Abstract
Push–pull osmotic pump (PPOP) tablets of a practically insoluble model drug were developed and the effect of various formulation and process parameters on tablet performance was evaluated in order to identify critical factors. The formulation factors such as the viscosity grade of polyethylene oxide as the primary polymer as well as the level and location of osmogen within the bilayer tablets led to a difference in performance of osmotic tablets and hence should be critically evaluated in the design of such dosage forms. Modification of granulation process, i.e., the granulating liquid composition or drying method of granules, did not impact the drug release from the osmotic tablets at the evaluated scale of this study. The influence of varying dose and aqueous solubility of other model drugs (i.e., theophylline, acetaminophen, and verapamil HCl) on the developed PPOP template was also investigated. Results showed that irrespective of the perceived complexity of development and manufacturing of osmotic pumps, the osmotic tablets in this study demonstrated a robust and yet flexible platform in accommodating different types of drug candidates, regardless of solubility, for the dose levels below 25% w/w of the pull layer formulation.
KEY WORDS: cellulose acetate, OROS, osmogen, polyethylene oxide, push–pull osmotic pump (PPOP)
INTRODUCTION
Solid oral dosage forms continue to be the most common and preferred delivery systems for most therapeutic agents. The popularity of the oral route is attributed to ease of administration and thus patient compliance, coupled with the accessibility of well-developed solid dosage systems which can confer accurate dosing, cost-effective manufacturing, and long shelf-life. For many drugs and therapeutic indications, conventional multiple dosing of immediate release formulations provides adequate clinical performance with an appropriate balance of safety and efficacy. However, it has long been known that extended release (ER) formulations for some drug types may significantly improve the therapeutic benefits, minimize the side effects as well as providing an opportunity for life-cycle management (1). Over the last decade, there has been an increasing interest in the development of oral osmotic devices in which the active pharmaceutical ingredient is delivered in a precise and sustained manner. The zero-order drug release from osmotic devices is generally independent of pH, ionic strength, agitation, and other physiological factors within the gastrointestinal tract. These attributes minimize patient-to-patient variability and allow accurate prediction of in vivo performance from in vitro dissolution profiles. The main clinical benefits of these systems are improving treatment tolerability and patient compliance, especially for drugs with narrow therapeutic index. However, access to the relevant technologies has been restricted due to perceived and real manufacturing constraints and the patent landscape (2–11).
Osmotic tablets consist of a drug core which is osmotically active and surrounded by a semipermeable membrane with delivery passage way(s). The first osmotic tablets containing a bilayer core were developed and introduced by Alza in 1982, commonly known as OROS push–pull devices (12). A push–pull osmotic pump (PPOP) tablet (Fig. 1) has two layers, pull and push, coated with a semipermeable membrane which is insoluble but permeable to water. A drug delivery orifice is drilled through the coating on the pull-layer side to facilitate the drug release from the tablets upon exposure to liquid. This design provides a controlled rate of drug delivery into the gastrointestinal lumen. The performance of PPOP tablets depends on an osmotic gradient between the contents of the bilayer tablet core and the fluid in the GI tract. The drug delivery rate is essentially constant as long as the osmotic gradient remains constant. During the terminal phase of drug release, there is a gradual decline in rate until the full drug payload is dispensed from the tablet. The semipermeable membrane remains intact with some of the push-layer components encased during GI transit and the remnant, known as “exhausted ghost”, is eliminated in the feces (13).
Fig. 1.

Components of a push–pull osmotic pump (PPOP) tablet. 1 Pull (drug) layer, 2 push layer, 3 semipermeable membrane, 4 drug delivery orifice, 5 topcoat/overcoat
In order to successfully formulate the bilayer tablet cores of the osmotic systems and to obtain satisfactory performance, the choice of excipients is essential. Although several polymers have been cited in the literature for use in osmotic tablets, such as sodium carboxymethyl cellulose and pectin (14), hypromellose, hydroxyethyl methylcellulose, methylcellulose, polyvinylpyrollidone, and polyethylene oxide, only a few have proven worthy of consideration in the design of the osmotic tablets (2,15). Polyethylene oxide (PEO) is the most commonly used polymer in the formulation of PPOP tablets due to its favorable hydration kinetics and swelling properties. The NF grades of polyethylene oxide, POLYOX Water Soluble Resins, are present in most of the osmotic pump tablets currently available in the market place. POLYOX is a white, tasteless nonionic hydrophilic polymer which is generally free flowing, very compressible, and available in different molecular weight/viscosity grades, rendering it a suitable and almost exclusive choice in formulation of both pull and push layers. The lower molecular weight grades (200,000–400,000 Da) generally serve as the primary component in the pull layer, while the higher molecular weight grades are used as a swelling agent in the push layer (3,000,000–7,000,000 Da) (2,16,17).
In order to maintain the osmotic gradient for the tablets and assure desirable release profiles, sodium chloride (NaCl) is commonly used as an osmogen since it is available, non-reactive, and provides high osmotic pressure across the semipermeable membrane (2). The quantity and presence of the osmogen in different layers of the osmotic tablets may affect the overall performance of such systems (4,18).
Another important component of an osmotic tablet is the semipermeable membrane since it acts as a barrier to control the diffusion of water into the tablet core and the drug release out of the delivery orifice. The membrane is generally composed of cellulose acetate as a water-insoluble, film-forming polymer in combination with polyethylene glycol (PEG) as a plasticizer and pore former (2,19).
Given the aforementioned advantages, the osmotic technology has been, and continues to be, used extensively in development of ER oral dosage forms; however, there have been only few references on critical evaluation of formulation and process parameters that may influence the performance of such dosage forms. The objectives of the present study, therefore, are systematic development and evaluation of PPOP tablets, providing process details. This includes investigation of the effect of various formulation factors for the bilayer tablet cores as well as different parameters related to high shear granulation process, used in manufacture of pull and push layers of this study, to identify critical factors which influence the drug release from the osmotic tablets. The study also presents the encountered process challenges for granulation and bilayer tablet compression and offers approaches to alleviate them. In this study, glipizide was selected as a practically insoluble model drug. Furthermore, the developed osmotic system was used as a template to investigate the influence of varying dose and aqueous solubility of different model drugs (theophylline, acetaminophen, and verapamil HCl) on performance of such system.
MATERIALS AND METHODS
Development and Evaluation of Push–Pull Osmotic Pumps
In this study, osmotic tablet formulations of glipizide were developed (11 mg, including 10% overage) (Table I). The manufacturing process for osmotic tablets is illustrated in Fig. 2. Manufacture of osmotic tablets involves a multi-step process train including preparation of formulation blends for pull layer and push layer, a granulation step, drying of granules, compression of bilayer tablets, preparation and application of semipermeable membrane, drying of coated tablets, and laser drilling a delivery orifice on the pull-layer side of the tablets.
Table I.
Formulation of Pull and Push Layers for Glipizide Osmotic Tablets
| Tablet core-Ingredients | Supplier | Quantity (% w/w) |
|---|---|---|
| Pull layer—ingredients | ||
| Glipizide | Ria International LLC, USA | 5.6 |
| Polyethylene oxide (POLYOX WSR N-80 NF) | The Dow Chemical Company, USA | 93.9 |
| Magnesium stearate (MgSt) | Mallinckrodt, USA | 0.5 |
| Total (200 mg) | 100 | |
| Push layer—ingredients | ||
| Polyethylene oxide (POLYOX WSR Coagulant NF) | The Dow Chemical Company, USA | 64.0 |
| Sodium chloride | Mallinckrodt, USA | 35.0 |
| Pigment, red iron oxide | Rockwood Pigments, Italy | 0.5 |
| Magnesium stearate (MgSt) | Mallinckrodt, USA | 0.5 |
| Total (130 mg) | 100 | |
Fig. 2.

General process train in manufacture of PPOP tablets
The individual pull- and push-layer ingredients, except for magnesium stearate, were added to a high shear granulator (Diosna P/VAC 10, Germany) (batch size, 1 kg) and dry blended for 3 min, followed by spray application of hydro-alcoholic granulating liquid composed of ethanol/water, 85:15 w/w, at the addition rate of 30 g/min for the pull-layer and 20 g/min for the push-layer blend. The application rate was slower for the push layer due to the higher viscosity grade of POLYOX Coagulant within this layer. The impeller and chopper were operated at 150 and 2,000 rpm, respectively. The granules were dried in a vacuum drying oven (VDL-115, BINDER, Germany) at 40°C for 16 h to achieve an initial equilibrium moisture content of ∼0.5% w/w and then milled (Quadro Comil, 1.18 mm grated screen) (Quadro Engineering, Canada), followed by lubrication with magnesium stearate for 1 min in a twin shell blender (Patterson Kelly, USA). Bilayer tablets were prepared on a rotary press (Piccola, Riva, Argentina) using standard, round, concave tooling (9.5 mm) at the target weight of 330 mg (pull/push layer, ∼2:1 w/w). A tamping force (pressure) of ∼0.7 kN (9.8 MPa) was used to compress the pull layer, followed by main compression force (pressure) of 7 kN (98 MPa) to compress the bilayer tablets. Tablets were coated to 8, 10, and 12% w/w weight gain (WG) using Opadry CA fully formulated osmotic coating system (Colorcon Inc., USA) consisting of cellulose acetate and PEG 3350 (9:1 w/w) in a solvent mixture of acetone and water at 7% w/w solids content. The coating process was performed in a Hi-Coater LDCS (Vector Corporation, USA) at a product temperature of 28°C. Coated tablets were dried in a vacuum oven at 40°C for 24 h to remove residual solvent and moisture. A delivery orifice was drilled on the pull-layer side using a laser machine (Cobalt 250, InkCupsNow, USA).
The dried granules were evaluated for physical characteristics (bulk and tapped density, compressibility index, and particle size distribution) and the bilayer tablet cores (prior to application of semipermeable coating) were evaluated for physical properties (weight variation, dimensions, hardness, and friability) based on the USP methods (20). The osmotic tablets were evaluated for in vitro drug release in a USP compliant dissolution bath using apparatus II at 50 rpm with sinkers, in 900 ml of simulated intestinal fluid without pancreatin, pH 7.5, and UV analysis at 275 nm (21). In addition, the push–pull patterns and media uptake of the osmotic tablets were examined over time by hydrating the tablets in the dissolution media, followed by physical and gravimetrical evaluation at pre-determined time intervals. This examination was carried out to assure that the push layer provided consistent push pattern to the drug layer which would ultimately result in constant drug release from the system.
The osmotic tablets were further evaluated for the level of residual solvents using gas chromatography (Agilent Technologies, USA) to assure the level of process related solvents, ethanol and acetone, are within the allowable limit as specified by the USP and ICH guidelines for Class III solvents (5,000 ppm per day) (20,22).
The developed osmotic tablets of this section (Table I) were used as “control tablets” for the following investigations.
Effect of Formulation and Processing Variables on Performance of the Osmotic Tablets
Various formulation factors and parameters were examined to identify potential critical factors affecting the bilayer tablet cores and hence the performance of the resulting osmotic tablets. In all instances, the osmotic tablets were manufactured per the process train shown in Fig. 2. The modifications in formulation or processing conditions are noted below. The bilayer tablet cores were evaluated for physical properties and the osmotic tablets were tested for in vitro drug release, as described earlier. To further investigate the significance of every variation in formulation or processing conditions, the drug release profiles of the resulting tablets were compared to the “control tablets”, using similarity factors (f2) (23).
Evaluation of Formulation Factors
Effect of Various Grades of POLYOX in Pull and Push Layers
To evaluate the effect of molecular weight of the polymer, various grades of POLYOX, i.e., WSR N-80 NF or N-750 NF for the pull-layer and POLYOX WSR 301 NF, Coagulant NF, or 303 NF for the push-layer formulations, were investigated (Table II). The formulation in Table I was used as the control formulation and individual grades of POLYOX were incorporated within the pull- and push-layer formulations at the same levels.
Table II.
Various Viscosity Grades of POLYOX Used in this Study (17)
| POLYOX NF grade | Approximate molecular weight (Da) | Viscosity at 25°C (cP) |
|---|---|---|
| WSR N-80 NF | 200,000 | 55–90 (5% solution) |
| WSR N-750 NF | 300,000 | 600–1,200 (5% solution) |
| WSR 301 NF | 4,000,000 | 1,650–5,500 (1% solution) |
| WSR Coagulant NF | 5,000,000 | 5,500–7,500 (1% solution) |
| WSR 303 NF | 7,000,000 | 7,500–10,000 (1% solution) |
Effect of Osmogen Quantity in Push Layer
Sodium chloride was used at varying levels of 0–75% w/w within the push-layer formulation. The control formulation in Table I was used as the base system; as the level of salt was increased, the level of polymer (POLYOX Coagulant) was decreased accordingly.
Effect of Osmogen Location
The effect of NaCl location was evaluated in push layer only (35% w/w), in pull layer only (22.8% w/w), and in both pull and push layers (11.4 and 17.5%, respectively). In all instances, NaCl level was kept constant (45 mg) within the bilayer tablet formulation. Incorporation of NaCl in the pull layer was achieved by lowering the level of POLYOX N-80 in the formulation.
Evaluation of Granulation Process
Effect of Solvent Ratio in Granulating Liquid
Blends for pull and push layers were prepared using the control formulation (Table I), followed by high shear granulation process, as described earlier. In order to evaluate the effect of composition of granulating liquid, three different ethanol/water ratios were used, i.e., 100:0, 85:15 (control), and 70:30 w/w. The resulting granules were dried, milled, and evaluated for physical properties, followed by manufacturing steps to prepare the osmotic tablets.
Effect of Drying Method
Individual blends for pull and push layers were prepared using a high shear granulation process, as described earlier. The effect of drying method for both pull- and push-layer granules was evaluated using tray drying and fluid bed drying. For the former, the granules were dried in a vacuum oven at 40°C for 16 h. For fluid bed drying, granules were dried in a Glatt GPCG-2 drier (Glatt Air Techniques, USA) at a product temperature of 22–25°C. In both methods, the granules were dried to achieve initial moisture content of the dry blends (∼0.5% w/w). The resulting granules were milled and evaluated for physical properties followed by manufacture into osmotic tablets.
The Influence of Dose and Solubility of Various Model Drugs on Drug Release From the Osmotic Tablets
Four model drugs, glipizide, theophylline, acetaminophen (APAP), and verapamil HCl (Table III), were incorporated in the pull layer of the osmotic tablets and evaluated at different dose levels, corresponding to a range of 5.6–60.0% w/w of drug within the pull layer. The higher dose levels were accommodated by reducing the POLYOX N-80 content within the pull-layer formulation (Table IV). Push-layer composition remained the same (Table V). Bilayer tablets were compressed at a target tablet weight of 330 mg, except for verapamil HCl, where tablets were compressed at 450 mg to accommodate higher dose levels. The ratio of pull/push layers (∼2:1 w/w) was constant for all osmotic tablets followed by coating with a semipermeable membrane and laser drilling, as noted earlier. The dissolution profiles of the resulting osmotic tablets were obtained in an apparatus II (50 rpm) dissolution bath with sinkers. Drug release profiles were compared using similarity factors.
Table III.
Model Drugs of Varying Dose and Solubility
| Model drug | Solubility (27) | Low dose (mg) | Medium dose (mg) | High dose (mg) |
|---|---|---|---|---|
| Glipizide | Practically insoluble (0.02 mg/ml) | 11 | 50 | – |
| Theophylline | Slightly soluble (8 mg/ml) | 11 | 50 | 100 |
| APAP | Sparingly soluble (14 mg/ml) | 11 | 50 | 100 |
| Verapamil HCl | Soluble (50 mg/ml) | 40 | 100 | 180 |
Table IV.
Formulation of Pull Layer Used in the Osmotic Tablets of Various Model Drugs at Different Dose Levels: Pull Layer for Glipizide, Theophylline, and APAP (A); Pull Layer for Verapamil HCl (B)
| Pull layer—ingredients | Quantity (% w/w) |
|---|---|
| (A) Dose levels: low, 11 mg; medium, 50 mg; high, 100 mg | |
| Model drug (glipizidea, theophylline, APAP) | 5.6, 25.0, 50.0 |
| Polyethylene oxide (POLYOX WSR N-80 NF) | 93.9, 74.5, 49.5 |
| Magnesium stearate | 0.5 |
| Total (200 mg) | 100 |
| (B) Dose levels: low, 40 mg; medium, 100 mg; high, 180 mg | |
| Model drug (verapamil HCl) | 13.3, 33.3, 60.0 |
| Polyethylene oxide (POLYOX WSR N-80 NF) | 86.2, 66.2, 39.5 |
| Magnesium stearate | 0.5 |
| Total (300 mg) | 100 |
aA high dose of glipizide was not evaluated due to the lack of sink condition in the dissolution medium.
Table V.
Formulation of Push Layer Used in the Osmotic Tablets of Various Model Drugs
| Push layer—ingredients | Quantity (% w/w) |
|---|---|
| Polyethylene oxide (POLYOX WSR Coagulant NF) | 64.0 |
| Sodium chloride | 35.0 |
| Pigment, red iron oxide | 0.5 |
| Magnesium stearate | 0.5 |
| Total (130 mg)a
a150 mg for verapamil HCl PPOP |
100 |
RESULTS AND DISCUSSION
The physical properties of dried granules for pull and push layers are displayed in Fig. 3 and Table VI. The bilayer tablet cores of glipizide showed a drug assay value of 100.4 ± 2.9%, indicating good content uniformity. Table VII shows the physical properties of bilayer tablet cores, indicating acceptable weight variation, tensile strength, and low friability which are attributed to the material properties of POLYOX, as the main component of the tablets.
Fig. 3.
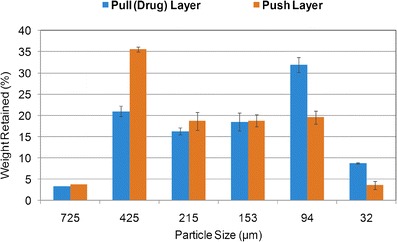
Particle size distribution of dried granules for pull and push layers of glipizide osmotic tablets
Table VI.
Physical Properties of Dried Granules
| Dried granules | Bulk density (g/cm3) | Tapped density (g/cm3) | Carr’s compressibility index (%) |
|---|---|---|---|
| Pull layer | 0.43 | 0.52 | 18.3 |
| Push layer | 0.47 | 0.58 | 18.8 |
Table VII.
Physical Properties of Glipizide Bilayer Tablet Cores (n = 10)
| Tablet properties | Values |
|---|---|
| Tablet weight (mg) | 332 ± 4.1 |
| Tablet thickness (mm) | 5.0 ± 0.1 |
| Tablet hardness (kp) (tensile strength (MPa)) |
9.4 ± 1.2 (1.36) |
| Tablet friability (%) | 0.0 |
Generally, tablets containing high levels of PEO tend to have lower mechanical strength than those typically observed for conventional tablet formulations (containing typical excipients, i.e., microcrystalline cellulose, dicalcium phosphate, or lactose) (2,24). For example, the crushing force values for bilayer cores of osmotic tablets, containing POLYOX in both layers (65–80% w/w), and for a conventional immediate release tablets of similar size and weight (9.5 mm, 440 mg) are 5–7 and 9–12 kp, respectively (2). The PEO-based tablets are also very robust with minimal friability. Such behavior is consistent with high ductility of this polymer and the tendency for PEO particles to yield as opposed to fracture under applied pressure. This ductility, however, could cause plastic deformation, leading to manufacturability issues, while processing the PEO-based formulations. Therefore, optimization of process parameters is critical in the manufacture of bilayer tablet cores, including granulation as well as tablet compression. In this study, during the granulation process, formation of a hard irregular shaped mass (i.e., corals or needles) was observed within 1 min into the operation. This could be attributed to compaction and/or melting of POLYOX under the impeller of the granulator and/or immediate local hydration of the polymer. The impeller speed used for the granulation process was initially set at 200 rpm. To overcome the aforementioned issue, the process was performed at lower impeller speed of 150 rpm which allowed for more homogenous mixing of the powder within the bowl of the granulator and resulted in more uniform granules for the batch size of this study.
During bilayer tablet compression, high ductility of PEO could result in crowning or flashing (flow around the punch) and ultimately lead to downstream coating defects. Crowning can be minimized by either decreasing the compression force or increasing the dwell time. Lowering the compaction pressure will generally alleviate the problem, but may also lead to lower mechanical strength of the tablets. In this study, application of low tamping force and main compression force (0.7 N for the first layer followed by 7 kN for the bilayer tablet) led to bilayer tablets with no crowning issues and yet acceptable mechanical strength (tablet hardness of ∼9 kp with no friability).
The levels of residual solvents for the osmotic tablets, after the drying step, were determined as 1,877 ppm of acetone and below 100 ppm for ethanol. These values are well below the daily allowable limit of 5,000 pm.
Figure 4 shows a typical release profile for osmotic tablets with an initial lag time followed by zero-order kinetics. The initial lag time indicates the time required for sufficient water ingress into the system and subsequent increase in hydrostatic pressure in order for the drug to be pushed through the delivery orifice. The lag time is mainly governed by the composition and thickness of the semipermeable membrane (2). The data points represent the mean value for six tablets and error bars signify the standard deviation. Drug release profiles from the osmotic tablets at various weight gains of semipermeable coating are also shown in Fig. 4, demonstrating that an increase in coating weight gain resulted in slower drug release. In addition, increasing the coating weight gain from 8 to 12% w/w led to prolonged lag time. For comparison, t10% was considered as lag time and defined as the time required for 10% of the labeled drug content to be released (18). The t10% values for glipizide osmotic tablets were determined as 2.4, 2.7, and 3.1 h for 8, 10, and 12% w/w semipermeable coating weight gain, respectively. Furthermore, the drug release rate was obtained from the slope of the linear portion of the release profiles with correlation coefficient, R2 >0.99. The release rate values were calculated as 9.0, 7.7, and 7.0%/h for 8, 10, and 12% w/w semipermeable coating weight gain, confirming that the higher weight gain led to slower drug release.
Fig. 4.
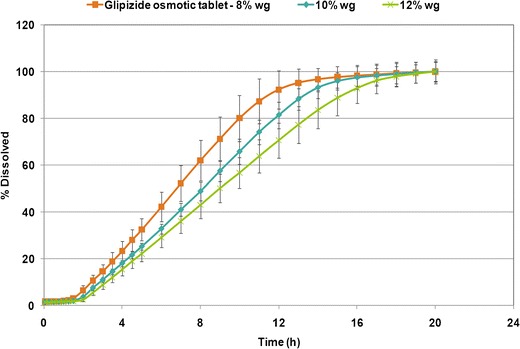
The influence of different coating weight gains of semipermeable membrane on drug release profiles from glipizide osmotic tablets (n = 6)
Figure 5 shows the behavior of the osmotic glipizide tablets coated to with 12% w/w weight gain of the semipermeable membrane over time with respect to drug release, tablet weight change (including media uptake and drug/excipient loss), and pull–push patterns upon hydration in dissolution media. Results demonstrated that the increase in the weight of the osmotic device was about 16% w/w after 2 h of hydration and remained almost steady over the period of the dissolution study (16 h). The first 2 h signify the lag time required for activation of the osmotic system, after which the drug is “pumped out” or released from the tablet at a constant rate. The viscosity balance between the pull and push layers within the osmotic tablet is critical for the performance of the osmotic dosage form, as it leads to desirable swelling and consistent pushing pattern of the push layer against the pull layer (25).
Fig. 5.

Drug release profile, percent tablet weight change, and pull–push pattern for glipizide osmotic tablets, coated to 12% w/w weight gain
In the evaluation of tablet core factors, varying the viscosity grades of POLYOX in pull (N-80 or N-750) and push layers (301, Coagulant, or 303) showed comparable physical properties among the bilayer tablet cores (tablet hardness of ∼10 kp). It has been reported that compressibility of the POLYOX is not significantly affected by molecular weight of the polymer (24). Drug release from the osmotic tablets was not significantly affected by varying the POLYOX grade in push layer (f2 > 74), while the use of higher viscosity grade of POLYOX in pull layer led to longer lag time (Fig. 6).
Fig. 6.

Drug release profiles of glipizide osmotic tablets, coated to 12% w/w weight gain, using various grades of POLYOX in pull and push layers (n = 6) (legend signifies the POLYOX grades used in formulation of pull–push layers)
An increase in NaCl level (osmogen) within push layer resulted in an increase in the density of the granules. The granulations containing varying levels of NaCl showed good to fair powder flow, as per the USP (20) (Carr’s compressibility index of 13.0–18.8%). Comparison of bilayer tablet cores showed that an increase in NaCl level and a subsequent decrease in POLYOX Coagulant led to lower mechanical strength of tablets, ranging from 12.6 kp (1.6 MPa) to 7.6 kp (1.2 MPa) for the bilayer tablets containing 10% and 75% w/w sodium chloride within the push-layer formulation, respectively. This may be expected as reducing the POLYOX content would lead to tablets of lower mechanical strength (2). Figure 7 shows that drug release was similar when varying the salt content in push layer in the range of 0–35% w/w (f2 ≥ 56). However, drug release was not complete and the linearity was compromised in the absence of NaCl within the osmotic tablets. The higher levels of NaCl (50 and 75% w/w) led to incomplete drug release, which was due to lower levels of POLYOX Coagulant within the push layer. The results of this investigation showed that the osmotic formulation for glipizide was the least sensitive to the push-layer NaCl levels of 10–35% w/w.
Fig. 7.
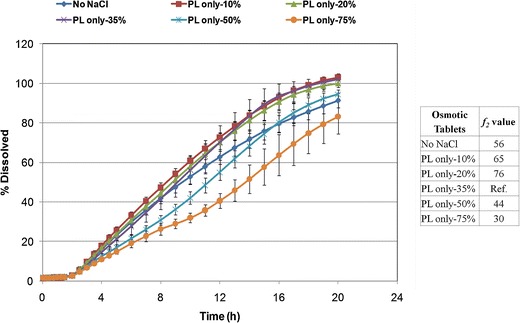
Drug release profiles of glipizide osmotic tablets, coated to 12% w/w weight gain, with varying levels of osmogen in push layer (PL) (n = 6)
Addition of NaCl to the pull-layer formulation resulted in granules with an increased Carr’s compressibility index (22.0–23.5%), indicating less powder flow as compared to the control formulation without NaCl. Tablet mechanical properties (hardness, friability, and weight variation) were similar, irrespective of NaCl presence in the pull or push layer. Inclusion of NaCl in the pull layer (Fig. 8) resulted in shorter lag time and greater drug release rate compared to the control tablets with no NaCl in pull layer (f2 < 49). The presence of NaCl and subsequently lower level of POLYOX N-80 within the pull layer may have led to quicker water ingress and overall lower viscosity of this layer. Figure 8 also shows that drug release for the osmotic tablets containing NaCl in pull layer was similar, when the salt content was varied between 11.4% and 22.8% w/w within the pull-layer formulation (f2 = 76).
Fig. 8.
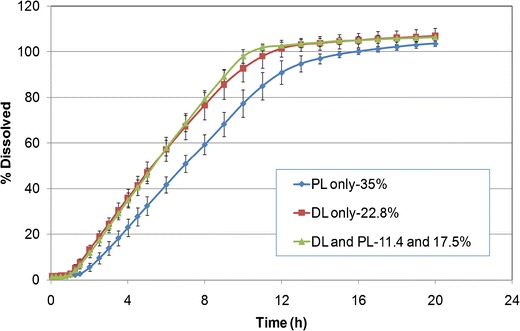
Drug release profiles of glipizide osmotic tablets, coated to 12% w/w weight gain, with constant level of osmogen at different locations (PL push layer; DL drug layer) (n = 6)
Evaluation of the granulation process showed that among different granulating liquids, the use of pure ethanol resulted in slightly higher density for the push-layer granules. Powder flow was good to fair for all granules with compressibility indices in the range of 15.5–18.9%. Use of ethanol/water at 70:30 w/w resulted in slightly larger particles, while use of pure ethanol led to generation of more fines. Mechanical properties of the bilayer cores (hardness, friability, and weight variation) were generally comparable, regardless of the composition of the granulating liquid. Use of pure ethanol resulted in slightly higher tablet hardness (∼11 kp compared to ∼9 kp). Figure 9 shows that drug release was not significantly affected when using different ethanol/water compositions in the granulation process (f2 > 81), indicating that in a laboratory scale the high shear granulation process is robust when using ethanol/water as the granulating liquid in the range of 100:0 to 70:30 w/w.
Fig. 9.
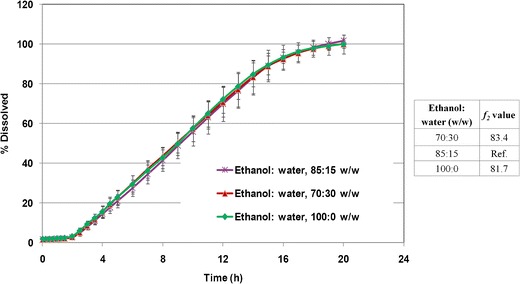
Drug release profiles of glipizide osmotic tablets, coated to 12% w/w weight gain, using different granulating liquid compositions (n = 6)
Tray drying or fluid bed drying methods resulted in comparable particle size distribution for granules with similar tablet properties (hardness, friability, and weight variation). Figure 10 shows that drug release was not significantly affected when using different drying methods in the preparation of pull- and push-layer granules (f2 = 92.8).
Fig. 10.

Drug release profiles of glipizide osmotic tablets, coated to 12% w/w weight gain, using different drying methods for granules (n = 6)
Different model drugs (glipizide, theophylline, APAP, and verapamil HCl) with varying dose and solubility were evaluated using the template PPOP formulation, described above. Characterization of pull-layer granules showed that the increase of dose (5.6–60.0% w/w of pull layer) and subsequent decrease of POLYOX N-80 (93.9–39.5% w/w of pull layer) resulted in poor granule flow as indicated by higher Carr’s compressibility indices (18.7–33.8%) which led to difficulties in tableting process. For all model drugs, irrespective of solubility, low-dose osmotic tablets (5.6–13.0% w/w of the pull layer) were successfully manufactured and resulted in typical osmotic drug release pattern (i.e., presence of lag time followed by zero-order kinetics). Figure 11 shows that using a similar core formulation (bilayer) and semipermeable membrane weight gain resulted in similar drug release profiles for glipizide, theophylline, and APAP at a low dose of 11 mg (f2 > 59). At a medium dose of 50 mg, manufacture of the osmotic tablets was challenging, and although the drug release followed the expected pattern, the release profiles were different among the model drugs (Fig. 12). At a high dose of 100 mg, in addition to compression challenges, drug release profiles deviated from a typical osmotic system which may be due to the imbalance of pull–push layers viscosities. Figures 13 and 14 show the drug release profiles for various doses of theophylline and verapamil HCl within the osmotic tablets, respectively. Release profiles from the osmotic tablets of other model drugs showed a similar pattern.
Fig. 11.
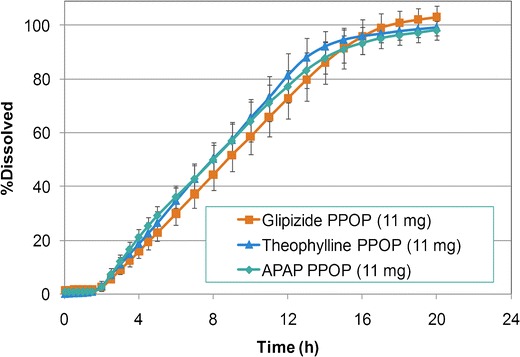
Comparative dissolution profiles for model drugs at low dose (11 mg)—semipermeable membrane: 12% w/w weight gain (n = 6)
Fig. 12.

Comparative dissolution profiles for all model drugs at medium dose (50 mg)—semipermeable membrane: 12% w/w weight gain (n = 6)
Fig. 13.
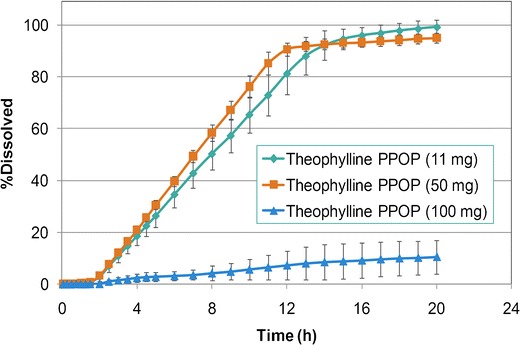
Comparative dissolution profiles for theophylline osmotic tablets—semipermeable membrane: 12% w/w weight gain (n = 6)
Fig. 14.

Comparative dissolution profiles for verapamil HCl osmotic tablets—semipermeable membrane: 8% w/w weight gain (n = 6)
CONCLUSIONS
Push–pull osmotic pump (PPOP) tablets of glipizide, as a practically insoluble model drug, were developed and evaluated. The bilayer tablet cores demonstrated good content uniformity and physical properties. The osmotic tablets showed desirable performance with respect to drug release profiles, media uptake, and push–pull pattern. The higher coating weight gain led to longer lag time and slower drug release rate. Various formulation factors and granulation processing conditions of glipizide osmotic tablets were investigated in order to identify the critical parameters in the formulation and manufacture of the osmotic tablets. The results demonstrated that using a higher viscosity grade of POLYOX within pull layer led to longer lag time, while using sodium chloride (osmogen) in formulation of pull layer resulted in shorter lag time and greater drug release rate. The push-layer formulation was not sensitive to the change in the POLYOX grade from 301 to 303 or the osmogen content at the inclusion levels of 10–35% w/w. Drug release was not complete and the linearity was compromised in the absence of salt in the push layer of the osmotic tablets. The higher levels of NaCl (50 and 75% w/w) within the push layer led to incomplete drug release which could be due to lower level of POLYOX and consequently insufficient swelling and pushing effect. Although the physical properties of the granules were slightly affected by variation of the granulation process conditions, the drug release from the osmotic tablets was not significantly affected when using granulating liquids of varying ethanol/water ratios or different drying methods (tray vs. fluid bed drying) for granules. In addition, the process challenges encountered during the high shear granulation and bilayer tablet compression were successfully addressed, resulting in satisfactory manufacture of osmotic tablets. Application of model drugs, with various dose levels and solubility, to the template PPOP formulation revealed that the standard osmotic system may be suitable for a wide range of drugs of varying solubility and doses (below 25% w/w of pull-layer formulation).
This study highlighted the robustness, and yet flexibility, of the osmotic system for various model drugs. To accommodate higher dose levels, the standard formulation, granulation step, and size of the tablets need to be modified in order to achieve zero-order release kinetics.
These investigations illustrated the robustness of the osmotic systems, which will assist in designing formulations and processes, as prescribed in ICH Q8, Pharmaceutical Development (26).
References
- 1.Tiwari SB, Rajabi-Siahboomi AR. In: Methods in molecular biology, vol. 437: drug delivery systems. Jain KK, editor. New York: Humana; 2008. pp. 217–243. [Google Scholar]
- 2.Shamblin SL. In: Oral controlled release formulation design and drug delivery: theory to practice. Wen H, Park K, editors. New York: Wiley; 2010. pp. 129–153. [Google Scholar]
- 3.Malaterre V, Ogorka L, Loggia N, et al. Oral osmotically driven systems: 30 years of development and clinical use. Eur J Pharm Biopharm. 2009;73:311–323. doi: 10.1016/j.ejpb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Malaterre V, Ogorka L, Loggia N, et al. Approach to design push–pull osmotic pumps. Int J Pharm. 2009;376(1–2):56–62. doi: 10.1016/j.ijpharm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Santus G, Baker RW. Osmotic drug delivery: a review of the patent literature. J Control Release. 1995;35:1–21. doi: 10.1016/0168-3659(95)00013-X. [DOI] [Google Scholar]
- 6.Wald R. Modern oral osmotic tablet technology. Tablets Capsules. 2009;7(6):22–27. [Google Scholar]
- 7.Qiu Y, et al. In: Developing solid oral dosage forms: pharmaceutical theory & practice. Qiu Y, Chen Y, Zhang GZ, et al., editors. Amsterdam: Elsevier; 2009. pp. 469–499. [Google Scholar]
- 8.Sathyan G, Dmochowski RR, Appell RA, et al. Effect of antacid on the pharmacokinetics of extended-release formulations of tolterodine and oxybutynin. Clin Pharmacokinet. 2004;43(14):1059–1068. doi: 10.2165/00003088-200443140-00008. [DOI] [PubMed] [Google Scholar]
- 9.Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Control Release. 2002;79(1–3):7–27. doi: 10.1016/S0168-3659(01)00550-8. [DOI] [PubMed] [Google Scholar]
- 10.Swanson DR, Barclay BL, Wong PS, et al. Nifedipine gastrointestinal therapeutic system. Am J Med. 1987;83(6B):3–9. doi: 10.1016/0002-9343(87)90629-2. [DOI] [PubMed] [Google Scholar]
- 11.Gupta BP, Thakur N, Jain NP, et al. Osmotically controlled drug delivery system with associated drugs. J Pharm Pharm Sci. 2010;13(4):571–588. doi: 10.18433/j38w25. [DOI] [PubMed] [Google Scholar]
- 12.Cortese R, Theeuwes F. Inventors; Alza Corporation, assignee. Osmotic device with hydrogel driving member. United States patent 4,327,725. 1982 May 4.
- 13.The Internet Drug Index, Available from: http://www.rxlist.com/.
- 14.Mishra B, Makesh BK, Sankar C. Oral push–pull osmotic pumps of pentazocine hydrochloride: development and evaluation. Indian J Pharm Sci. 2006;68(1):85–87. doi: 10.4103/0250-474X.22971. [DOI] [Google Scholar]
- 15.Gupta S, Singh RP, Sharma R, et al. Osmotic pumps: a review. Pharmacie Globale Int J Compr Pharm. 2011;2(6):1–8. [Google Scholar]
- 16.Thombre AG, Appel LE, Chidlaw MB, et al. Osmotic drug delivery using swellable-core technology. J Control Release. 2004;94(1):75–89. doi: 10.1016/j.jconrel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17.POLYOX water-soluble resins NF in pharmaceutical applications, The Dow Chemical Company. Available from: http://msdssearch.dow.com/ 2004.
- 18.Malaterre V, Ogorka J, Loggia N, et al. Evaluation of the tablet core factors influencing the release kinetics and the loadability of push–pull osmotic systems. Drug Dev Ind Pharm. 2009;35:433–439. doi: 10.1080/03639040802425230. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J, Dunn D, Clipse NM, et al. Formulation effects on the thermomechanical properties and permeability of free films and coating films. Pharm Technol. 2009;33(3):88–100. [Google Scholar]
- 20.USP 35—NF 30, United States Pharmacopoeial Convention, Inc. Rockville, 2012.
- 21.FDA-Recommended Dissolution Methods. Available from: www.accessdata.fda.gov, 2012.
- 22.International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use; Impurities: Guideline for residual solvents Q3C (R4), 2009.
- 23.Moore JW, Flanner HH. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm Technol. 1996;20(6):64–74. [Google Scholar]
- 24.Yang L, Venkatesh G, Fassihi R. Characterization of compressibility and compactibility of poly (ethylene oxide) polymers for modified release application by compaction simulator. J Pharm Sci. 1996;85(10):1085–1090. doi: 10.1021/js960039v. [DOI] [PubMed] [Google Scholar]
- 25.Malaterre V, Metz H, Ogorka J, et al. Benchtop-magnetic resonance imaging (BT-MRI) characterization of push–pull osmotic controlled release systems. J Control Release. 2009;133(1):31–36. doi: 10.1016/j.jconrel.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Guidance for Industry, Q8 (R2) Pharmaceutical development, International Conference on Harmonisation, 2009.
- 27.Clark’s analysis of drugs and poisons, Moffat AC, Osselton M, Widdop B, editors. London: Pharmaceutical Press, 2004.


