INTRODUCTION
The American Association for Pharmaceutical Scientists (AAPS) Workshop on Predicting and Monitoring Impurities in API and Drug Products: Product Development and Regulatory Issues was held on October 13–14, 2012 at the McCormick Place in Chicago, IL, USA. The goal of the workshop was to discuss control strategies of chemical and physical changes of active pharmaceutical ingredients (API) and drug products in the drug development process. These changes can affect both the safety and efficacy of drugs; therefore, the ability to rapidly predict and assess the potential for drug product performance changes for impurity formation and the associated safety concerns are important parts of speeding the development of innovative drug therapies.
The workshop consisted of four different sessions. Each session focused on separate fundamental issues to build a comprehensive understanding of the physical and chemical processes that impact drug degradation, the control of impurities and the impact of these factors on safety and regulatory areas. Taken together, this comprehensive understanding is used to achieve a more robust development process that enables predictability with a concomitant assurance of safety and efficacy. Innovative methodologies for development of effective stability control strategies were also presented.
This article summarizes Sessions 1 and 2 of the American Association for Pharmaceutical Scientists (AAPS) Workshop on Predicting and Monitoring Impurities in API and Drug Products: Product Development and Regulatory Issues and addresses of predicting degradation related impurities and impurity considerations for pharmaceutical dosage forms.
Sessions 3 and 4 of the American Association for Pharmaceutical Scientists (AAPS) Workshop on Predicting and Monitoring Impurities in API and Drug Products: Product Development and Regulatory Issues are summarized in Recent Trends in Product Development and Regulatory Issues on Impurities in Active Pharmaceutical Ingredient (API) and Drug Products Part 2: Safety Considerations of Impurities in Pharmaceutical Products and Surveying the Impurity Landscape published separately.
SESSION I: STRESS TESTING: PREDICTING DEGRADATION RELATED IMPURITIES
The first session of the workshop consisted of four talks specifically focused on various aspects of predicting degradation pathways using stress testing with and without in silico/computational input. Prediction of drug degradation (which encompasses both pathways and kinetics) is an area with a great deal of interest, especially considering the evolving pharmaceutical interest in Quality-by-Design (QbD) approaches.
The first talk was given by Mark Kleinman (GlaxoSmithKline) on the topic of using stress testing as a predictive tool. This talk highlighted the current realities (both power and limitations) of in silico tools to predict “real world” degradation products of drugs and formulated products. The talk also highlighted stress testing conditions that have been proven to be effective in discovering degradation pathways available to a particular drug molecule, with emphasis on the more complex areas of oxidation and photodegradation pathways. Finally, Dr. Kleinman discussed practical approaches to predictions of the kinetics of drug degradation using empirical data from stress testing.
The second talk was given by Chris Foti (Pfizer), focusing on the analytical considerations when conducting stress testing studies. This talk provided a comprehensive look at carrying out a stress testing protocol and the accompanying analytical methodology. A detailed case study illustrated the process and provided practical recommendations for interpreting the results.
In the third talk, Steve Baertschi (Lilly) provided an in-depth look at mass balance, including the importance of mass balance in developing stability-indicating methods and the hidden complexities involved in assessing mass balance in a formulated product. Particular emphasis was given to the importance of understanding the degradation reactions involved, the changes in molecular weight (especially when excipient adducts are formed) and the stoichiometry of degradation reactions.
The fourth and final talk was provided by Karen Alsante (Pfizer), looking at recent advances in the understanding of drug degradation chemistry. In this presentation, the major mechanisms of chemical decomposition were examined in the context of common functional groups. Of particular interest was the analysis of the frequency of various molecular weight changes resulting from known drug degradation pathways, providing insight into which pathways are most common as well as those pathways that are rare or complex (e.g., involving multiple steps). The utility and continuing refinement of the chemical degradation prediction software package Zeneth® (www.lhasalimited.org/products/zeneth.htm) was also described.
STRESS TESTING: A PREDICTIVE TOOL (Contributed BY MARK KLEINMAN, GLAXOSMITHKLINE)
The stability of organic molecules and small pharmaceutical entities follows many rules defined by classic organic reaction mechanisms. Each rule governs a single transformation. Many small-molecule active pharmaceutical ingredients are inherently complex and have diverse functional groups that can undergo multiple reactions either simultaneously or in sequence. Thus, the degradation of pharmaceuticals is an area that is complex. The goal is to discuss the possibility of predicting degradation products that may form from small-molecule organic pharmaceuticals and to offer insights into the design of forced degradation (stress testing) studies.
The prediction of drug degradation is still in its infancy. Hypothetical degradation products are defined as those that are predicted in silico or through literature searches. Additionally, potential degradation products are those that are observed in stress tests; while actual degradation products are those formed under real-time (e.g., 30°C/65%RH) or accelerated stability studies (40°C/75%RH) (1). In an ideal scenario, the actual degradation products are completely predictable and observed in the stress tests—a true subset. Realistically, while almost all actual degradation products are observed in stress tests, there are still a significant number that are not predicted (Fig. 1).
Fig. 1.
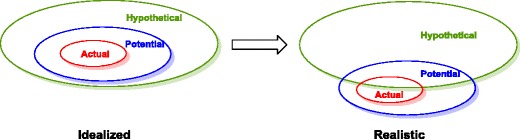
Ideal versus realistic scenarios for degradation product prediction
In essence, relative to in silico predictions, stress testing is a better predictor and yields greater specificity to actual degradation results. Zeneth® is a relatively new predictive tool from Lhasa Ltd. (developers of METEOR® and DEREK®) (2). Currently, one of the main uses of Zeneth is to expand the number of hypothetical degradation products for consideration. This allows for researchers to gain a view of the potential degradation products, help to define stress conditions and elucidate the tentative structures by matching mass/charge ratios in mass spectrometry studies.
The field of pharmaceutical stress testing has recently come to the forefront in many areas (3–6). The design of successful and efficient stress testing studies is difficult due to the fact that “one size does not fit all”. Even so, a typical set of starting conditions for solution stress tests (e.g., acid and base) are generally accepted. It is noted that oxidative and photolytic conditions require special considerations. There are a plethora of oxidative mechanisms (e.g., auto oxidation, peroxidation, electron transfer, and photo-oxidation). A successful oxidative stress test depends on rendering the actual degradation products. Therefore, appropriate oxidative conditions should be utilized. One relatively new condition uses N-methylpyrrolidinone (NMP) which affords a wide range of oxidative products (7). Similarly, pharmaceutical photodegradation is more difficult to predict due to the relative lack of expertise in the area compared to thermal processes. Photostability has become even more important to understand due to the relationship between photostability and phototoxicity (4,5,8,9). It is recommended that in addition to ICH Q1B confirmatory testing with solid API, a stress test with two to five times ICH Q1B light exposure is carried out with the solid. In addition, it is suggested to more fully understand the mechanism of photodegradation and the potential for phototoxicity by exposing a solution of API to a light exposure similar to that in the 3T3 Neutral Red Uptake model (10). This will help evaluate any potential photo-liabilities as well as explore the link to phototoxicity, assuming that the API absorbs significantly (>1,000 M−1 cm−1) at wavelengths greater than 290 nm (11).
The other key topic explored is the assessment of kinetic equivalence. Understanding kinetic equivalence is paramount to determining how long the stress should be applied to the solid or solution. The rate of degradation depends on the energy of activation of the reaction. Using the Arrhenius principle for solution or a modified Arrhenius treatment in solids (12), one can show the expected duration of stress relative to 6 months at 40°C for any activation energy (12–30 kcal/mol are typically reported for pharmaceuticals (13)). It is important to consider the kinetic equivalence as it affords a scientific rationale to complete a stress study even in the absence of obtaining the recommended 5–20% degradation. In summary, key areas for growth in stress testing are in silico predictivity/specificity, oxidative stress testing and a better mechanistic understanding of the photodegradation of active pharmaceutical ingredients.
ANALYTICAL CONSIDERATIONS FOR STRESS TESTING (CONTRIBUTED BY CHRIS FOTI, PFIZER INC.)
Stress testing plays an important role in the drug development process by providing an understanding of the chemistry of the drug substance and drug product and facilitates the development of stability-indicating analytical methodology. Although some recommendations for stress testing are given in the ICH [Q1A(R2)] and Q1B guidelines (1,14) on the stability testing of drug substances and drug products, the guidance given is very general concerning scope and timing and is not particularly useful. This paper is a short summary from the presentation on Analytical Considerations for Stress Testing given as part of the 2012 AAPS Workshop on Predicting and Monitoring Impurities in API and Drug Products: Product Development and Regulatory Issues conference to provide practical guidance concerning the design, setup and analytical aspects (i.e., data gathering, sample preparation) of carrying out stress testing studies for an API in late stage development. A specific focus was given to hydrolysis conditions at various pH values, oxidative reagents, and photostress (15). These concepts were illustrated through a stress testing case study of the Eli Lilly LY334370 API shown below in Fig. 2. Also, chromatographic method development to measure the loss of parent compound as well as the levels of degradation products or impurities formed under the stress conditions was discussed.
Fig. 2.
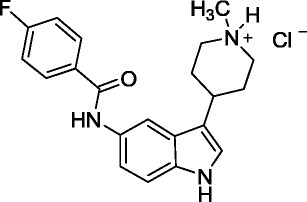
The chemical structure of LY334370
The first part of data gathering is to obtain information on solubility and to identify co-solvents that are suitable and compatible with the API through a simple semi-quantitative screen. It is especially important to select a dissolving solvent that is compatible with the chromatographic conditions, if samples are to be injected directly from the stressed solutions. Results for the LY334370 API indicate that an acetonitrile co-solvent was needed to achieve solution solubility at the extreme pH values, with greater than 4 mg/mL solubility in water and acetonitrile/water mixtures. Prediction of degradation products using tools such as Lhasa’s in silico Zeneth® software (16) combined with an assessment based on chemistry knowledge (in cerebro) can also be an important part of data gathering. For this API, the potential sites of reactivity are: (1) oxidation of aryl fused pyrrole, (2) hydrolysis of the amide, (3) oxidation of benzylic carbon and oxidation of the amine functional groups. Stress testing studies were conducted to confirm these predicted stability liabilities. Solid API samples were subjected to thermal and thermal/humidity and light exposure (Options 1 and 2). API was stressed in solution using 0.1 N NaOH or 0.1 N HCl conditions, solution-based azobisisobutyronitrile (AIBN) radical oxidation (to simulate autoxidation), solution-based peroxide oxidation and solution-based light exposure (Options 1 and 2). These stress testing studies were conducted in volumetric glassware to facilitate quantitative assessments as needed. Low reactivity was observed for the API under all of the solid-state stress conditions (data not shown) in contrast to the solution studies, which showed degradation of the parent from 2–21% (Table I).
Table I.
Overall Solution Stress Testing Results for LY334370
| Degradation condition | AIBN radical oxidation(40°C, 7 days) | H2O2 Oxidation (room temperature, 7 days) | 0.1 N HCl (70°C, 3 days) | 0.1 N NaOH (70°C, 3 days) | Photodegradation option 1 (1.3× ICH Vis and 4× ICH UV) | Photodegradation option 2 (4.5× ICH Vis) |
|---|---|---|---|---|---|---|
| High reactivity (>10%) | 18% | 21% | 19% | |||
| Moderate reactivity (1–10%) | 2% | 6% | 5% |
Values represent percent of degradation by loss of parent based on peak area percent by LC
High reactivity was observed for the API in solution under hydrolysis conditions at the extreme pH values using 0.1 N HCl and 0.1 N NaOH. Moderate reactivity was observed in solutions containing hydrogen peroxide or a radical initiator, suggesting that LY334370 may be susceptible to oxidative degradation. The API in solution is also very sensitive to light-catalyzed degradation. More details on this case study and guidance for the design and setup of stress testing studies can be found in Pharmaceutical Stress Testing (3) and other references (17,18)
There are a variety of separation techniques and detectors available to measure potency and purity of stressed samples. However, within the pharmaceutical industry, the most often utilized methodology is LC with UV detection for quantitation. Specifically, method development is facile, with selectivity being impacted by column stationary phase, buffer, and pH and these methods are typically rugged with the equipment being readily available. In addition, the introduction of ultra high performance liquid chromatography (UHPLC) allows the analytical scientist to optimize speed and resolution. The degradation conditions in Table I represent the key degradation sample set that was used to evaluate the stability-indicating nature of the analytical methodology.
This case study provides practical guidance for the design and execution of an API stress testing with highlighted analytical considerations. It is also expected that this field will continue to expand with different ways to conduct API and drug product stress testing. In the next 3 to 5 years, degradation prediction could impact the experimental design of stress testing studies with both API and drug product.
REVISITING OLD CONCEPTS: NEW INSIGHTS INTO THE CONCEPT OF MASS BALANCE IN DRUG PRODUCTS (CONTRIBUTED BY STEVE BAERTSCHI, ELI LILLY AND COMPANY)
The concept of “mass balance” with regard to stability-indicating analytical methodologies is an old topic in pharmaceutical analysis (19–21), as well as in synthetic chemistry. During the process of conducting stability studies, the important question to address is: Can the analytical method(s) used account for the entire parent drug “mass” that was present at the initial time point in the stability studies?
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) defines mass balance as “the process of adding together the assay value and levels of degradation products to see how closely these add up to 100% of the initial value, with due consideration of the margin of analytical precision” (19). In a balanced reaction, the stoichiometry is balanced such that the moles of reactants are accounted for in the moles of products and the stoichiometry allows direct translation into mass balance. In the case of drug product degradation, while the parent drug structure is known and the mass (the amount present) can be readily measured, the structure and amounts of the reactants and degradation products typically is not known a priori. Common reactants are water, peroxide, molecular oxygen, excipients or excipient impurities. If a reactant adds to the parent drug, there is an increase in mass associated with that degradation product, which can alter the calculation of mass balance.
Consider the example illustrated in Fig. 3, when pregabalin is formulated with lactose as an excipient. The combination may form a condensation degradation product, as is common with amines and reducing sugars, and is representative of the extensively researched Maillard Reaction (22). Using a detector that responds approximately equally to mass (e.g., the charged aerosol detector or CAD), the parent drug shows a loss of 5% of the potency value and a degradation product (the drug–excipient adduct) corresponding to a peak area of 5% by area. As shown in Fig. 4a, the conclusion would be that mass balance has been achieved (95% + 5% = 100% on a mass basis).
Fig. 3.
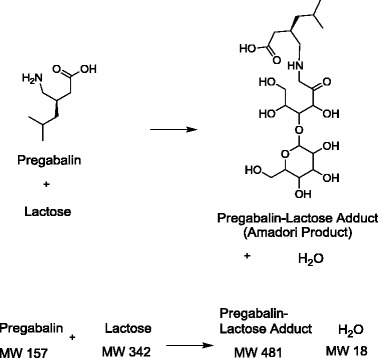
Hypothetical reaction of pregabalin, a primary amine, with lactose, a reducing sugar to form the Amadori product (pregabalin-lactose product) and water
Fig. 4.
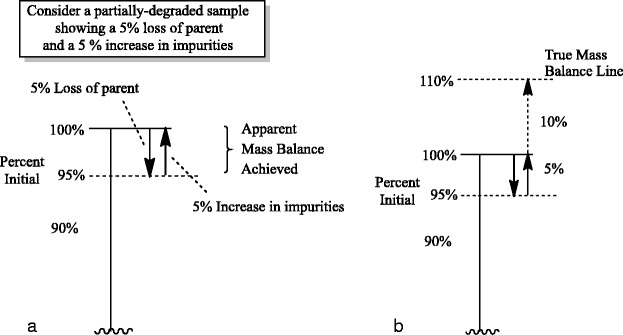
a Apparent mass balance in the case of a 5% loss of parent and a 5% increase in degradation product peak area, using a charged aerosol detector (CAD). b Based on the added mass from lactose in the lactose adduct, a true mass balance using the charged aerosol detector would require a total peak area corresponding to 110% of the peak area of the initial (undegraded) pregabalin timepoint
However, once the structure of the degradation product is determined, it is apparent that there is added mass from the lactose addition to pregabalin. Using the balanced equation in the bottom of Fig. 2, a 5% loss of pregabalin should correspond to 0.157 mg of pregabalin reacted, 0.342 mg of lactose consumed and 0.481 mg of lactose adduct formed. Thus, the mass of the lactose adduct detected by the CAD should be approximately three times that of the pregabalin reacted (481/157∼3). The peak area of the degradation product would need to be 15%, not 5%, in order to have complete mass balance. Since only one third of the mass needed for mass balance is being detected, there is a significant mass balance problem, with two thirds of the lost mass from pregabalin unaccounted for in the analysis (see Fig. 4b). In order to have complete confidence in mass balance calculations, a complete understanding of the degradation pathways, including corresponding degradation product structures, is required.
There are at least seven analytical causes of “mass imbalance” when using HPLC and there are some practical ways to investigate or address these issues. The seven causes are outlined below in Table II, along with practical suggestions or concepts to guide investigations.
Table II.
Common Analytical Causes of Mass Imbalance and Suggested Actions
| Potential analytical cause of mass imbalance | Practical suggestions to guide mass imbalance investigations |
|---|---|
| Impurities are not eluted from the HPLC column | • Use gradient HPLC with wide polarity range and longer hold time with strongest mobile phase condition • Analyze sample using reverse phase or normal-phase thin-layer chromatography (TLC) • Analyze sample(s) using hydrophilic interaction liquid chromatography (HILIC) or normal-phase HPLC • Analyze sample(s) using capillary electrophoresis (CE) |
| Impurities are poorly separated and are “missed”(20) | • Use gradient HPLC with a steep gradient • Use isocratic HPLC with very strong mobile phase condition, with and without a column in place • Use UV spectrophotometry without any analytical separation |
| Impurities are co-eluting with the parent compound | • Change HPLC stationary phases, solvent, or gradient • Use an orthogonal (different) separation method • Look for peak purity using a PDA-UV detector with a UV homogeneity algorithm • Look for peak purity using LC/MS techniques |
| Impurities are not detected by the detector | • Use PDA-UV (200–400 nm) detection to increase “universality”; monitor at low wavelength (e.g., 205–210 nm) (20) • Use UV-transparent solvents and buffers • Consider alternative detector e.g., evaporative light scattering detector (ELSD), mass spectrometry (MS), chemiluminescence nitrogen detector (CLND), corona, charged aerosol detector (CAD), refractive index or flame ionization detector (FID) • Analyze sample using alternate/orthogonal detection method • Use reverse phase (RP) or normal-phase (NP) thin-layer chromatography (TLC) –Use different options / chemistries for developing TLC spots or fluorescent-impregnated TLC plates • Use CE (often can look as low as 190 nm, more universal wavelength) |
| There is poor analytical recovery of the impurities | • Consider insolubility of impurities in analytical phases • Careful visual observation • Consider different solvents for sample preparation • Isolate solid material and analyze using other technique (e.g., probe-MS) • Consider possibility of reactions with insoluble excipients • Considered volatility (20) • Consider other analytical techniques (e.g., GC-headspace) • Consider adsorption losses • Compare results using different containers (e.g., glass and polypropylene) • Change sample/extraction solvent (e.g., different pH, different solvent) • Consider possibility of instability during the analytical preparation or workup |
| There is poor analytical recovery of the parent | |
| There is inaccurate quantification due to differences in response factors. | • Examine UV spectra of detected impurities (PDA detector) • Consider alternative detector—evaporative light scattering, MS, Corona CAD, chemiluminescence nitrogen detector (CLND), FID or LC/NMR • Determine response factors (20,21) • Isolate, purify, and determine using conventional means • Use CLND(23) or CAD (without isolation of impurities) • Use quantitative nuclear magnetic resonance (qNMR) (20) |
Mass balance can be measured and expressed in a variety of ways, but the concepts of Absolute and Relative Mass Balance has been advanced and discussed in detail by Nussbaum et al. (23). Absolute mass balance deficits (AMBD) can be expressed as the difference between the mass of parent drug consumed and mass of the parent contained in the degradation products recovered. Relative mass balance deficit (RMBD) can be expressed as the AMBD divided by the mass of the parent consumed and is expressed as a percentage
The topic of mass balance in relation to stability-indicating analytical methods is underappreciated and can be more complex than might be expected. An understanding of both reactant and products structures is essential to developing a reliable mass balance assessment when drug degradation occurs.
REVIEWING ADVANCES IN CHEMISTRY OF DRUG DEGRADATION (CONTRIBUTED BY KAREN ALSANTE, PFIZER INC.)
Stability has long been recognized as critically important in the drug development process, affecting both the safety and efficacy of drugs. The ability to rapidly predict and assess the potential for stability and safety concerns is an important part of speeding the development of innovative drug therapies. Degradation prediction enables understanding of labile functionalities critical in designing less reactive, more stable analogs. With efforts to reduce time and cost to market, the potential for stability issues increases dramatically. Degradation studies conducted by a chemistry-guided predictive stability approach enable analysts to deliver stability-indicating methodology more efficiently.
In this presentation, the major mechanisms of chemical decomposition of pharmaceuticals in the context of common functional groups were examined. The major mechanisms of chemical decomposition of pharmaceuticals include hydrolysis, dehydration, oxidation, isomerization/epimerization, decarboxylation, dimerization, polymerization, and photolysis and transformation products involving reaction with excipients/salt forms. While many pathways of degradation are obvious from basic organic chemistry principles, it is not uncommon to find surprising degradation chemistry leading to unexpected degradation products and pathways (13,24–27).
The chemistry explored in this presentation was extracted from actual degradation examples available in an online structure searchable drug degradation database tool, Pharma D3. This chemical structure searchable database was started by Dr. Alsante and Dr. Baertschi, in collaboration with Cambridgesoft™ (maker of ChemDraw™, Cambridge, MA) in 2005. The intent of the database is to be populated with drug degradation examples published either in the scientific literature or presented at scientific conferences. The database allows structure and name-based searching of the parent drug or the degradation product, and the conditions of the degradation and publication reference are included in the database. The database also allows for searching by molecular weight (MW) change, that is, the difference between the MW of the parent and the degradation products. As this compilation grows, the data should provide a useful tool for the field of degradation chemistry, enabling searches of specific drugs and molecular scaffolds as well as uncovering patterns of degradation of specific functional groups and of drugs in general.
As an example of the power of such an exercise, a search was conducted to determine the most common/frequent degradation pathways as a function of changes in MW from the parent to the degradation products. Thus, the database (28) was searched for all examples of degradation products that show MW changes from the parent of −60 to +60 amu, in 1 amu increments (29). The results of this effort are captured in Fig. 5, where the number of degradation products is plotted versus MW change. The plot shown reveals that there are patterns in degradation pathways, with certain MW changes occurring more frequently than others and many of the MW changes are not surprising to the seasoned degradation scientist.
Fig. 5.
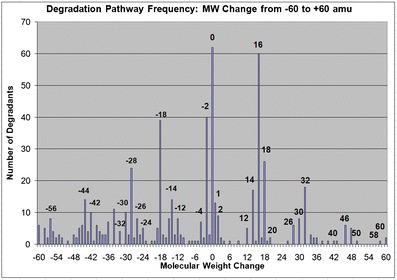
A plot of the frequency of specific MW changes from parent observed in degradation products contained in the Pharma D3 database in 2009. The height of the specific bar graphs indicate the most frequent MW changes from parent as a result of drug degradation processes
For example, changes in the MW of +16 and +32 amu occur frequently, corresponding to the addition of 1 and 2 oxygen atoms, respectively. Likewise, a change in the MW of +18 or −18 amu can readily be explained by the addition or loss of water. The most common instance is where there is no MW alteration, net change 0 amu, and this instance is represented by more than 60 examples found in the database, resulting from epimerization and rearrangements. The presentation then transitioned to understanding the impact of Zeneth® in silico software. Zeneth® is the only commercially available program designed to predict degradation pathways of pharmaceutical compounds and is a key tool to aid in degradation protocol design and structure elucidation. Zeneth® uses a high quality knowledge base and reasoning engine (16) to produce detailed tree depictions, showing chemical degradation pathways that include a level of likelihood, chemical formula, exact mass, and degradation pathway description.
The presentation concluded with Zeneth® benchmarking studies based on disguised industry examples. Zeneth® prediction captured to date demonstrated we are progressing in our ability to predict degradation products with this in silico tool. However, the demonstration revealed there is room for improvement, especially with respect to excipient-related degradation prediction capabilities. Obviously, there is still a need for wet chemistry in degradation related studies, but the power of prediction to aid in focusing stress testing protocols was clearly demonstrated. While many pathways of degradation are obvious from basic organic chemistry principles, it is not uncommon to find surprising degradation chemistry leading to unexpected degradation products and pathways. As the field of degradation chemistry matures, and degradation pathways of various drug scaffolds, ring systems, and functional group arrangements are documented in the literature, the predictability of degradation pathways will dramatically improve and patterns will begin to emerge. The concluding life cycle schematic (Fig. 6) depicts the evolution of the degradation workflow lifecycle and where we will continue to grow in our degradation knowledge.
Fig. 6.
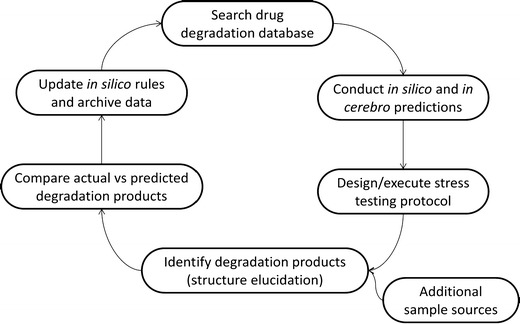
Degradation workflow lifecycle to improve process and incorporate lessons learned
SESSION 2: IMPURITY CONSIDERATIONS FOR PHARMACEUTICAL DOSAGE FORMS
The next session of the workshop transitioned to the pharmaceutical dosage form space expanding the complexity beyond impurities in the API alone to impurities in the excipients, degradation of the API via reactions with excipients and impurities in excipients as a few examples. This is a critical area of importance in the pharmaceutical industry as scientists must develop products that have acceptable chemical and physical stability over the course of the product shelf-life. It is quite common that the API will show stability issues when blended with excipients. There are often significant challenges with surprise degradation chemistry due to unknown impurities in excipients that cause the formation of degradants at levels of concern as per ICH Guidelines for Drug Products (ICH Q3B).
Margaret Landis (Pfizer) kicked off the session with the fundamentals and latest approaches on excipient compatibility testing. This is a critical area of understanding as knowledge of reactivity changes when the API is mixed with excipients is critical to formulation development. Since gathering real-time stability data on pharmaceutical dosage forms is impractical, the accelerated testing outlined is important for the understanding the stability of the drug product and for development of stability-indicating methodology.
Venkatramana Rao (Bristol-Myers Squibb) continued the focus on reactive impurities in pharmaceutical excipients and their impact on product robustness. These impurities include aldehydes/reducing sugars, peroxides, nitrates, nitrites, metals and solvents present at trace yet variable levels. However, control strategies using compendial methods do not appear feasible. A risk-based strategy involving early identification of incompatibilities between reactive excipient impurities and the drug followed by designing a drug product that can withstand the variability in excipients is provided as guidance.
The session transitioned to a discussion of complex dosage formulations, including nanoscale technology by Paul Meers (Rutgers University). Liposomes are one of the first “nanoscale” technologies used in drug delivery and have become an important formulation option in the field as liposomes can aid targeting and optimizing the pharmacokinetic profile.
The session closed with a presentation on combination therapies by Dan Reynolds (GlaxoSmithKline). Combination products constitute dosage forms that contain more than one API. Since reactions between the APIs becomes of particular concern with these products, these reactions should be investigated during product development. Most combination products contain APIs that are already in existing marketed products. Consequently, the degradation of the individual APIs is typically well understood but the interactions between the APIs represent potential novel drug–excipient and drug–drug reactions that require further study.
SOLID STATE EXCIPIENT COMPATIBILITY TESTING (CONTRIBUTED BY MARGARET LANDIS, PFIZER INC.)
Excipient compatibility is a broad term utilized for a variety of studies at many different stages of drug development. Compatibility screening with excipients can encompass early, simple studies aimed at evaluating compatibility of a drug with excipient to support initial biological efficacy studies, screening to ensure compatible and robust formulations for pre-clinical toxicology, short term compatibility studies to support early clinical dosage forms for Phase 1 or more complex compatibility studies needed to support larger, longer clinical studies, such as Phase 2A/2B and Phase 3 clinical formulations. Extensive and definitive compatibility studies are needed to support commercial formulation development and product launch. Finally, additional, specialized compatibility studies may be initialed to investigate alternative dosage formulations, such as controlled release and combination products.
Design of excipient compatibility studies will be unique to each molecule and drug therapy in question. It should be carefully considered how the excipient compatibility data will be used and clearly define the scope and limits of the compatibility information that will be gained from each study. Different stages of dosage form development will require varying size and types of compatibility studies. Prior to initiation of any compatibility studies, a thorough review of relevant drug substance information available at the time of the compatibility studies is extremely important. This evaluation should include a review of structural understanding of the molecular scaffold and sites of known reactivity, a detailed review of the synthetic route, a review of the API solution state stability data (pH, thermal, and photostability challenges), any and all forced degradation data, metabolite formation information and output from predictive models of degradation (Zeneth®, etc.).
In addition, a strong recommendation for compatibility studies includes generating a pre-compatibility profile of the drug candidate and the excipients. A detailed list of potential parameters to be evaluated and tracked is described in Table III below.
Table III.
Important API and Excipient Attributes Relevant to Excipient Compatibility Studies
| Important API Attributes relevant to Excipient Compatibility Studies | Important Excipient Attributes relevant to Excipient Compatibility Studies |
|---|---|
| API Impurity Profile (lot specific): water, solvent, metals and amorphous content, acidic/basic impurities, process-related impurities, alternate forms of the API present in small quantities (free forms or higher energy polymorphic forms) | Excipient impurity profile (lot specific): water, solvent, metals, amorphous content, acidic/basic impurities, reactive impurities (peroxides, aldehydes, organic acids) |
| Thermal and thermal/humidity solid-state stability (chemical and physical) | Thermal and thermal/humidity solid-state stability (chemical and physical) |
| Hygroscopicity profile | Equilibrium moisture content and hygroscopicity profile (1) |
| Particle attributes: size, shape, distribution, surface area | Particle attributes: size, shape, distribution, surface area |
| Effects of mechanical aggravation and processing | Effects of mechanical aggravation and processing |
| Crystal packing information (actual or predicted) | Details of excipient manufacture and processing |
| Effective pH in water (30) | |
| Spectroscopic properties | |
| Age and storage history of excipients (lot specific) |
The lot-specific parameters listed above should be considered and potentially evaluated for every drug substance and excipient evaluated in compatibility studies, especially the assessment of the levels of potentially reactive impurities present in common tableting excipients. The reactive impurities, including peroxides (31,32) and aldehydes (33,34), are known to cause chemical instability in dosages forms (35)
The four core aspects of excipient compatibility studies include sample design and preparation, sample composition, storage and stress conditions and methods of stability analysis. Sample design and preparation include aspects such as levels of API loading, blend preparation, compaction options, use and extent of mechanical aggravation utilized. These parameters can be chosen as conditions that represent a realistic state of the dosage form or the most challenged state (i.e., a worst-case scenario). Sample composition aspects include choices between the use of binary versus multi-component compatibility samples, spiking of samples with reactive impurities or water to induce or accelerate degradation, the use of statistical design or design of experiments for sample composition and the use of various forms of the drug (salts, free forms, polymorphic, or amorphous forms). Storage and stress conditions include the use of real-time or accelerated stress conditions involving humidity, heat and light stress conditions, the incorporation of open or special packaging (use of desiccants, blister packing, etc.) and the time course of the study. Methods of stability analysis need to address both physical and chemical stability during compatibility studies. Aspects to consider include the employment of destructive and/or non-destructive analysis, the choice of most applicable spectroscopic techniques, the number of samples needed per sampling timepoint and sensitivity of the methods employed. The stability data gathered during compatibility analysis may be used to dictate optimal excipient composition of a dosage form or generate stability–time profiles. Stability information gathered from compatibility studies serves to confirm real-time stability of a dosage form or to predict longer term shelf-life performance, such as via the use of the isoconversion-based accelerated stability assessment paradigm (ASAP) (36).
New frontiers for the science of excipient compatibility testing focus on the ability to acquire accurate compatibility information faster, utilizing less materials, experimentation and resources. Technological advances in the area will focus on the efficient automation of compatibility studies, the ability to predict and model degradation of drug substances in pharmaceutical dosage forms, the development of more efficient analytical techniques that can evaluate very low level physical and chemical changes of the API in excipient compatibility matrices. Future challenges for the field will come from the emergence of formidable new chemical entities, such as complex drug conjugates (i.e., antibody–drug conjugates, ADCs) and biopharmaceuticals. These systems will require new technology and paradigms for assessing compatibility in pharmaceutical dosage forms.
REACTIVE IMPURITIES IN PHARMACEUTICAL EXCIPIENTS AND THEIR IMPACT ON PRODUCT ROBUSTNESS (CONTRIBUTED BY VENKATRAMANA M. RAO, BRISTOL-MYERS SQUIBB COMPANY)
A robust drug product must accommodate typical variations in raw materials, i.e. API and excipients, operational elements (processing, equipment, etc.) and ambient conditions.(37) Excipients that directly react with drugs are eliminated during the excipient compatibility or stability studies, i.e. prior to the drug product design. That is why a large portion of reported excipient incompatibilities with drugs is due to reactions between drugs and “reactive” components within excipients (38). The main challenge for product robustness arises because these impurities are at trace yet variable levels and a control strategy based solely on compendial requirements (USP/NF, Ph.Eur., J.P.) is often not adequate. Additionally, very little information is available about these “reactive impurities” as manufacturing processes of excipients are trade secrets and not public information. Some of the most common reactive impurities include aldehydes/reducing sugars, peroxides, nitrates, nitrites, metals and solvents. These impurities could be introduced during the manufacturing processes of excipients or generated during storage or use. The analytical methods to quantify these trace level impurities need to be sensitive to detect such low levels and selective to differentiate between different “forms” of the impurities such as hydroperoxide versus hydrogen peroxide, etc. as the reactivity between drugs and these species may vary.
An approach to assess and mitigate the risks posed by the interactions between the impurities in excipients and drugs is proposed to be able to predict and/or determine the potential degradation pathways the drug candidate can undergo. This may be accomplished with prior knowledge, predictive modeling tools or experimental studies. Knowledge of reactive impurities that may be present in the specific excipients that are being considered in the product design is also essential.
The risk assessment approach involves combining the knowledge of reactive impurities in excipients along with an understanding of drug degradation pathways. Other factors such as drug to excipient ratio, crystal form of the API, environmental conditions, surface acidity or microenvironmental pH must also be considered during the assessment and mitigation of risk. The mitigation strategies include “designing out” the incompatibilities through formulation design, packaging configurations or putting in place a control strategy beyond compendial testing. Setting specific acceptance criteria for the excipients on a particular dosage form requires a strong and transparent relationship between excipient vendor and the drug product manufacturer. Arriving at the specification limits also requires an understanding of quantitative relationship between levels of impurities in the excipients and product stability. This may be challenging as procuring samples of excipients with varied levels of impurities may require the excipient vendor to produce batches that are not “typical” and spiking of the “reactive impurity” into the excipient by the end-user may not be practical. Early identification of incompatibilities between reactive excipient impurities and the drug followed by designing a drug product that can withstand the variability in excipients is the best approach to avoid undesirable surprises in subsequent stages of development and commercialization of the drug product.
LIPOSOMAL DEGRADATION (CONTRIBUTED BY PAUL MEERS, RUTGERS UNIVERSITY)
Liposomes represent one of the first nanoscale technologies used in drug delivery and have become an increasingly important formulation choice to address a number of pharmaceutical needs. As nanoscale to microscale supramolecular assemblies of lipids that encapsulate or associate with an active pharmaceutical ingredient, liposomes can aid targeting and optimize the pharmacokinetic profile. Truly liposomal formulations comprise one or more lipid bilayers that completely enclose or encapsulate an aqueous space (Fig. 6). Phospholipids, cholesterol, polymer-grafted lipids and cationic lipids are the major structural components of many liposomal formulations in development, and appropriate stress conditions and tests for chemical changes need to be identified (Fig. 7).
Fig. 7.
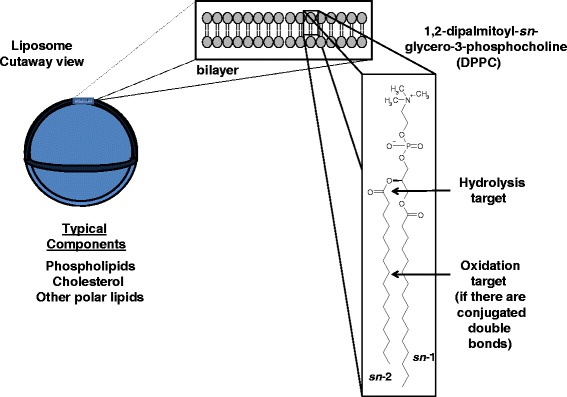
Schematic representation of a liposome. A liposome composed of the lipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) with a diameter of approximately 100 nm and a membrane thickness (phosphorus to phosphorus) of approximately 3.8 nm is shown. Top inset space filling models of DPPC bilayer arranged in a typical bilayer organization. Far right inset chemical structure of DPPC with stereo-specific numbering (sn) for the glycerol backbone attachment of acyl chains. Arrows show site of possible hydrolysis and oxidation sites
The primary degradation products for the many common liposomal components result from well-known reactions. For example, hydrolytic degradation products of phospholipids often include lyso-lipids that are produced by the cleavage of an acyl chain at the ester linkage of the sn-2 position, which is catalyzed by acid or base (39,40). Oxidation products can occur near the double bonds of unsaturated or especially polyunsaturated phospholipid acyl chains, or the 7-carbon position of cholesterol (41) (Fig. 1). Standard stress protocols include non-neutral pH and/or heat for hydrolysis, and free radical initiators and propagators for oxidation (42).
Because liposomes are particulate, it is the physical characteristics of the assembly that primarily determine the pharmacological delivery activity. In this sense, “impurities” include not only the chemical degradation products of the constituent lipids, but also alternate physical organizations of these lipids. Regulatory guidance therefore strongly recommends characterization of a number of physical properties of the liposomes that include surface charge, “leakiness”, size, and lamellarity (number of bilayers)(43) (Table IV).
Table IV.
Methods to Test Important Liposome Physical Properties
| Test parameter/(relevance) | Analytical methods | Stress methods |
|---|---|---|
| Captured volume (encapsulation efficiency, release rate) |
• Electron Spin Resonance (ESR) with CAT1 spin probe (volume exclusion method) (44) • Encapsulation of water soluble probe |
Chemical degradation; heat, detergents, osmolarity change |
| Drug encapsulation (encapsulation efficiency, dose) | • Field flow fractionation • Filtration • Size exclusion chromatography • Sedimentation • Fluorescent probes • NMR probes |
Chemical degradation; heat (phase transition temps), detergents, mechanical stress, sonication, osmolarity change |
| Liposome size (tissue targeting) | • Photon correlation spectroscopy (PCS) • Size exclusion chromatography • Field flow fractionation • Freeze fracture electron microscopy • Cryo-electron microscopy |
Chemical degradation; heat, detergents, mechanical stress, sonication, osmolarity change, ionic milieu |
| Lamellarity (release rate) | • 31P NMR • PCS w/captured volume • Fluorescent probes • Chemical surface labeling |
Chemical degradation; heat, detergents, mechanical stress, sonication |
| Surface charge (tissue targeting) | • Electrophoretic mobility | Chemical degradation; ionic milieu |
Importantly, the relevant chemical degradation products can affect the physical parameters of the liposomal assembly and vice versa. The tendency of lipids to organize in bilayers is highly dependent on their detailed chemistry, and even small chemical changes in the lipids can cause a change in the liposomal physical properties. Conversely, the physical and structural properties of the liposome can dictate the stability of the lipid components. For instance, while hydrolysis of phospholipid acyl chains directly affects parameters such as the leakage of encapsulated substances (45), the liposomal surface charge can affect the rate of these hydrolytic processes under certain conditions (46).
Design of pharmaceutical liposomes should be guided by some of the known degradation pathways to yield robust, stable formulations that can be easily characterized physically and chemically. The impetus to apply liposomal strategies to more complex delivery problems will lead to more challenges in performing appropriate stress tests. New chemical stress test protocols have become necessary as non-phospholipid constituents such as cationic lipids have become more prevalent, particularly for delivery of novel biopharmaceuticals (47). Furthermore, new devices for alternative routes of administration may have important effects on the physical parameters of the formulation. For instance, the development of liposomes for inhalation leads to unique parameters for stress testing involving analysis of the effects of nebulization on the aerosol distribution of resulting physical degradation products (48).
Addressing stress protocols for any particular formulation will require an informed and specific conglomeration of appropriate chemical and physical tests. The knowledge base in this relatively new field continues to develop and can be expected to significantly improve over the coming years as more drug delivery issues are addressed by products utilizing liposomes.
PRACTICAL ASPECTS OF STRESS TESTING ON SMALL MOLECULE PARENTERAL PRODUCTS (CONTRIBUTED BY ANDREAS ABEND, MERCK & CO., INC.)
Small molecule injectables are typically administered to patients by a physician and they are available as sterile powders lyophilized for injection or as a sterile liquid for injection. Compared to solid oral dosage forms, these formulations demand additional understanding of their stability since they may require reconstitution or dilution with a variety of diluents prior to their use. The product may be administered directly with a syringe or injected into a drip-bag and slowly infused into the vein of a patient. In addition, extractable and leachable studies are necessary to ensure no impurities are introduced into the product upon storage or during the final patient administration process (49).
Lyophilization, or “freeze-drying”, is the process of making sterile formulations for injection. This formulation approach is used to stabilize drugs that are otherwise sensitive to hydrolysis. The impact of moisture on the stability of freeze-dried products was presented in two case studies (Cases 1 and 2).
The formulation presented in Case 1 showed hydrolysis of the drug substance stemming from residual moisture in the freeze-dried product upon storage. The chemical nature of the degradation product was not a concern, but its limited solubility in commonly used diluents and the levels anticipated based on the available kinetic data at the end of the proposed product shelf-life may have posed a patient safety risk. The rate of hydrolysis was ultimately controlled by the excipients, the residual cake moisture at the end of the lyophilization process and the storage temperature.
The second case study highlighted a physical stability risk in a freeze-dried formulation. Here, controlling the amount of water released from the rubber closure turned out to be a significant risk during product development. Karl Fischer (KF) titration is normally the method of choice for determination of moisture levels in stoppers because of its simplicity and fast turnaround. KF is usually sensitive and suitable enough for the development of a stopper drying process for most lyophilized products. However, this method may not be sensitive enough to detect small differences in residual stopper moisture that may cause unacceptable changes upon storage in products with a relatively small mass (50). Relatively short stability studies with stoppers dried over a period of time and then placed on product vials can be used to gauge stopper drying effectiveness with much higher sensitivity. The relative humidity inside the stoppered vials can be measured by field modulated IR spectroscopy (51). Assessing the headspace moisture in vials with product as function of stopper drying time has shown to be predictive of physical stability of the lyophilized product, whereas KF measurements on stoppers alone was not (52). Once the critical headspace moisture in the vial has been established, these experiments can be used for example to evaluate the effectiveness of the stopper drying process during process scale-up development. A disadvantage of the headspace moisture experiments is the time it takes for the system to reach equilibrium. On the other hand, these experiments are still short in comparison to performing long term studies each time the stopper drying process is changed or the stopper composition or vendor is changed.
Case studies 3 and 4 discussed the impact of light on products in solution. ICH (Q1B) photostability studies are usually part of the battery of stress testing performed during formulation development and part of the stability data provided for regulatory submissions. Case study 3 showed that for a drug substance that is stable when exposed to light, the sensitivity of the formulation towards photodegradation increased over time (53). The root cause of this increased photodegradation was the amount of Fe3+ ions that had leached from the glass vial. The increased amounts of iron ions leaching into the product was promoted by the presence of chelators in the formulation. Most soluble organic Fe3+ chelated complexes absorb ultraviolet-A and visible light. The actual formulation may not show a noticeable UV–Visible light absorption profile due to the very low levels of these metal complexes. However, the presence of dissolved oxygen and exposure to light give rise to formation of hydrogen peroxide and Fe2+(54). The hydrogen peroxide is subsequently reduced by Fe2+ ions to hydroxyl radicals (55). The hydroxyl radicals then account for the observed degradation when the product is exposed to light.
Finally, Case 4 discussed the assessment of photodegradation during patient treatment, or in-use photodegradation. For drug products that are considered photosensitive (based on the photostability assessment outlined in ICH Q1B), the risk of unacceptable degradation during product administration is considered very low. This is due to the fact that the time of actual exposure to light is very short in comparison to the photostress study. Case 4 highlighted the level of additional product understanding one might consider when developing a highly light sensitive product especially with respect to patient in-use. The drug product can be routinely manufactured with virtually no degradation products and product storage in secondary package comfortably supports a 2-year shelf-life. Once removed from the protective secondary package and reconstituted, the product slowly undergoes photodegradation inside the primary package. The main issue with the drug is that when exposed to ambient light in a normal clear glass syringe, degradation is fast, even if the drug is administered right away. Experiments to gauge the extent of photodegradation included performing light measurements in hospital emergency rooms under various scenarios under which the drug may be administered. With an understanding of the conditions under which the drug would be administered, studies were performed to assess the amount of degradation during the administration process. These studies showed that the drug product can be safely administered to patients but requires clear instructions about the exposure time and tight measures to protect the product from direct light exposure.
STRESS TESTING OF COMBINATION THERAPIES (CONTRIBUTED BY DAN REYNOLDS, GLAXOSMITHKLINE)
Combination therapies contain more than one API. Several regulatory entities including International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Food and Drug Administration (FDA), and the World Health Organization (WHO) maintain that the possibility of reactions between APIs in combination therapies should be investigated during product development. Most combination therapies contain APIs that are already in existing marketed products. Consequently, what usually remains to be understood are potential novel drug–excipient and drug–drug reactions. Associated concerns are development of stability-indicating methods (SIMs), adequate packaging and shelf-life, etc.
A survey of the literature prior to 2002 showed that most investigators developing SIMs for combination therapies did not investigate the possibility of drug–drug reactions. However, a survey in 2010 showed the possibility of such drug–drug reactions were being taken into account by a majority albeit in various and inconsistent ways.
Little has been reported in the literature concerning the chemistry of drug–drug reactions in combination therapies. Examples are illustrated in Schemes 1, 2, and 3 below and include: the effect of Timolol Maleate on the stability of pilocarpine (56,57) (Scheme 1), the reaction of isoniazid with a degradation product of rifampicin (58) (Scheme 2) and transacetylation reactions of aspirin (59) with codeine and sulfadiazine (Scheme 3).
Scheme 1.
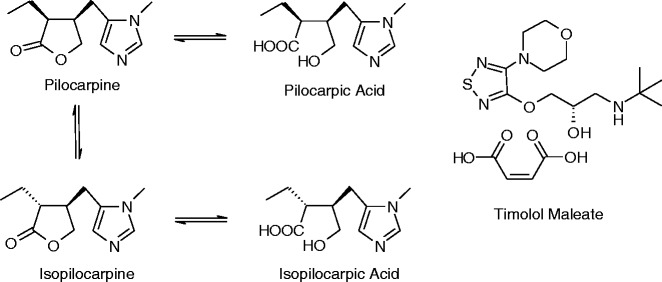
Degradation pathways of pilocarpine accelerated in the presence of timolol maleate
Scheme 2.
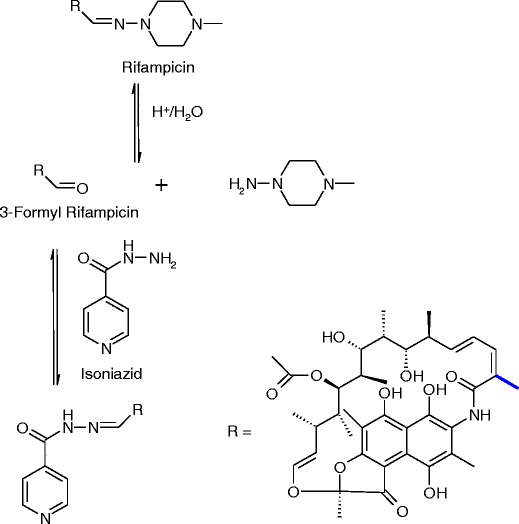
Degradation of combination therapy containing rifampicin and isoniazid
Scheme 3.
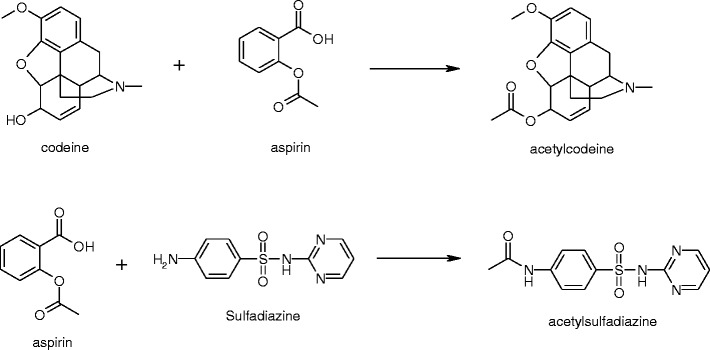
Transesterification degradation reactions of aspirin in combination products with codeine and sulfadiazine
GlaxoSmithKline (GSK) developed a triple combination tablet of the HIV therapies abacavir sulfate, lamivudine, and zidovudine. Prior to the NDA submission, the FDA requested in a letter that GSK stress the three APIs together in solution (acid/base, oxidation) and the solid-state (heat, heat/humidity, light). GSK complied with that request and also stressed the product tablets. The results of the degradation studies were reported to the FDA; no questions concerning degradation chemistry were asked. This approach has also been used for Advair (fluticasone propionate, salmeterol xinafoate) MDI, Combivir (lamivudine, zidovudine) tablets, and Treximet (naproxen sodium, sumatriptan) tablets. These studies were also reported in the respective NDAs without questions from regulators.
A recommended experimental approach to combination therapies includes stressing the combined APIs (1:1 mole ratio) in 0.1 N HCl; NMP/water (7) under N2, air, and O2; in 0.1 N NaOH; in the solid-state with ambient and 75% RH; and in the solid-state with >2× ICH light storage conditions. All samples (except light storage) may be stored at 60°C for 7 weeks and/or 80°C for 2 weeks (kinetic equivalent) or until the most labile API has degraded 10%, whichever comes first. This protocol does not apply to combination therapies where the APIs are not in physical contact with each other. In that case, drug–drug reactions are not considered an issue.
Results of API degradation studies should be reported in section 3.2.S.7.3 of the Common Technical Document (CTD) while drug product studies are reported in section 3.2.P.8.3. Suggested contents for the API module include a description of stress conditions, a scheme of the degradation pathways for each API, quantitative results (table format) for solution and solid-state samples (mass balance), results of chiral testing results (may refer to previous studies), chromatograms from HPLC testing on key samples, a discussion of the formation of each significant degradation product (conditions, mechanism), and a summary of peak homogeneity experiments on each API. It is recommended to dismiss any insignificant peaks observed in stress studies that are below Q3A identification thresholds in formal stability studies. The same information is suggested for the drug product module plus the description of the formulation and discernment between drug and excipient-related peaks, as Q3B also dictates identification thresholds for drug products. When the degradation products observed in the drug product on stability are the same as seen in stress studies of the APIs, it may not be necessary to file a drug product stress testing module (60).
CONCLUSION
The conference sessions summarized in this white paper (Part 1 of 2) covered a variety of important aspects involved in the study of drug degradation, both as the drug substance and in the drug product. Important advances in technology to predict drug degradation were investigated and reviewed. Focus was drawn to specific analytical and mass balance considerations involved in conducting successful and informative forced degradation studies of drug substances. Investigation of degradation in pharmaceutical dosage forms was shown to include a consideration of both the strategic design of solid-state compatibility studies and the need to understand reactive impurities present in pharmaceutical excipients. Stress testing of liposomal and small-molecule parenteral products requires in-depth understanding of these complex systems and need to be approached with concerns of both chemical and physical stability. Finally, a timely discussion of recommendations for stress testing of combination drug products was presented. Overall, the sessions served to highlight the importance of having the ability to rapidly predict and assess the potential for impurity formation in drug products, which can lead to performance, regulatory, efficacy, and safety concerns.
Acknowledgments
The organizing committee would like to thank all the speakers for sharing their work at this conference and through this publication.
Disclaimer
The views and opinions in this article are only those of the authors and do not necessarily reflect the views or policies of the companies, organizations or agencies cited herein.
Footnotes
Dedication
This manuscript is dedicated to the memory of the late Karen Russo, Ph.D. (United States Pharmacopeial Convention)
References
- 1.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Stability Testing of New Drug Substances and Products Q1A (R2). ICH Harmonized Tripartite Guidelines. 2003.
- 2.Parenty ADC, Button WG, Ott MA. An expert system to predict the forced degradation of organic molecules. Molecular Pharmaceutics. 2013;in press. [DOI] [PubMed]
- 3.Baertschi SW, Reynolds DW, Jansen P, Alsante K, Santafianos D, Kimmer Smith W, et al. Pharmaceutical stress testing: predicting drug degradation. Second ed. Baertschi SW, Alsante KM, Reed RA, editors: InformaHealth Sciences; 2011. 624 p.
- 4.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals ICH M3 (R2). ICH Harmonized Tripartite Guidelines. 2009.
- 5.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Photosafety Evaluation of Pharmaceuticals S10: Draft ICH Consensus Guideline. ICH Harmonized Tripartite Guidelines. 2013.
- 6.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Guideline on assessment and control of dna reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7: Draft Consensus Guideline. ICH Harmonized Tripartite Guidelines. 2013.
- 7.Reynolds DW, Galvani M, Hicks SR, Joshi BJ, Kennedy-Gabb SA, Kleinman MH, et al. The use of N-methylpyrrolidone as a cosolvent and oxidant in pharmaceutical stress testing. J Pharm Sci. 2012;101:761–776. doi: 10.1002/jps.22793. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman MH, Smith MD, Kurali E, Kleinpeter S, Jiang K, Zhang Y, et al. An evaluation of chemical photoreactivity and the relationship to phototoxicity. Regul Toxicol Pharmacol. 2010;58:224–232. doi: 10.1016/j.yrtph.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Onoue S, Hosoi K, Wakuri S, Iwase Y, Yamamoto T, Matsuoka N, et al. Establishment and intra-/inter-laboratory validation of a standard protocol of reactive oxygen species assay for chemical photosafety evaluation. J Appl Toxicol. 2012;13(10). [DOI] [PubMed]
- 10.Spielmann H, Balls M, Brand M, Doring B, Holzhutter HG, Kalweit S, et al. EEC/COLIPA project on in vitro phototoxicity testing: first results obtained with a Balb/c 3T3 cell phototoxicity assay. Toxicol In Vitro. 1994;8(4):793–796. doi: 10.1016/0887-2333(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 11.Henry B, Foti C, Alsante K. Can light absorption and photostability data be used to assess the photosafety risks in patients for a new drug molecule? J Photochem Photobiol B. 2009;96(1):57–62. doi: 10.1016/j.jphotobiol.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Waterman KC, Carella AJ, Gumkowski MJ, Lukulay P, MacDonald BC, Roy MC, et al. Improved protocol and data analysis for accelerated shelf-life estimation of solid dosage forms. Pharm Res. 2007;24(4):780–790. doi: 10.1007/s11095-006-9201-4. [DOI] [PubMed] [Google Scholar]
- 13.Baertschi SW, Jansen PJ, Alsante KM. Stress testing: a predictive tool (Chapter 2). In: Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical Stress Testing: Predicting Drug Degradation: Informa Life Sciences; 2011. 10-48.
- 14.Baertschi SW, Alsante KM, Tonnesen HH. A critical assessment of the ICH guideline on photostability testing of new drug substances and products (Q1B): Recommendation for revision. J Pharm Sci. 2010;99(7):2934–2940. doi: 10.1002/jps.22076. [DOI] [PubMed] [Google Scholar]
- 15.Jansen PJ, Smith WK, Baertschi SW. Stress testing: Analytical considerations (Chapter 4) In: Baertschi S, Alsante K, Reed R, editors. Pharmaceutical Stress Testing. New York, NY: Informa Healthcare; 2011. [Google Scholar]
- 16.Lhasa Limited. Zeneth. Leeds UK: Lhasa Limited,; 2013; Version 5:(Available from: www.lhasalimited.org/zeneth/.
- 17.Albini A, Anderson NH, Baertschi S, Boxhammer J, Byard SJ, Carter PL, et al. In: Photostability of drugs and drug formulations. 2. Tonnesen H, et al., editors. Boca Raton, Florida: CRC Press; 2004. [Google Scholar]
- 18.Piechocki JT, Tonnesen H, Allen JM, Allen SK, Gauglitz G, Hubig SM, et al. In: Pharmaceutical photostability and stabilization technology. 1. Piechocki JT, Thoma K, et al., editors. New York, NY: Informa Healthcare; 2007. [Google Scholar]
- 19.Lukulay P, Hokanson G. Reconciling mass balance in forced degradation studies. Pharm Tech. 2005; 106-12.
- 20.Baertschi SW. Analytical methodologies for discovering and profiling degradation-related impurities. TrAC Trends Anal Chem. 2006;25(8):758–767. doi: 10.1016/j.trac.2006.05.012. [DOI] [Google Scholar]
- 21.Nussbaum MA, Kaerner A, Jansen PJ, Baertschi SW. Role of "mass balance" in pharmaceutical stress testing (Chapter 9). In: Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical Stress Testing: Predicting Drug Degradation (Drugs and the Pharmaceutical (Sciences). Second Edition ed: Informa Life Sciences; 2011. p. 233-53.
- 22.Nursten HE, Royal Society of Chemistry. The Maillard Reaction: Chemistry, Biochemistry, and Implications: Royal Society of Chemistry; 2005.
- 23.Nussbaum MA, Baertschi SW, Jansen PJ. Determination of relative UV response factors for HPLC by use of a chemiluminescent nitrogen-specific detector. J Pharm Biomed Anal. 2002;27(6):983–993. doi: 10.1016/S0731-7085(01)00545-3. [DOI] [PubMed] [Google Scholar]
- 24.Baertschi SW, Alsante KM, Santafianos D. The chemistry of drug degradation (Chapter 3). In: Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical Stress Testing: Predicting Drug Degradation: Informa Life Sciences; 2011. p. 49-141.
- 25.Alsante KM, Baertschi SW, editors. Reviewing Advances in Knowledge of Drug Degradation Chemistry. Forced Degradation for Pharmaceuticals : Conference Proceedings; 2012 January 18-19, 2012; United Kingdom.
- 26.Alsante KM, Ando A, Brown R, Ensing J, Hatajik TD, Kong W, et al. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv Drug Del Rev. 2007;59:29–37. doi: 10.1016/j.addr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds DW, Facchine KL, Mullaney JF, Alsante KM, Hatajik TD, Motto MG. Available guidance and best practices for conducting forced degradation studies. Pharm Tech. 2002;26(2):48–54. [Google Scholar]
- 28.Alsante KM, Snyder KD, Swartz M, Parks C. Applications development using out-of-the-box software: a structure searchable degradation/impurity database. Scientific Computing and Instrumentation. June 2012:30-7.
- 29.Boyd DB, Sharp TR. The Power of Computational Chemistry to leverage Stress Testing of Pharmaceuticals. In: Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical stress testing. Predicting Drug Degradation: Informa Life Sciences; 2011. [Google Scholar]
- 30.Handbook of Pharmaceutical Excipients. Seventh ed. Rowe RC, Sheskey PJ, Cook WG, Fenton ME, editors: Pharmaceutical; 2012.
- 31.Wasylaschuk W, Harmon P, Wagner G, Harman AB, Templeton AC, Xu H, et al. Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci. 2007;96(1):106–116. doi: 10.1002/jps.20726. [DOI] [PubMed] [Google Scholar]
- 32.Yue H, Bu X, Huang M-H, Young J, Raglione T. Quantitative determination of trace levels of hydrogen peroxide in crospovidone and a pharmaceutical product using high performance liquid chromatography with coulometric detection. Int J Pharm. 2009;375:33–40. doi: 10.1016/j.ijpharm.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Jacobus LK, Wuelfing WP, Golden M, Martin GP, Reed RA. Detection and quantification of low-molecular-weight aldehydes in pharmaceutical excipients by headspace gas chromatography. J Chromatogr A. 2006;1104(1–2):1–10. doi: 10.1016/j.chroma.2005.10.084. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Kozlowski BM, Chang EP. Analysis of aldehydes in excipients used in liquid/semi-solid formulations by gas chromatography-negative chemical ionization mass spectrometry. J Chromatogr A. 2007;1160(1–2):299–305. doi: 10.1016/j.chroma.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 35.Hartauer KJ, Arbuthnot GN, Baertschi SW, Johnson RA, Luke WD, Pearson NG, et al. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Technol. 2000;5(3):303–310. doi: 10.1081/PDT-100100545. [DOI] [PubMed] [Google Scholar]
- 36.Waterman KC. The application of the Accelerated Stability Assessment Program (ASAP) to quality by design (QbD) for drug product stability. AAPS PharmSciTech. 2011;12(3):932–937. doi: 10.1208/s12249-011-9657-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glodek M, Liebowitz S, McCarthy R, McNally G, Oksanen C, Schultz T, et al. Product robustness—a PQRI white paper. Pharm Eng. 2006;26(6):1–11. [Google Scholar]
- 38.Wu Y, Levons J, Narang AS, Raghavan K, Rao VM. Reactive impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility. AAPS PharmSciTech. 2011;12(4):1248–1263. doi: 10.1208/s12249-011-9677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensil CR, Dennis EA. Alkaline hydrolysis of phospholipids in model membranes and the dependence on their state of aggregation. Biochemistry. 1981;20(21):6079–6085. doi: 10.1021/bi00524a025. [DOI] [PubMed] [Google Scholar]
- 40.Zuidam NJ, Crommelin DJ. Chemical hydrolysis of phospholipids. J Pharm Sci. 1995;84(9):1113–1119. doi: 10.1002/jps.2600840915. [DOI] [PubMed] [Google Scholar]
- 41.Schnitzer E, Pinchuk I, Lichtenberg D. Peroxidation of liposomal lipids. Eur Biophys J. 2007;36(4–5):499–515. doi: 10.1007/s00249-007-0146-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JA, Pawelchak J. Effect of pH, ionic strength and oxygen burden on the chemical stability of EPC/cholesterol liposomes under accelerated conditions. Part 1: Lipid hydrolysis. Eur J Pharm Biopharm. 2000;50(3):357–364. doi: 10.1016/S0939-6411(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 43.Food and Drug Administration (FDA). Guidance for Industry: Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation (Draft Guidance). 2002.
- 44.Moll KP, Stosser R, Herrmann W, Borchert HH, Utsumi H. In vivo ESR studies on subcutaneously injected multilamellar liposomes in living mice. Pharm Res. 2004;21(11):2017–2024. doi: 10.1023/B:PHAM.0000048192.13398.ac. [DOI] [PubMed] [Google Scholar]
- 45.Zuidam NJ, Gouw HK, Barenholz Y, Crommelin DJ. Physical (in) stability of liposomes upon chemical hydrolysis: the role of lysophospholipids and fatty acids. Biochim Biophys Acta. 1995;22(1):101–110. doi: 10.1016/0005-2736(95)00180-5. [DOI] [PubMed] [Google Scholar]
- 46.Grit M, Crommelin DJ. The effect of surface charge on the hydrolysis kinetics of partially hydrogenated egg phosphatidylcholine and egg phosphatidylglycerol in aqueous liposome dispersions. Biochim Biophys Acta. 1993;17(1):49–55. doi: 10.1016/0005-2760(93)90216-V. [DOI] [PubMed] [Google Scholar]
- 47.MacLachlan I. Liposomal formulations for nucleic acid delivery (Chapter 9) In: Corooke ST, editor. Antisense drug technologies: principles, strategies, and applications. 2. Boca Raton, FL: CRC; 2007. pp. 237–269. [Google Scholar]
- 48.Li Z, Zhang Y, Wurtz W, Lee JK, Malinin VS, Durwas-Krishnan S, et al. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J Aerosol Med Pulm Drug Deliv. 2008;21(3):245–254. doi: 10.1089/jamp.2008.0686. [DOI] [PubMed] [Google Scholar]
- 49.Abend A, Duersch B, Fiszlar K. Small molecule parenteral drugs: practical aspects of stress testing (Chapter 12). In: Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical Stress Testing: Predicting Drug Degradation: Informa Life Sciences; 2011. p. 322-42.
- 50.Abend A, Templeton A. The impact of stopper drying for lyophilized drug products. Am Pharm Rev. 2010;25(6):201–206. [Google Scholar]
- 51.Carlisle CB, Cooper DE. Tunable diode laser frequency modulation spectroscopy through an optical fiber: high‐sensitivity detection of water vapor. Appl Phys Lett. 1990;56:805–807. doi: 10.1063/1.102669. [DOI] [Google Scholar]
- 52.Templeton AC, Placek J, Xu H, Mahajan R, Hunke WA, Reed RA. Determination of the moisture content of bromobutyl rubber stoppers as a function of processing: implications for the stability of lyophilized products. PDA J Pharm Sci Technol. 2003;57(2):75–87. [PubMed] [Google Scholar]
- 53.Reed RA, Harmon P, Manas D, Wasylaschuk W, Galli C, Biddell R, et al. The role of excipients and package components in the photostability of liquid formulations. PDA J Pharm Sci Technol. 2003;57(5):351–368. [PubMed] [Google Scholar]
- 54.Faust BC, Zepp RG. Photochemistry of aqueous iron(III)-polycarboxylate complexes: roles in the chemistry of atmospheric and surface waters. Environ Sci Technol. 1993;27(12):2517–2522. doi: 10.1021/es00048a032. [DOI] [Google Scholar]
- 55.Haber F, Weiss J. Uber die Katalyse des Hydroperoxydes. Naturwissenschaften. 1932;20(51):948–950. doi: 10.1007/BF01504715. [DOI] [Google Scholar]
- 56.Pilatti C, Torre MC, Chiale C, Spinetto M. Stability of pilocarpine ophthalmic solutions. Drug Dev Ind Pharm. 1999;25(6):801–805. doi: 10.1081/DDC-100102241. [DOI] [PubMed] [Google Scholar]
- 57.Al-Badr AA, Aboul-Enein HY. Pilocarpine. In: Florey K, editor. Analytical profiles of drug substances. Academic; 1983. p. 385–432.
- 58.Singh S, Mariappan TT, Sharda N, Kumar S, Chakraborti AK. The reason for an increase in decomposition of rifampicin in the presence of isoniazid under acid conditions. Pharm Pharmacol Commun. 2000;6(9):405–410. doi: 10.1211/146080800128736277. [DOI] [Google Scholar]
- 59.Yoshioka S, Stella VJ. Stability of drugs and dosage forms. First ed: Springer; 2000.
- 60.Food and Drug Administration (FDA). Questions and Answers on Current Good Manufacturing Practices, Good Guidance Practices, Level 2 Guidance—Laboratory Controls. Guidance, Compliance, & Regulatory Information [Internet]. 2011. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm124785.htm.


