pieces. After incubation at 34 °C for 15 min, released sperm cells were suspended to obtain a homogeneous mixture. Sperm numbers and motility were analyzed under a light microscope.
Assessment of serum and testicular testosterone
Immediately after decapitation, a 2 ml blood sample was collected and serum sample was obtained by centrifugation at 2,000 rpm for 15 min and stored at −20 °C for assay within 1 month.
Testes were homogenized according to manufacturer’s instructions (Pierce, Rockford, IL). Samples were centrifuged at 10,000 rpm for 10 min; supernatants were collected and stored at −70 °C for assay within 2 months.
Serum and testicular samples were used to measure testosterone concentration using a Testosterone Assay kit (R&D Systems, Wiesbaden, Germany). Testicular testosterone content was calculated as ng/ml/mg of testis.
Assessment of oxidative stress markers
Testes were homogenized in T-PER Reagent according to manufacturer’s instructions (Pierce, Rockford, IL, USA). Samples were centrifuged to pellet tissue debris.
Malondialdehyde (MDA) content was measured using a MDA Assay kit (Cayman, Ann Arbor, USA) according to the protocol provided with the kit. MDA content in testis was calculated as μmol/mg of protein.
An Amplex Red Hydrogen peroxide/Peroxidase Assay Kit (Molecular Probes, Eugene, Oregon, USA) was used to measure H2O2 levels. Briefly, 5 μM Amplex Red and 15 μg/ml horseradish peroxidase were included in the incubations. H2O2 was detected by the formation of the fluorescent Amplex Red oxidation product resorufin using excitation and emission wavelengths of 563 and 587 nm, respectively. H2O2 level in testis was calculated as nmol/mg of protein.
Protein carbonyl content was determined as described previously [18]. Carbonyl content was calculated as nmol/mg of protein.
Measurement of protein-bound nitrotyrosine content was performed using a Nitrotyrosine Assay Kit (Cayman, Ann Arbor, USA) according to the protocol provided with the kit. Nitrotyrosine content was calculated as pmol/mg of protein.
Determination of caspase-3 activity
Caspase-3 activity in the testicular tissues was measured with the colorimetric Caspase assay system (Promega, Madison, WI, USA). Briefly, samples were lysed and centrifuged at 10,000 rpm for 10 min. The supernatants were collected and the protein concentrations were determined using Bradford Protein Assay. The protein lysates were added to the caspase-3 assay buffer containing 20 mmol/L of caspase-3 substrate Ac-DEVD-pNA and incubated at 37 °C for 4 h. Reaction mixtures without testis extract were used as negative controls. Production of the yellow color released from the substrate upon cleavage by caspase-3 was monitored with spectrofluorometry (Hitachi U-1500) at 405 nm.
Statistical analysis
Quantitative data were expressed as mean ± SD. Differences between groups were determined with a one-way ANOVA followed by a Student-Newman-Keuls test. A value of P < 0.05 was considered to denote statistical significance.
Results
Effects of treatment with hydrogen-rich saline, vitamin C and vitamin E on the epididymal spermatozoa of nicotine-treated mice
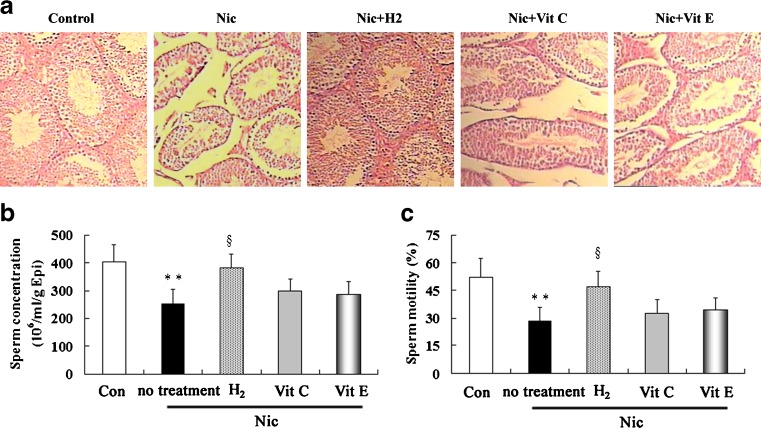
The testes of in control group indicated the presence of normal testicular structure and uniform seminiferous tubular morphology with normal spermatogenesis and the presence of primary and secondary spermatocytes, spermatids, and spermatozoa (Fig. 1a). In nicotine group, there were seminiferous tubules with severe disruption of the seminiferous epithelium. Treatment with hydrogen-rich saline showed an improved histological appearance. The effect of neither vitamin C nor vitamin E was obvious.
Fig. 1.
Histopathological evaluation by hematoxylin-eosin staining (a) of testis, and sperm cell counts (b) and motility (c) in epididymis in control and nicotine-treated mice without and with treatment by hydrogen-rich saline, vitamin C and vitamin E. Con: control, Nic: nicotine, Vit C: vitamin C, Vit E: vitamin E, Epi: epididymis. ** P < 0.01 vs. control group, §P < 0.05 vs. nicotine-treated group. n = 12 in each group
It was found that sperm number (Fig. 1b) and motility (Fig. 1c), as two of the most important indexes of male reproductive capacity, decreased in nicotine-treated group when compared to control group. Treatment with hydrogen-rich saline resulted in significant elevation of sperm number and motility compared to nicotine-treated group. Nevertheless, treatment with vitamin C and vitamin E increased the sperm number and motility, but not significantly.
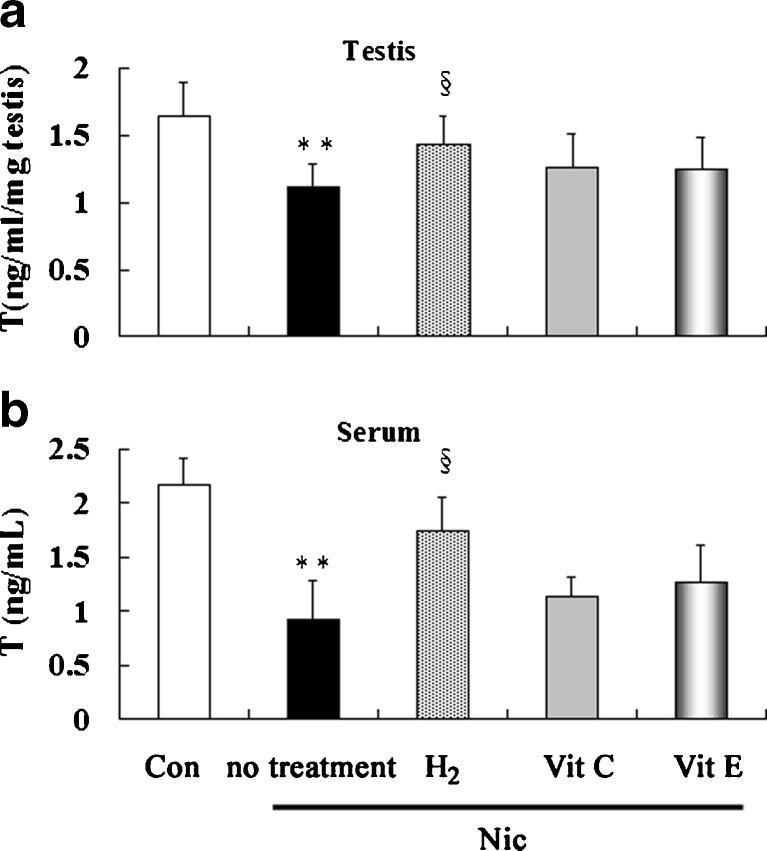
Effects of treatment with hydrogen-rich saline, vitamin C and vitamin E on the testosterone levels in nicotine-treated mice
It was observed that testicular (Fig. 2a) and serum (Fig. 2b) testosterone levels, as another important index of male reproductive capacity, decreased in nicotine-treated group compared to control group. Treatment with hydrogen-rich saline significantly increased both testicular and serum testosterone levels compared to nicotine-treated group. Treatment with vitamin C and vitamin E increased testosterone level to some extent, but not significantly.
Fig. 2.
Testicular (a) and serum (b) concentrations of testosterone in control and nicotine-treated mice without and with treatment by hydrogen-rich saline, vitamin C and vitamin E. Con: control, Nic: nicotine, Vit C: vitamin C, Vit E: vitamin E, T, testosterone. ** P < 0.01 vs. control group, §P < 0.05 vs. nicotine-treated group. n = 12 in each group
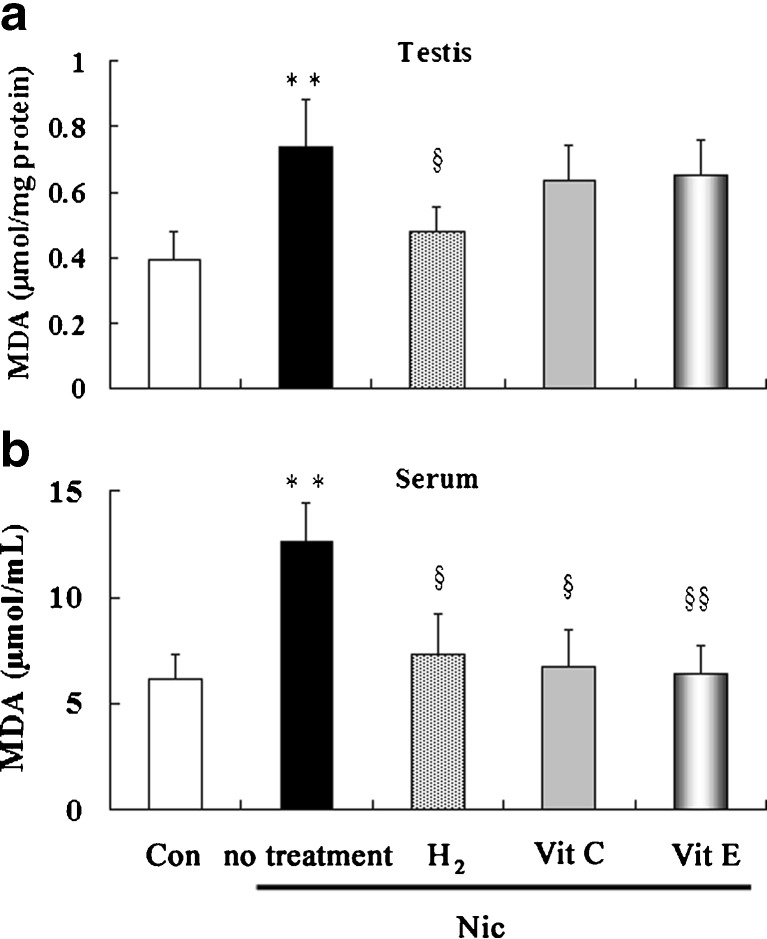
Effects of treatment with hydrogen-rich saline, vitamin C and vitamin E on the malondialdehyde (MDA) levels in nicotine-treated mice
It has been well known that MDA content is a marker of lipid peroxidation. Therefore, the levels of MDA in testicular and serum were determined. Just as shown in Fig. 3, Testicular (Fig. 3a) and serum (Fig. 3b) MDA levels were significant lower in nicotine-treated group than those in control group. Treatment with vitamin C and vitamin E lowered the serum MDA levels, but did not significantly increase the testicular MDA levels. Treatment with hydrogen-rich saline decreased both testicular and serum MDA levels significantly, which indicated that hydrogen-rich saline was more protective against oxidative stress in testis than vitamin C and vitamin E.
Fig. 3.
Testicular (a) and serum (b) levels of MDA in control and nicotine-treated mice without and with treatment by hydrogen-rich saline, vitamin C and vitamin E. Con: control, Nic: nicotine, Vit C: vitamin C, Vit E: vitamin E, MDA, malondialdehyde; ** P < 0.01 vs. control group, §P < 0.05 vs. nicotine-treated group, §§P < 0.01 vs. nicotine-treated group. n = 12 in each group
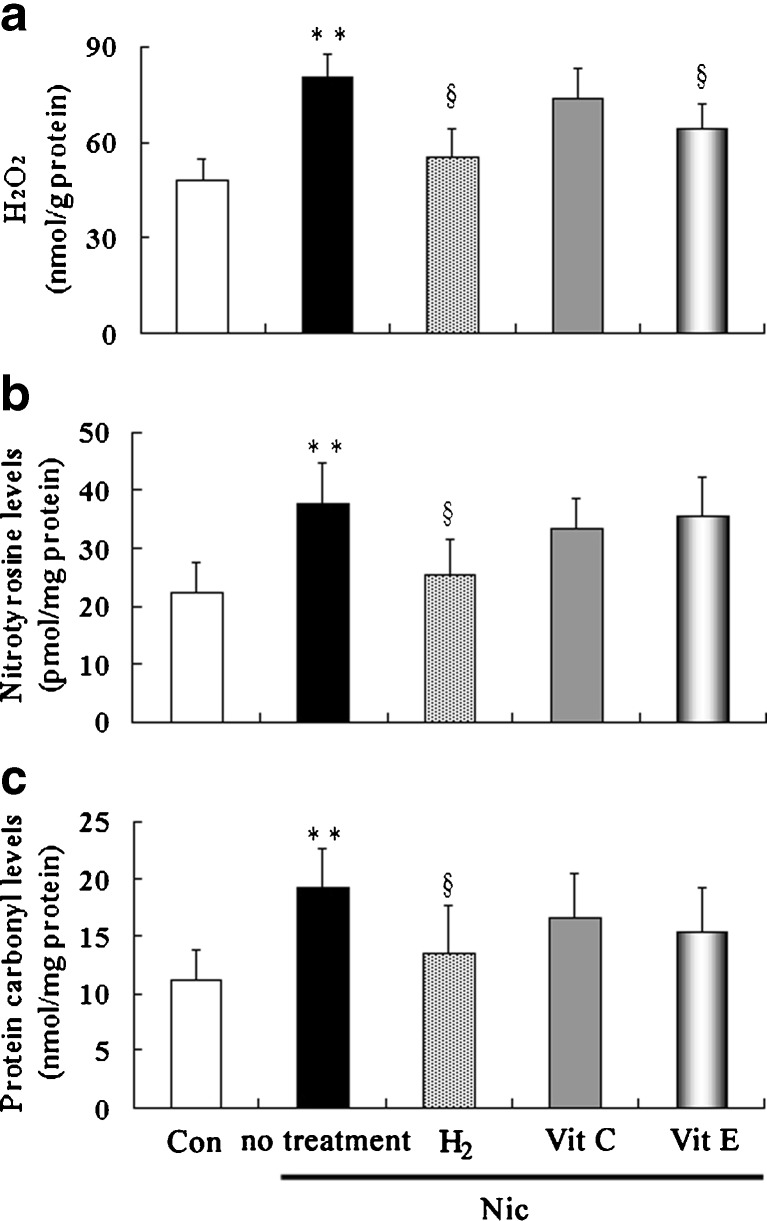
Effects of treatment with hydrogen-rich saline, vitamin C and vitamin E on the testicular H2O2, nitrotyrosine and protein-carbonyl levels in nicotine-treated mice
Testicular levels of H2O2 (Fig. 4a), one of the ROS secreted by macrophages that were seen closely aligned with Leydig cells in the testicular interstitium, increased in nicotine-treated group compared to control group. Treatment with hydrogen-rich saline and vitamin E significantly decreased the H2O2 level; treatment with vitamin C did not. Testicular nitrotyrosine level (Fig. 4b) (a marker of reactive nitrogen species) and protein carbonyl level (Fig. 4c) (a biomarker of protein oxidation) were significantly higher in nicotine-treated group compared to control group. Treatment with vitamin C and vitamin E did not influence them significantly, whereas treatment with hydrogen-rich saline resulted in a significant reduction of both nitrotyrosine and protein carbonyl levels in testis.
Fig. 4.
Testicular H2O2 (a), nitrotyrosine (b) and protein-carbonyl (c) levels in control and nicotine-treated mice without and with treatment by hydrogen-rich saline, vitamin C and vitamin E. Con: control, Nic: nicotine, Vit C: vitamin C, Vit E: vitamin E, MDA, malondialdehyde; ** P < 0.01 vs. control group, §P < 0.05 vs. nicotine-treated group. n = 12 in each group
Effects of treatment with hydrogen-rich saline, vitamin C and vitamin E on the testicular caspase-3 activity in nicotine-treated mice
Since we observed that hydrogen-rich saline treatment caused significant increasing in sperm number and motility, we next examined if the protective effects involved reverse of apoptosis. Testicular caspase-3 activity (Fig. 5), a marker of apoptosis, was higher in nicotine-treated group than it in control group. Treatment with hydrogen-rich saline decreased caspase-3 activity compared to nicotine-treated group; treatment with vitamin C and vitamin E had no significant influence on it. The results indicated that hydrogen-rich saline effectively protected against nicotine-induced damage in testis through anti-apoptotic pathway.
Fig. 5.
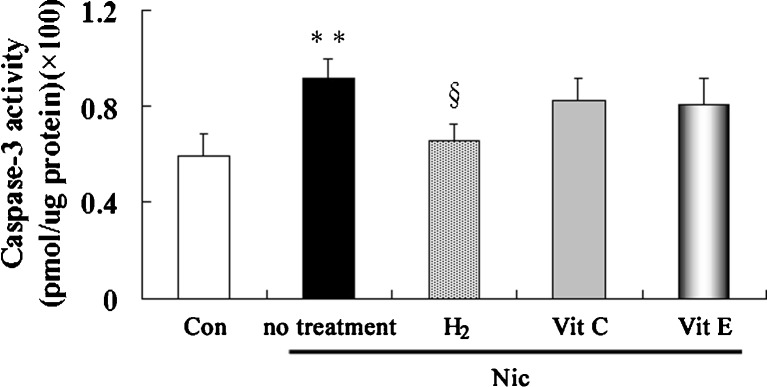
Testicular caspase-3 activities in control and nicotine-treated mice without and with treatment by hydrogen-rich saline, vitamin C and vitamin E. Con: control, Nic: nicotine, Vit C: vitamin C, Vit E: vitamin E, ** P < 0.01 vs. control group, §P < 0.05 vs. nicotine-treated group. n = 12 in each group
Discussion
The main new findings of the present study are summarized as follows: (1) Nicotine exposure not only reduced epididymal sperm numbers and motility, but also depressed testicular and serum testosterone levels in mice. (2) Nicotine exposure induced oxidative stress and promoted apoptosis in testes of mice. (3) Treatment with hydrogen-rich saline abated oxidative stress, decreased apoptosis, increased sperm numbers and motility and raised testosterone levels in nicotine-treated mice. (4) Compared to vitamin C or vitamin E, hydrogen exhibited more effective to attenuate oxidative levels induced by nicotine in testis.
ROS generation induced by nicotine in testis might be one important cause which contributes to decreased sperm counts and motility accompanied with the reduction of plasma and testicular testosterone concentration. The formation of reactive oxygen species in cells leads to the formation of free radicals in metabolic processes. These harmful species cause oxidative damage to many molecules such as lipid, protein and nucleic acid. Many researchers have demonstrated that nicotine contributed to reactive oxygen species production in patients and animals [19–22]. It is reported that chronic nicotine treatment decreases the cytochrome P450 I.E. and glutathione reductase activities, increases free radical formation and leads to oxidative damage in rats [11, 21, 23–26].
In our present study, three kinds of antioxidants including hydrogen-rich saline, vitamin C and vitamin E were used to treat the oxidative damage induced by nicotine in mice. All of them attenuated circulating oxidative levels, but hydrogen-rich saline could attenuate testicular oxidative levels in mice exposed to nicotine more effectively. It could be explained by 2 possibilities: (1) Blood-testis barrier, a specialized barrier in the testis, between the interstitial blood compartment and the adluminal compartment of the seminiferous tubules, could stop vitamin C or vitamin E from entering testis. In addition, membrane of mitochondrion, which is one source of ROS, is also a barrier to antioxidants. However, hydrogen, different from normal antioxidants, is electrically neutral and much smaller, so it could easily penetrate blood-testis barrier and enter cells and organelles such as the nucleus and mitochondria. (2) Hydrogen is able to selectively reduce the hydroxyl radical and ONOO-, the most cytotoxic chemicals of ROS, and effectively protect cells, but does not react with other ROS, which possess physiological roles [27].
The present study did not test the effect of hydrogen on pituitary gonadotropins, because it was reported that hydrogen could penetrate blood–brain barrier and abated oxidative stress in brain, which was our study limitation and required further research.
In conclusion, long-term treatment with hydrogen-rich saline could attenuate testicular oxidative levels and improve male reproductive dysfunction induced by nicotine in mice.
Footnotes
Capsule Long-term treatment with hydrogen-rich saline could attenuate testicular oxidative level and improve male reproductive function in nicotine-treated mice.
Shu Li and DanDan Lu contribute equally to this work. Both persons are the first authors.
References
- 1.Takenawa J, Kaneko Y, Okumura K, Nakayama H, Fujita J, Yoshida O. Urinary excretion of mutagens and covalent DNA damage induced in the bladder and kidney after passive smoking in rats. Urol Res. 1994;22:93–97. doi: 10.1007/BF00310998. [DOI] [PubMed] [Google Scholar]
- 2.Sofikitis N, Miyagawa I, Dimitriadis D, Zavos P, Sikka S, Hellstrom W. Effects of smoking on testicular function, semen quality and sperm fertilizing capacity. J Urol. 1995;154:1030–1034. doi: 10.1016/S0022-5347(01)66968-4. [DOI] [PubMed] [Google Scholar]
- 3.Kavitharaj NK, Vijayammal PL. Nicotine administration induced changes in the gonadal functions in male rats. Pharmacology. 1999;58:2–7. doi: 10.1159/000028262. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Isoyama E, Sofikitis N, Miyagawa I. Effects of smoking on testicular function and fertilizing potential in rats. Urol Res. 1998;26:45–48. doi: 10.1007/s002400050022. [DOI] [PubMed] [Google Scholar]
- 5.Aydos K, Guven MC, Can B, Ergun A. Nicotine toxicity to the ultrastructure of the testis in rats. BJU Int. 2001;88:622–626. doi: 10.1046/j.1464-4096.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- 6.Stillman RJ, Rosenberg MJ, Sachs BP. Smoking and reproduction. Fertil Steril. 1986;46:545–549. doi: 10.1016/s0015-0282(16)49628-7. [DOI] [PubMed] [Google Scholar]
- 7.Pacifici R, Altieri I, Gandini L, Lenzi A, Pichini S, Rosa M, et al. Nicotine, cotinine, and trans-3-hydroxycotinine levels in seminal plasma of smokers: effects on sperm parameters. Ther Drug Monit. 1993;15:358–362. doi: 10.1097/00007691-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Yeh J, Barbieri RL, Friedman AJ. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. J Steroid Biochem. 1989;33:627–630. doi: 10.1016/0022-4731(89)90051-4. [DOI] [PubMed] [Google Scholar]
- 9.Jana K, Samanta PK, De DK. Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci. 2010;116:647–659. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Londonkar R, Reddy S, Patil SB. Testicular changes due to graded doses of nicotine in albino mice. Indian J Physiol Pharmacol. 1998;42:276–280. [PubMed] [Google Scholar]
- 11.Erat M, Ciftci M, Gumustekin K, Gul M. Effects of nicotine and vitamin E on glutathione reductase activity in some rat tissues in vivo and in vitro. Eur J Pharmacol. 2007;554:92–97. doi: 10.1016/j.ejphar.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH, et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 16.Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH, et al. Hydrogenrich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med. 2009;234:1212–1219. doi: 10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 18.Lyras L, Evans PJ, Shaw PJ, Ince PG, Halliwell B. Oxidative damage and motor neurone disease difficulties in the measurement of protein carbonyls in human brain tissue. Free Radic Res. 1996;24:397–406. doi: 10.3109/10715769609088038. [DOI] [PubMed] [Google Scholar]
- 19.Guemouri L, Lecomte E, Herbeth B, Pirollet P, Paille F, Siest G, et al. Blood activities of antioxidant enzymes in alcoholics before and after withdrawal. J Stud Alcohol. 1993;54:626–629. doi: 10.15288/jsa.1993.54.626. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins RR, Goldfarb A. Introduction: oxidant stress, aging, and exercise. Med Sci Sports Exerc. 1993;25:210–212. [PubMed] [Google Scholar]
- 21.Leanderson P, Tagesson C. Cigarette smoke-induced DNA damage in cultured human lung cells: role of hydroxyl radicals and endonuclease activation. Chem Biol Interact. 1992;81:197–208. doi: 10.1016/0009-2797(92)90034-I. [DOI] [PubMed] [Google Scholar]
- 22.Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, et al. Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med. 1995;18:877–882. doi: 10.1016/0891-5849(94)00221-5. [DOI] [PubMed] [Google Scholar]
- 23.Anandatheerthavarada HK, Williams JF, Wecker L. The chronic administration of nicotine induces cytochrome P450 in rat brain. J Neurochem. 1993;60:1941–1944. doi: 10.1111/j.1471-4159.1993.tb13424.x. [DOI] [PubMed] [Google Scholar]
- 24.Ashakumary L, Vijayammal PL. Lipid peroxidation in nicotine treated rats. J Ecotoxicol Environ Monit. 1991;1:283–290. [Google Scholar]
- 25.Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3- pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–839. doi: 10.1016/S0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 26.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 27.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]