Introduction
Female fertility preservation before chemotherapy has become a common issue among postpubertal and premenopausal women who desire children. Von Wolff et al. [1] recently published a practical guideline for different fertility preservation techniques, based on data from the FertiPROTEKT network database. The published practical guideline particularly addresses women with breast cancer, Hodgkin disease, or borderline ovarian tumour. The following are the different fertility preservation techniques covered: (1) hormonal stimulation and cryopreservation of unfertilised and fertilised oocytes, (2) cryopreservation of ovarian tissue, and (3) administration of gonadotropin-releasing hormone agonists (GnRHa). There is one or more than 1 option available for cancer patients, depending on cancer type, partnership, and individual desire.
The classical Hodgkin’s lymphoma presents with an enlargement of lymph nodes and unspecific symptoms like night sweats, unexplained weight loss, fatigue and others. It is classified histopathologically by the typical Sternberg-Reed-Cells and may be cured with modern polychemotherapy in more than 80 % [2]. Women with Morbus Hodgkin receiving COPP/ABVD (cyclophosphamide, vincristine [O], procarbazine, prednisolone/adriamycin, bleomycin, vinblastine, and dacarbazine) or BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine [O], procarbazine, and prednisolone) have a high risk of amenorrhoea after chemotherapy [1, 3, 4]. The incidence of Hodgkin disease in pregnancy is low, with an estimated prevalence of 1 per 6,000 pregnancies [5]. If the diagnosis is made during the first trimester, the pregnancy usually needs to be interrupted [5]; however, with low-risk lymphoma, the pregnancy might be prolonged until labour. Even if a woman has given birth to a healthy child, she may still have the desire for more children. Therefore, fertility preservation techniques should be considered, especially for young mothers. To the best of our knowledge, no report has been published on fertility preservation in pregnant women or women who had given birth just a few days before an anti-cancer treatment.
Case report
A 29-year-old woman (gravida I) in the 40th gestational week presented to our gynaecologic department with a newly diagnosed Morbus Hodgkin disease. Until this date, she had an uneventful pregnancy with routinely check-up (every 4 weeks until 34th week of gestation, every 2 weeks 34th -39th week of gestation). We needed to terminate the pregnancy to start further diagnostic examinations such as lymph node extirpation and computed tomography before chemotherapy. In addition, the patient requested for fertility preservation and we informed her about the possible preservation techniques including the hormonal stimulation and cryopreservation of unfertilised and fertilised oocytes, the cryopreservation of ovarian tissue, and the administration of gonadotropin-releasing hormone agonists.
Induction of labour (IOL) was started at 39 weeks 5 days of gestation. When the IOL was unsuccessful, caesarean section was performed 1 day later (CBC [complete blood count] on the day of the Caesarean: White blood cells 12.4giga/l, Haemoglobin 10.9 g/dl, platelets 369 giga/l). During the operation, half of the left ovary was removed for cryopreservation using the slow-freezing protocol [6]. A biopsy of the tissue showed normal ovarian tissue with antral follicles and without any signs of metastasis (Up to now, the risk of a relapse of the Hodgkin’s lymphoma after re-transplantation is very low [7], however the patient was informed about this small risk of metastasis). After 1 week of recovery, controlled ovarian hyperstimulation was started using the short GnRHa protocol. Hormonal analyses were performed before and during the stimulation process. Initially (on the second day after the caesarean delivery), the human chorionic gonadotropin (hCG) level was high (2131.0 IU/L), continuously decreasing during stimulation (18th day after the caesarean delivery, 8.1 IU/L). The anti-Müllerian hormone (AMH) level was <0.2 μg/L before the hormonal stimulation (day 2 after the caesarean delivery). The results of the detailed hormonal analyses are presented in Table 1.
Table 1.
Hormonal analyses after the caesarean delivery (day 0)
| day 2 | day 4 | day 7 | day 11 | day 14 | day 16 | day 18 | |
|---|---|---|---|---|---|---|---|
| Days of GnRHa application | start | 5th day | 8th day | 10th day | 12th day | ||
| Days of gonadotropin stimulation | 3rd day | 6th day | 8th day | 10th day | |||
| LH (IU/L) | <0.10 | <0.10 | 0.50 | 3.19 | 3.55 | 3.33 | 2.55 |
| E2 (ng/L) | 58.7 | 34.7 | 24.5 | 161.8 | 417.5 | 597.3 | 922.9 |
| Progesterone (μg/L) | 7.50 | 2.64 | 0.79 | 0.49 | – | 0.86 | 0.95 |
| Testosterone (μg/L) | 0.567 | – | 0.480 | 0.214 | 0.223 | 0.249 | 0.365 |
| TSH (mIU/L) | 3.870 | 5.450 | 3.920 | 2.510 | 1.250 | 1.280 | 1.440 |
| AMH (μg/L) | <0.2 | – | – | – | – | <0.2 | – |
| βhCG (IU/L) | 2131.0 | 617.7 | 145.7 | 34.7 | 15.8 | 10.8 | 8.1 |
LH luteinising hormone
E2 estradiol
TSH thyroid-stimulating hormone
AMH anti-Müllerian hormone
βhCG beta human chorionic gonadotropin
GnRHa Gonadotropin Releasing Hormone Agonist
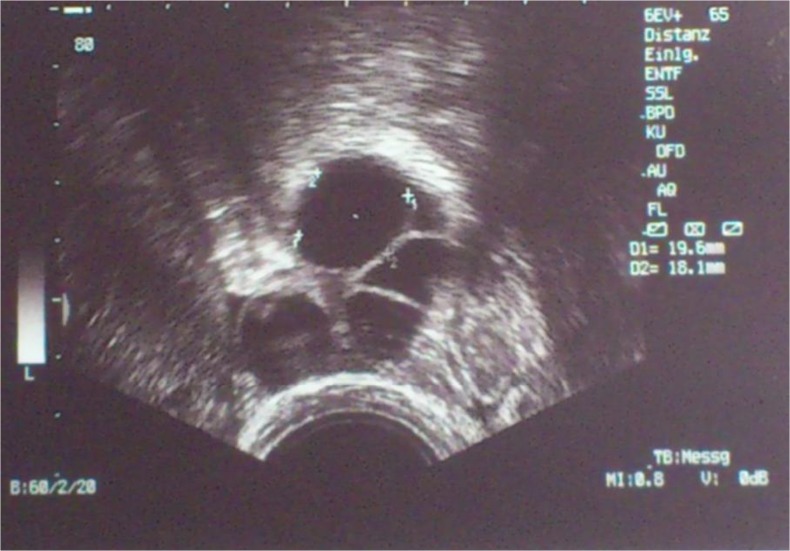
The GnRH agonist flare-up protocol was performed [8] and intranasal GnRHa (Synarela (0,23 mg Nafarlinacetat/0,1 ml): Pfizer, Berlin, Germany) administration twice daily (0,2 ml/d) was started on day 7 after the caesarean delivery. After another 2 days (9 days after the caesarean delivery), human menopausal gonadotropin (hMG, Menogon HP: Ferring, Saint-Prex, Switzerland) administration at 225 IE/day was started. On the eighth day of stimulation, the hMG dosage was increased to 300 IE/day (14 days after the caesarean delivery). On the 14th day of stimulation (20 days after the caesarean delivery), the leading follicle was 18–19 mm in diameter (Fig. 1); hence, ovulation induction using 10.000 IE hCG (Brevactid: Ferring, Saint-Prex, Switzerland) was performed, followed by oocyte retrieval 36 h later. We obtained 4 oocytes in total, of which 2 were metaphase I and the other 2 were used for intracytoplasmic sperm injection. One oocyte (PN, day 2) was fertilised and cryopreserved.
Fig. 1.
Sonogram obtained on the day of induction of ovulation (day 20 after the caesarean delivery)
The patient received a depot of GnRHa (Trenantone Gyn: Takeda Pharma, Cerano, Italy) on the day of oocyte retrieval (day 22 after the caesarean delivery); she was informed about the inconclusive data about GnRHa and about the experimental design of this method [9]. She wished to receive GnRHa and the potential benefit of the following amenorrhea during chemotherapy encouraged us to fulfil her desire. She was transferred to the haematology department. After final examinations, Morbus Hodgkin stage IIIA was diagnosed, and the BEACOPP protocol was started 25 days after the caesarean delivery.
Discussion
This is the first report on fertility preservation techniques performed during and shortly after childbirth in a woman with Hodgkin disease. Our case demonstrates that the combination of cryopreservation of ovarian tissue, oocyte fertilisation, and GnRHa application is feasible even for women in early childbed.
Ovarian stimulation and oocyte retrieval
Different types of protocols are available for ovarian stimulation in assisted reproduction techniques. The choice of protocol depends on the woman’s age, medical history (e.g. polycystic ovaries, endometriosis, or previous poor responder), and ovarian reserve. Recently, ovarian stimulation even in the luteal phase for fertility preservation was demonstrated to produce as many oocytes as the regular stimulation in the follicular phase [1, 10]. However, ovarian stimulation after childbirth within childbed has not been reported. We chose the short GnRHa protocol because we expected a low response after removal of a half ovary, with a rather low AMH level and an already decreased progesterone level after birth. Ovarian stimulation monitored by sonography and hormonal analysis was within the expected ranges. The measured serum hCG levels decreased rapidly postpartum but not lower than the detection threshold. The immature oocytes obtained may be because of the late induction of ovulation. Nevertheless, we retrieved 2 oocytes that were suitable for intracytoplasmic sperm injection with 1 fertilised oocyte. Although ovarian stimulation shortly after caesarean section is experimental, our case serves as a proof of principle.
Time of cancer treatment delay
The final histological examination results of the lymph nodes were obtained 11 days after caesarean delivery, and cancer treatment started 14 days later (25 days after the caesarean delivery), which was proposed by haematologists to be within a justifiable range for the treatment of Hodgkin lymphoma. Therefore, the completion of fertility preservation via cryopreservation of ovarian tissue, ovarian stimulation with cryopreservation of fertilised oocytes, and GnRHa application is feasible and does not delay the cancer treatment for too long.
Questions for the future
This is the first report on the combination of 3 fertility preservation techniques performed shortly after caesarean delivery. It remains unclear whether the cryopreservation of only 1 fertilised egg was due to the timing after the pregnancy and the low hCG level or low response after half-ovary removal. The pregnancy outcome after cancer treatment completion needs to be further investigated.
Acknowledgement
None.
Footnotes
Capsule The combination of fertility preservation techniques shortly after delivery. A case report of a primigravida woman with a newly diagnosed Morbus Hodgkin disease and a proof of principle.
References
- 1.Von Wolff M, et al. Fertility preservation in women: a practical guide to preservation techniques and therapeutic strategies in breast cancer. Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284(2):427–435. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diehl V, et al. Hodgkin’s lymphoma: biology and treatment strategies for primary, refractory, and relapsed disease. Hematology Am Soc Hematol Educ Program. 2003;2003:225–247. doi: 10.1182/asheducation-2003.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Behringer K, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23(30):7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169(1–2):123–131. doi: 10.1016/S0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 5.Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Lancet. 2012;379(9815):580–587. doi: 10.1016/S0140-6736(11)61348-2. [DOI] [PubMed] [Google Scholar]
- 6.Isachenko V, et al. Cryobanking of human ovarian tissue for anti-cancer treatment: comparison of vitrification and conventional freezing. Cryo Lett. 2009;30:449–454. [PubMed] [Google Scholar]
- 7.Dolmans MM, et al. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99(6):1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Kahraman K, et al. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer cycle. Fertil Steril. 2009;91(6):2437–2444. doi: 10.1016/j.fertnstert.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Loren AW, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedoschi GM, et al. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet. 2010;27(8):491–494. doi: 10.1007/s10815-010-9429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]