Abstract
Although conventional pharmaceuticals have many drug dosage forms on the market, the development of new therapeutic molecules and the low efficacy of instant release formulations for the treatment of some chronic diseases and specific conditions encourage scientists to invent different delivery systems. To this purpose, a supramolecular hydrogel consisting of the tri-block copolymer PLGA-PEG-PLGA and α-cyclodextrin was fabricated for the first time and characterised in terms of rheological, morphological, and structural properties. Naltrexone hydrochloride and vitamin B12 were loaded, and their release profiles were determined.
KEY WORDS: α-Cyclodextrin, controlled release, naltrexone hydrochloride, PLGA-PEG-PLGA, supramolecular hydrogel, vitamin B12
INTRODUCTION
Supramolecular hydrogels holds great promise as novel drug delivery systems. The potential of this technology lies in their ability to release drugs in a controlled manner; their biodegradability; their biocompatibility due to the large amount of water they absorb; their low toxicity, as they consist of safe materials such as α-cyclodextrin (α-CD) and low amounts of polymer; and their remarkable rheological properties, shear thinning and thixotropy, which makes them entirely injectable, even through a fine needle (1–3). In addition, one of the promising characteristics of these systems is their potential to substitute for conventional drug formulations in the administration of new therapeutic entities, such as oligonucleotides (4), peptides, and proteins (5,6).
Supramolecular hydrogel is usually an inclusion complex of cyclodextrins and polymer chains (7–11). Various supramolecular hydrogels have been developed and investigated as drug delivery systems. Some examples include blending linear polymers such as poly(ethylene oxide) (12); grafting copolymers composed of dextran as a backbone and poly(ethylene glycol) (PEG) as a linear side chain (13);and di-/tri-block copolymers consisting of poly caprolactone and PEG with α-CD (7,14,15).
Poly lactide glycolic acid (PLGA)-PEG-PLGA is a tri-block copolymer composed of PLGA and PEG; it was synthesised for the first time by Zentner et al. (16). In addition, we synthesized this copolymer under microwave irradiation in a short period of time (5 min), with high-quality results (17). It was degraded by hydrolysis and the rate of degradation changes from week to year depending on the variables in the formulation such as the molecular weight of copolymer and the ratio of lactide to glycolide (18).
Copolymer solutions convert abruptly to a gel when the temperature is elevated above the lower critical solution temperature, but it only occurs with the highest copolymer concentration (19–21).
We anticipated that by adding α-CD to a diluted PLGA-PEG-PLGA solution, a supramolecular hydrogel could be fabricated via threading of α-CD onto PEG and hydrophobic cross-links between PLGA blocks. Naltrexone hydrochloride, an opioid antagonist (22), and vitamin B12 were loaded into the supramolecular hydrogel.
MATERIALS AND METHODS
Materials
Poly(ethylene glycol) Mn = 6,000, D,L-lactide, and acetic acid were obtained from Merck Co., Darmstadt, Germany. Stannous octoate and glycolide and lactide were purchased from Sigma Aldrich Co., St. Louis, MO USA. α-CD was obtained from Fluka Co., Buchs, Switzerland, and acetonitrile high-performance liquid chromatography (HPLC) grade was purchased from Duksan Pure Chemicals Co., Korea. B12 (United States Pharmacopeia (USP) grade) was kindly donated by Iran Hormone Pharmaceutical Co., and naltrexone hydrochloride (USP grade) was obtained from Razak Co., Iran.
Methods
Synthesis and Purification of PLGA-PEG-PLGA
The tri-block copolymer PLGA-PEG-PLGA, with an D,L-lactide (LA)/glycolide (GA) ratio of 3:1,was prepared according to a previously reported method (17). Briefly, PEG 6,000 (60 g), LA (28.36 g), GA (7.82 g), and stannous-2-ethyl hexanoate (0.1 ml) were loaded into a flask, and the system was entirely sealed. The mixture was incubated under microwave irradiation, using a Milestone MicroSYNTH at 800 W and 150°C, while it was stirred at 250 rpm for 5 min. The obtained copolymer was purified by dissolving it in 4 ± 1°C water, precipitating it at 60 ± 10°C, and discarding the supernatant, consecutively (20). The product froze in −20°C and then dried by lyophilisation and at the end, stored at −20°C before use.
Investigation of Tri-Block Copolymer Structure
Gel permeation chromatography (GPC) was performed to determine the molecular weight and polydispersity of the copolymer, using an Agilent GPC Add-on apparatus and a RID-A refractive index signal detector coupled to PLgel® columns. A calibration curve was achieved using standard solutions of polystyrene. Tetrahydrofuran served as an eluent, at a flow rate of 1 ml/min (21).
1H nuclear magnetic resonance (1H NMR) was used to confirm the synthesis of the purposed copolymer, PLGA-PEG-PLGA, and to measure the average molecular weight number (Mn). In addition, using the obtained spectrum, LA/GA was calculated, and the suitability of the copolymer preparation method was evaluated (23). The procedure was run using a Bruker AC-80 NMR instrument and at room temperature in dimethyl sulfoxide-d6.
Preparation of Supramolecular Hydrogels and Evaluation of Gel Formation Time
First, various concentrations of PLGA-PEG-PLGA (2, 4, 8, 10, 20, and 30% w/v) and drugs (0, 0.2 and 1.0% w/v) were prepared in distilled water (solution A). Different concentrations of α-CD (4, 8, 12, 16, 20, 24, and 28% w/v) were prepared in distilled water, separately (solution B). Then, equal volume of the copolymer–drug solutions (solution A) was added into the α-CD solutions (solution B) while the system was being mixed by a magnetic stirrer with 60 rpm, until the supramolecular hydrogels developed, up to 24 h. The final concentrations of drugs, copolymer and α-CD were shown in Table I. To determine whether the supramolecular hydrogels had formed, the vials were inverted, and when the mixture did not flow, it was assumed to be the gel formation time. This test was conducted at room temperature (25°C), after which the temperature of the gel was raised to 37°C to determine whether it would retain its gel form at body temperature. Finally, the supramolecular hydrogels with the formulation of F4 and F6 (Table I) were frozen in −20°C for 24 h and then lyophilised for removing the water from the formulations. The drug was inside the formulation F4 from the beginning.
Table I.
Different Formulations of Supramolecular Hydrogels
| Drug | No. | Drug concentration % w/v | α-CD concentration % w/v | Copolymer concentration % w/v |
|---|---|---|---|---|
| Vitamin B12 | F1 | 0.1 | 12 | 5 |
| F2 | 0.5 | 12 | 5 | |
| F3 | 0.1 | 12 | 10 | |
| F4 | 0.5 | 12 | 10 | |
| F5 | 0 | 12 | 5 | |
| F6 | 0 | 12 | 10 | |
| Naltrexone hydrochloride | F7 | 0.1 | 12 | 2 |
| F8 | 0.5 | 12 | 2 | |
| F9 | 0.1 | 8 | 5 | |
| F10 | 0.5 | 8 | 5 | |
| F11 | 0 | 12 | 2 | |
| F12 | 0 | 8 | 5 |
α-CD α-cyclodextrin
Investigation of Supramolecular Hydrogel and Physical Mixture Morphology
Via scanning electron microscopy (SEM; LEO1350vp, Germany), the surface morphology of the supramolecular hydrogel (F4) and the physical mixture of the α-CD, copolymer, and vitamin B12 were studied. The images were prepared from freeze-dried samples, before and after hydrating.
Swelling Ratio Measurement
To determine the swelling ratio of the supramolecular hydrogel, 100 mg of dried hydrogel (F6) were immersed in a beaker of containing 250-mL distilled water. After removing the excess water from the hydrogel, it was weighed every 15 min until the weight did not change. The swelling ratio was then estimated by dividing the weight of the supramolecular hydrogel before hydrating by the weight after hydrating (24).
X-ray Powder Diffraction
To confirm the formation of the supramolecular hydrogel, the samples of PLGA-PEG-PLGA copolymer, α-CD, and B12 (F4), their physical mixture, the supramolecular hydrogel, and the drug-loaded supramolecular hydrogel were structurally investigated using a Philips Analytical x-Ray B.V. (USA), consisting of a PW3710 diffractometer and an x-ray tube (30 mA and 40 kV)with a copper anode. The test was conducted at an angle of 2θ and at a range of 5°–70° on 1 mg of each sample (24).
Differential Scanning Calorimetry
The arrangement of the supramolecular hydrogel was confirmed via thermal analysis of the samples, using a differential scanning calorimeter (Mettler Toledo DSC 822e, USA). To conduct the test, 5-mg samples of the copolymer, α-CD, and B12, their physical mixture, and the drug-loaded supramolecular hydrogel (F4) were inserted into the pan and warmed under nitrogen gas from 25°C to 175°C, at a rate of 20°C/min (24).
Evaluating the Rheological Behaviour of the Supramolecular Hydrogel
Flow characterisations of the supramolecular hydrogel (F4) were conducted by measuring viscosity at a wide range of shear rate at 25°C, using an R/S Plus Mo8-219 Rheometer (Brookfield, USA). By plotting logarithmic transformations of viscosity against shear rate and shear stress vs shear rate, the thixotropic behaviour of the hydrogel was evaluated. Syringeability of the formulations was determined by passing them through a 25-gauge syringe at 25°C.
In Vitro Release Study
The drug-loaded supramolecular hydrogels were prepared as shown in Table I. Briefly, various concentrations of naltrexone hydrochloride or vitamin B12 were dissolved in the PLGA-PEG-PLGA solutions, and then α-CD solution was added gradually, under vigorous stirring. Supramolecular hydrogels without drugs were prepared as blanks. All solutions were prepared in distilled water. Then, 2 ml of each formulation were loaded into a 10-ml tube, and 4 ml of phosphate buffer solution (PBS, pH = 7.4) were poured over the formulation as a release medium. The tubes were transferred to a shaker incubator water bath (Farazteb, Iran) at 37°C ± 0.1°C and shaken at 40 ± 2 rpm. At sampling times 0, 2, 4, 6, 8, and 12 h, and every 24 h thereafter, a 1-ml aliquot of release medium was withdrawn to determine the amount of drug released, and the aliquot was replaced by fresh PBS (19). The vitamin B12 samples were analysed with a UV-160A Shimadzu (Germany) UV–vis spectrophotometer at 361 nm (25). The naltrexone hydrochloride samples were assayed using the reversed-phase HPLC method, with a Young Lin (South Korea) Acme 9000 system consisting of an ODS C18 (4.6 × 250 mm, 5 μm) column, a CTS30 column oven, an SP930D solvent delivery module, an SDV50A solvent mixing vacuum degasser, and a UV730 dual wavelength UV/VIS detector; the analysis was performed using Autochro-3000 software. The column temperature was fixed at 50°C. The mobile phase, composed of 14% and 0.5% (v/v) acetonitrile and acetic acid, respectively, in deionised water, was conducted at a flow rate of 0.8 ml/min by the isocratic method; 20 μl of each sample were injected and evaluated at 280 nm.
Statistics
Each test was repeated at least three times. The results were reported as mean ± SD and analysed by one-way ANOVA, with a significance level of p < 0.05.
Comparisons of the drug release profiles of the different formulations were accomplished by calculating the difference factor (f' 1) and similarity factor (f 2); f 2 value 50–100 and f'1 value 0–15 were assumed to indicate a similarity between two profiles(26).
RESULTS
Tri-Block Copolymer Characterisations
PLGA-PEG-PLGA was synthesised using a microwave-assisted method with a yield of approximately 91%.
The 1H NMR copolymer spectrum, indicating the accurate preparation of PLGA-PEG-PLGA, is shown in Fig. 1. The signals that appeared at 4.4 (a) and 4 (c) ppm are attributed to the -CH of LA and -CH2 of GA, respectively. Two-CH2 of PEG appeared at 3.6 (e) and 2.5 (d) ppm. The signals at 1.7 (f) and 0.6 (b) are related to the -OH and -CH3 of LA. The obtained integrations of signals pertaining to each monomer were used to estimate the Mn(NMR) and LA/GA ratio of the copolymer, which are listed in Table II.
Fig. 1.
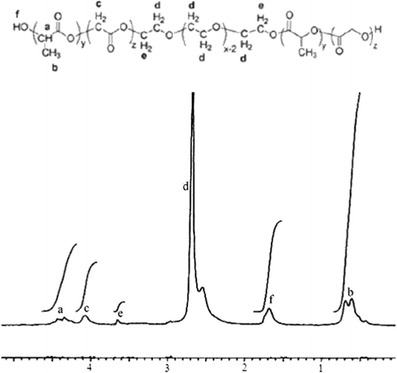
The 1H NMR spectrum of PLGA-PEG-PLGA
Table II.
Characterization of PLGA-PEG-PLGA by 1H NMR and GPC
| 1H NMR | GPC | |||
|---|---|---|---|---|
| Mn(NMR) | LA/GA | Mn(GPC) | Mw | Mw/Mn(GPC) |
| 9,127 | 3.7 | 9,055 | 5,421 | 0.59 |
LA D,L-lactide, GA glycolide, 1 H NMR 1H nuclear magnetic resonance, GPC gel permeation chromatography, Mn (NMR) number average molecular weight of copolymer determined by 1H NMR, Mn (GPC) number average molecular weight of copolymer measured by GPC, Mw/Mn (GPC) indicates the polydispersity of copolymer
A nearly symmetric peak in the GPC chromatogram (Fig. 2) indicates the low polydispersity of the copolymer. Mw, Mn(GPC), and polydispersity (Mw/Mn(GPC)) are shown in Table II.
Fig. 2.
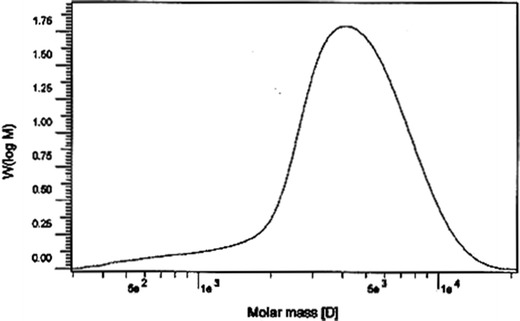
The GPC chromatogram of PLGA-PEG-PLGA showing the unimodal peak that confirms the low polydispersity of synthesized copolymer
Supramolecular Hydrogel Formation
The effects of the copolymer and α-CD concentrations on the gelling behaviour of the formulations were investigated, and it was found that at least 12% w/v of α-CD and 2% w/v of copolymer were needed to obtain gel shapes. Table III shows the formulations that turned into the gel and the length of time they took to shape at 25°C. All the formulations remained in gel form at 37°C. The formulations that were able to turn into a gel over a short period of time were selected for the subsequent experiments.
Table III.
Gelling Time of Different Formulations
| α-CD concentration % w/v | Copolymer concentration % w/v | Hydrogel at 25°C | Gelling time (min) |
|---|---|---|---|
| 12 | 1 | No | – |
| 8 | 2 | No | – |
| 10 | 2 | No | – |
| 12 | 2 | Yes | 18 |
| 6 | 5 | Yes | 1,440 |
| 8 | 5 | Yes | 720 |
| 10 | 5 | Yes | 17 |
| 6 | 10 | Yes | 1,200 |
| 12 | 10 | Yes | 4 |
| 14 | 10 | Yes | 0.5 |
| 4 | 15 | No | – |
| 12 | 15 | Yes | 0.166 |
| 14 | 15 | Yes | 0.083 |
| 6 | 20 | No | – |
No didn’t convert to the hydrogel, Yes converted to the hydrogel
Swelling Ratio Measurement
Since supramolecular hydrogels possess a porous structure, they are able to absorb high amounts of liquid. As shown in Fig. 3, the weight of the supramolecular hydrogel (F6, see Table I) increased 2.5 times in the presence of water after about 10 min.
Fig. 3.
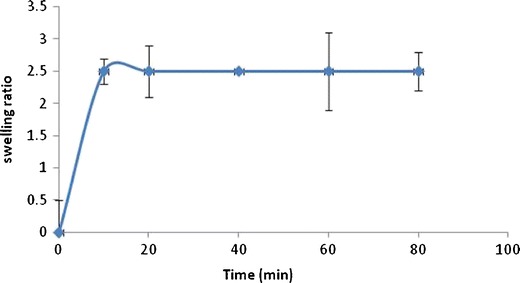
Swelling ratio of supramolecular hydrogel immersed in water, after 10 min, weight of supramolecular hydrogel elevated 2.5 times
SEM Imaging
The morphology of the freeze-dried supramolecular hydrogel (F4) before and after hydrating, compared with the physical mixture of components, was investigated by SEM. Figure 4 shows the structures of the non-hydrated supramolecular hydrogel and the physical mixture. The supramolecular hydrogel exhibited a porous structure despite the physical mixture. As shown in Fig. 5, the supramolecular hydrogel was observed to be more porous, as evidenced by the swelling. Although the physical mixture appeared somewhat porous after hydrating, it was negligible compared with the hydrated supramolecular hydrogel.
Fig. 4.

SEM: non-hydrated physical mixture (a ×5,000 and c ×2,000) and supramolecular hydrogel F4 (b ×2,000 and d ×2,000)
Fig. 5.

SEM: hydrated physical mixture (a ×5,000 and c ×10,000) and supramolecular hydrogel F4 (b ×5,000 and d ×10,000)
XRPD
The x-ray powder patterns of the pure components, their physical mixture, and the supramolecular hydrogel formulations (F4 and F6) are shown in Fig. 6. The signal at d = 4.46Ao, 2θ = 19.8 confirmed α-CD threading onto copolymer blocks in the supramolecular hydrogel (24).
Fig. 6.
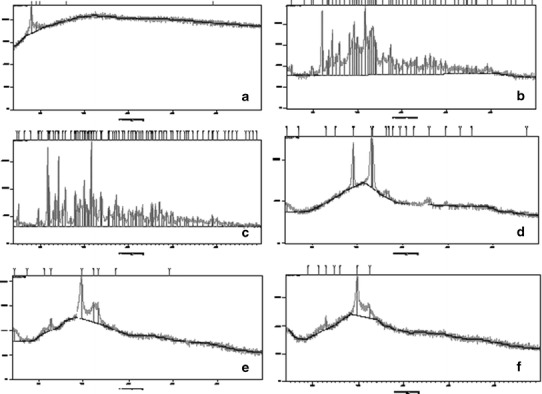
The x-ray powder patterns of pure vitamin B12 (a), α-CD (c), and PLGA-PEG-PLGA (e); the physical mixture (vitamin B12, α-CD and PLGA-PEG-PLGA) (b); supramolecular hydrogel formulations, F4 (d) and F6 (f)
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) thermograms of the pure components, their physical mixture, and the supramolecular hydrogel (F6) are shown in Fig. 7. The endothermic peaks that were observed in the pure component chromatograms disappeared entirely in the supramolecular hydrogel formulations. However, in the physical mixture, a peak at about 50°C, attributed to the copolymer, was detected.
Fig. 7.

The DSC thermograms of vitamin B12 (a), α-CD (b), copolymer (c), the physical mixture (vitamin B12, α-CD and PLGA-PEG-PLGA) (d), and the supermolecular hydrogel F6 (e)
Rheological Properties of Supramolecular Hydrogels
Shear stress and logarithmic transformations of viscosity against shear rate were plotted to evaluate the rheological behaviour of F6 and the formulation containing 12% w/v of copolymer and 8% w/v of α-CD (Fig. 8 and 9). The formulations passed easily through a 25-gauge needle and were injectable.
Fig. 8.

Plot of shear stress vs shear rate showing thixotropic behaviour of F6 (a) and supramolecular hydrogel composed of 12% w/v of copolymer and 8% w/v of α-CD (b)
Fig. 9.

Plot of apparent viscosity (log scale) vs rate of shear for F6 and supramolecular hydrogel composed of 12% w/v of copolymer and 8% w/v of α-CD showing pseudoplastic behaviour of hydrogels
In Vitro Release Study
The release profiles of the different formulations, which are presented in Table I, are shown in Figs. 10 and 11. As can be observed in the B12 release profile, about 20–35% of the loaded drug released after 700 h, depending on the concentrations of the copolymer and the drug. The release rate of naltrexone hydrochloride was more than that of B12, and 85–99% of the drug released in approximately 500 h. The amount of burst release was less than 5% in the B12 profile and 5–16% for naltrexone hydrochloride.
Fig. 10.

Release profiles of supramolecular hydrogel loaded by vitamin B12 (F1, F2, F3, and F4)
Fig. 11.

Release profiles of supramolecular hydrogel loaded by naltrexone hydrochloride (F7, F8, F9, and F10)
F′1 and f2of comparisons between every two formulations are shown in Table IV. Because the f 2 value of 50–100 and thef′1 value of 0–15 indicate a similarity between the two profiles (26), no significant differences between the drug release rates of the various formulations were found in the naltrexone release profiles, except F7 vs F8, which elucidates the effects of drug concentration on the rate of release in these formulations. However, in the case of B12, it seems that the release profile is absolutely affected by formulation variables, such as copolymer and drug concentrations.
Table IV.
Comparisons between Release Profile of Different Formulations
| Comparisons | f′1 | f 2 |
|---|---|---|
| F1 vs F2 | 33.43 | 21.3 |
| F1 vs F3 | 42.5 | 25.2 |
| F1 vs F4 | 42.89 | 17.9 |
| F2 vs F3 | 28.1 | 40.8 |
| F2 vs F4 | 26.3 | 43.2 |
| F3 vs F4 | 20 | 44.3 |
| F7 vs F8 | 23.4 | 43.5 |
| F7 vs F9 | 9.5 | 79.34 |
| F7 vs F10 | 12.3 | 80.4 |
| F8 vs F9 | 14.2 | 57.2 |
| F8 vs F10 | 6.5 | 83.4 |
| F9 vs F10 | 5.3 | 86.8 |
f′ 1 difference factor, f 2 similarity factor
DISCUSSION
Tri-Block Copolymer Preparation and Characterisations
The tri-block copolymer PLGA-PEG-PLGA was prepared via ring-opening polymerisation (16), using microwave irradiation. In spite of the conventional method, microwave-assisted synthesis of materials is less time consuming and leads to products that have a higher yield and fewer impurities (27). The obtained PLGA-PEG-PLGA was perfectly purified by only one purification procedure. The low polydispersity of the copolymer and the unimodal GPC trace indicated that purification was sufficient and that the copolymer was ready for the physical properties to be studied (21).
The 1H NMR spectrum was similar to the previously reported one (28), and it confirmed the synthesis of the copolymer. Because the LA/GA of the copolymer was very close to the intended ratio, and the Mn was almost the same as the theoretical Mn = 9,618, based on the amount of initial materials added to the reaction, we concluded that the copolymer was efficiently polymerised. According to the results of a PLGA-PEG-PLGA stability study conducted by Zentner et al. (16), to avoid the quick hydrolysis of the copolymer in the presence of water, it was lyophilised and stored in a refrigerator. It was more crucial that our copolymer possess more hydrophilicity, due to the higher Mn of the PEG block (PEG 6000) and low LA/GA ratio. In a study performed by Yu et al., PLGA-PEG-PLGA with a lower LA/GA ratio, 3:1, was hydrolysed more quickly than a less hydrophilic copolymer, LA/GA 5:1 and 10:1 (29).
Supramolecular Hydrogel Preparation and Characterisations
Supramolecular hydrogel is a necklace-like inclusion complex assembled by threading the α-CD onto the PEG blocks and forming a hydrophobic crosslink between hydrophobic blocks (30). In this study, different concentrations of α-CD and PLGA-PEG-PLGA were examined to find the appropriate formulations that turn into gel in a short time and retain their structure at body temperature. The results demonstrated that increasing the concentration of each component caused the hydrogel to take shape more rapidly, due to the greater level of interactions between PLGA blocks and increased threading of α-CD onto PEG blocks (14). The influence of α-CD concentration on reducing the time of gel formation was greater than that of the copolymer. By incorporating long PEG blocks into the α-CD cavity, the probability of interaction between the PLGA blocks increased due to the reduction of copolymer hydrophilicity. Unless this phenomenon occurs, the perfect hydrophilic–lipophilic balance, which is a critical parameter for gelling, will not be provided (1,10,12).
To conduct the DSC, x-ray powder diffraction (XRPD), and SEM analysis, the supramolecular hydrogel was dried, using lyophilisation. The powder formulation, instead of a solution or gel, not only increases the stability, but it also becomes more applicable and portable as a drug delivery system (31).
One of the exclusive properties of supramolecular hydrogels is their high capacity for water absorption and swelling in the presence of liquids, which contributes to the biocompatibility of the system (1), due to its extensive porosity and hydrophilicity. While the liquid is entering the hydrophilic sites of system, the hydrophobic bonds strengthen the structure. After drying, as shown in Fig. 2, the hydrogel still keep its porous structure, a characteristic that was not observed in the physical mixture. Noticeably, few holes appeared in the structure of the physical mixture after hydrating, presumably because of the dissolving of components in water.
The disappearance of the component’s endothermic peaks in the DSC chromatogram confirmed the embedding of PEG blocks in the α-CD cavities and the formation of the supramolecular hydrogel (24).
Formation of the supramolecular hydrogel was also verified using XRPD, by obtaining a sharp signal at d = 4.46Ao, 2θ = 19.8, which is the identity of a necklace-like structure. As most of the physical mixture consisted of α-CD (12% w/v), the achieved XRPD pattern was very similar to the α-CD pattern (24). Loading B12 into the formulation did not have a noticeable effect on the patterns of the physical mixture and the supramolecular hydrogel.
Supramolecular hydrogels are pseudoplastic and thixotropic materials (1). These shear thinning materials lose their high viscosity as the shear rate increases, and they regain it when the shear rate decreases. By applying the shear rate, the physical cross-links between the copolymer temporarily break, water moves out of the structure, and consequently, the fluidity of the formulation increases (7,14), thereby facilitating the injectability of the formulations. The supramolecular hydrogel prepared in this study exhibited such rheological behaviour, with a hysteresis loop. The viscosity of both examined formulations decreased and they turned into sol when the shear rate increased. When the shear rate decreased, they became semi-solid again within 1–2 min.
Previous studies have shown that in spite of being a copolymer, α-CD concentration does not have a noticeable effect on viscosity as we found with the supramolecular hydrogel in the current study (14). Besides component concentrations, the polymer structure, such as Mw and length of each block, might have a critical role in the rheological properties and viscosity (1).
In Vitro Release
The graphs ( Fig. 10 and 11) demonstrate a controlled release of drugs at various rates, depending on the formulation’s composition.
Release of B12 from the supramolecular hydrogel occurred slowly, with a slight burst release. B12 is a large molecule, so it seems that in addition to diffusion, polymer degradation is the main mechanism of release, particularly for molecules trapped in the inner part of the supramolecular hydrogel network (17). As we observed in this study, the rate of supramolecular hydrogel erosion, which started after about 20 days, was F4 ≪ F3 < F2 ≪ F1. By increasing the concentrations of the B12 and the copolymer, the rate of release decreases significantly (p < 0.05), perhaps because of the higher viscosity of the concentrated supramolecular hydrogel and the lower water activity in it. The higher amount of copolymer is the higher amount of PLGA blocks, which, besides causing more hydrophobic interactions, reduces the hydrophilicity of systems, and consequently, hydrolysis and degradation of the systems occur more slowly (19,29). It has previously been reported that the presence of hydrophilic molecules and water-soluble salts by a salting out mechanism can reduce water activity and the polymer degradation rate (32). In addition, higher viscosity is in opposition to diffusion, which is possibly the main release mechanism for drug molecules loaded onto the surface of a supramolecular hydrogel (33). It was observed that the red color of the loaded B12 gradually disappeared due to diffusion of the drug molecules and probably degradation of the supermolecular hydrogel structure. The rate of diffusion was increased by the increased degradation of the system, due to the higher porosity levels. Table V indicates the kinetic profiles of B12 that confirmed our hypothesis regarding the release mechanism. Considering the R2 associated with Higuchi and zero order models, both diffusion and degradation played important roles in the B12 release profiles (34).
Table V.
Kinetic Profiles of Different Formulations
| Formulation No. | Zero order | Higuchi | ||
|---|---|---|---|---|
| Slope | R 2 | Slope | R 2 | |
| F1 | 0.056 | 0.932 | 1.607 | 0.983 |
| F2 | 0.032 | 0.814 | 0.981 | 0.951 |
| F3 | 0.041 | 0.883 | 1.222 | 0.979 |
| F4 | 0.027 | 0.929 | 0.788 | 0.981 |
| F7 | 0.217 | 0.881 | 4.793 | 0.99 |
| F8 | 0.191 | 0.831 | 4.329 | 0.982 |
| F9 | 0.235 | 0.854 | 5.219 | 0.971 |
| F10 | 0.162 | 0.806 | 3.688 | 0.967 |
The Higuchi model was well fitted to obtain the data of the release of hydrochloride salt of naltrexone, which is a water-soluble salt with a small hydrophobic molecule. Reported slopes related to the rate of release indicated the rapid diffusion of drug molecules in about 20 days, which is before the time that visible erosion or degradation occurred in the formulations. According to the calculated f′1 and f 2 factors, no significant differences were found between formulations except for F7 and F8, which both contained 12% w/v of the α-CD, 2% w/v of the copolymer, and 0.1 and 0.5% w/v of the drug, respectively. Because diffusion is the main release mechanism for naltrexone hydrochloride, increasing drug concentration was expected to increase the rate of release, but that did not occur in this study, which may have been the result of higher viscosity in formulations with higher drug concentration. These results are consistent with our previously reported results regarding the effects of increasing drug concentration (naltrexone hydrochloride and B12) on the rate of release from PLGA-PEG-PLGA hydrogels (17,20).
It is worth noting that increasing and decreasing α-CD concentrations, along with decreasing and increasing copolymer concentration, respectively, had a negligible effect on the release profile, and that the formulations may possess almost the same characteristic parameters, which have an effect on diffusion rate.
CONCLUSION
The microwave-assisted method provides an accelerated synthesis of PLGA-PEG-PLGA with a high yield of reaction and low level of impurities. It is possible to prepare a supramolecular hydrogel in a short amount of time, with a low quantity of copolymer, by increasing the α-CD concentration. The supramolecular hydrogels demonstrated shear thinning and thixotropic behaviour and regained their networks less than 2 min after passing through a fine needle. Due to the porous construction of supramolecular hydrogels, they swell in the presence of fluids and absorb large quantities of water, thereby making the system comprehensively biocompatible. Results of the present study indicate that a supramolecular hydrogel composed of α-CD and PLGA-PEG-PLGA is an appropriate drug delivery system that releases drug molecules in a sustained manner.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by Mashhad University of Medical Sciences for this study. The results described in this paper were part of a Pharm D thesis of Haydar Esmaeel.
Contributor Information
Farzin Hadizadeh, Email: hadizadehf@mums.ac.ir.
Sayyed A. Sajadi Tabassi, Email: sajadia@mums.ac.ir.
References
- 1.Li J, Loh XJ. Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery. Adv Drug Deliv Rev. 2008;60(9):1000–1017. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev. 2013;65(1):104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv Drug Deliv Rev. 1999;36(1):125–141. doi: 10.1016/S0169-409X(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 4.Ma D, Zhang H-B, Chen D-H, Zhang L-M. Novel supramolecular gelation route to in situ entrapment and sustained delivery of plasmid DNA. J Colloid Interface Sci. 2011;364(2):566–573. doi: 10.1016/j.jcis.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 5.Ma D, Zhang L-M, Xie X, Liu T, Xie M-Q. Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme. J Colloid Interface Sci. 2011;359(2):399–406. doi: 10.1016/j.jcis.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T, Hirayama F, Misumi S, Arima H, Uekama K. Design and evaluation of polypseudorotaxanes of pegylated insulin with cyclodextrins as sustained release system. Biomaterials. 2008;29(28):3866–3871. doi: 10.1016/j.biomaterials.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Zhao SP, Feng ZG, Piao DX. Synthesis and characterization of photopolymerized supramolecular-structured hydrogels of alpha-CD assemblies. Chem J Chin Univ-Chin. 2003;24(1):186–188. [Google Scholar]
- 8.Zhao S, Lee J, Xu W. Supramolecular hydrogels formed from biodegradable ternary COS-g-PCL-b-MPEG copolymer with α-cyclodextrin and their drug release. Carbohydr Res. 2009;344(16):2201–2208. doi: 10.1016/j.carres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Simões SMN, Veiga F, Torres-Labandeira JJ, Ribeiro ACF, Sandez-Macho MI, Concheiro A, et al. Syringeable pluronic–α-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur J Pharm Biopharm. 2012;80(1):103–112. doi: 10.1016/j.ejpb.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27(22):4132–4140. doi: 10.1016/j.biomaterials.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Huh KM, Cho YW, Chung H, Kwon IC, Jeong SY, Ooya T, et al. Supramolecular hydrogel formation based on inclusion complexation between poly(ethylene glycol)-modified chitosan and α-cyclodextrin. Macromol Biosci. 2004;4(2):92–99. doi: 10.1002/mabi.200300037. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ni X, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and α-cyclodextrin. J Biomed Mater Res Part A. 2003;65A(2):196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 13.Huh KM, Ooya T, Lee WK, Sasaki S, Kwon IC, Jeong SY, et al. Supramolecular-structured hydrogels showing a reversible phase transition by inclusion complexation between poly (ethylene glycol) grafted dextran and α-cyclodextrin. Macromolecules. 2001;34(25):8657–8662. doi: 10.1021/ma0106649. [DOI] [Google Scholar]
- 14.Zhao S-P, Zhang L-M, Ma D. Supramolecular hydrogels induced rapidly by inclusion complexation of Poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) block copolymers with alpha-cyclodextrin in aqueous solutions. Journal of Physical Chemistry B. 2006;110(25):12225–12229. doi: 10.1021/jp057506u. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Li J. Supramolecular hydrogels based on inclusion complexation between poly(ethylene oxide)-b-poly (ε-caprolactone) diblock copolymer and α-cyclodextrin and their controlled release property. J Biomed Mater Res Part A. 2008;86A(4):1055–1061. doi: 10.1002/jbm.a.31710. [DOI] [PubMed] [Google Scholar]
- 16.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72(1–3):203–215. doi: 10.1016/S0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 17.Khodaverdi E, Tekie FSM, Mohajeri SA, Ganji F, Zohuri G, Hadizadeh F. Preparation and investigation of sustained drug delivery systems using an injectable, thermosensitive, in situ forming hydrogel composed of PLGA–PEG–PLGA. AAPS PharmSciTech. 2012;13(2):590–600. doi: 10.1208/s12249-012-9781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt C, Mäder K, Kissel T. The degradation, swelling and erosion properties of biodegradable implants prepared by extrusion or compression moulding of poly(lactide-co-glycolide) and ABA triblock copolymers. Biomaterials. 2000;21(9):931–938. doi: 10.1016/S0142-9612(99)00262-8. [DOI] [PubMed] [Google Scholar]
- 19.Qiao MX, Chen DW, Ma XC, Liu YJ. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1–2):103–112. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Khodaverdi E, Hadizadeh F, Tekie FSM, Jalali A, Mohajeri SA, Ganji F. Preparation and analysis of a sustained drug delivery system by PLGA–PEG–PLGA triblock copolymers. Polym Bull. 2012;69(4):429–438. doi: 10.1007/s00289-012-0747-5. [DOI] [Google Scholar]
- 21.Khodaverdi E, Akbari A, Tekie FS, Mohajeri SA, Zohuri G, Hadizadeh F. Sustained delivery of amphotericin B and vancomycin hydrochloride by an injectable thermogelling tri-block copolymer. PDA J Pharm Sci Technol. 2013;67(2):135–45. [DOI] [PubMed]
- 22.Crabtree B. Review of naltrexone, a long-acting opiate antagonist. Clin Pharm. 1984;3(3):273. [PubMed] [Google Scholar]
- 23.Jeong B, Bae YH, Kim SW. Biodegradable thermosensitive micelles of PEG-PLGA-PEG triblock copolymers. Colloids Surf B-Biointerfaces. 1999;16(1–4):185–193. doi: 10.1016/S0927-7765(99)00069-7. [DOI] [Google Scholar]
- 24.Abu Hashim II, Higashi T, Anno T, Motoyama K, Abd-ElGawad AEGH, El-Shabouri MH, et al. Potential use of γ-cyclodextrin polypseudorotaxane hydrogels as an injectable sustained release system for insulin. Int J Pharm. 2010;392(1):83–91. doi: 10.1016/j.ijpharm.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Khodaverdi E, Rajabi O, Farhadi F, Jalali A, Tekie FSM. Preparation and investigation of (N-isopropylacrylamide-acrylamide) membranes in temperature responsive drug delivery. Iran J Basic Med Sci. 2009;13:1–8. [Google Scholar]
- 26.Costa P, Manuel J, Lobo S. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 27.Sosnik A, Gotelli G, Abraham GA. Microwave-assisted polymer synthesis (MAPS) as a tool in biomaterials science: how new and how powerful. Prog Polym Sci. 2011;36(8):1050–1078. doi: 10.1016/j.progpolymsci.2010.12.001. [DOI] [Google Scholar]
- 28.Chen S, Pieper R, Webster DC, Singh J. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288(2):207–218. doi: 10.1016/j.ijpharm.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Chang GT, Zhang H, Ding JD. Injectable block copolymer hydrogels for sustained release of a PEGylated drug. Int J Pharm. 2008;348(1–2):95–106. doi: 10.1016/j.ijpharm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 30.He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127(3):189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Kasper JC, Winter G, Friess W. Recent advances and further challenges in lyophilization. Eur J Pharm Biopharm. 2013;85(2):162–169. doi: 10.1016/j.ejpb.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Khodaverdi E, Golmohammadian A, Mohajeri SA, Zohuri G, Mirzazadeh Tekie FS, Hadizadeh F. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive Poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) hydrogel. ISRN Pharm. 2012;976879. [DOI] [PMC free article] [PubMed]
- 33.Peppas NA. Mathematical modelling of diffusion processes in drug delivery polymeric systems. Control Drug Bioavailab. 1984;1:203–237. [Google Scholar]
- 34.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50(10):874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]


