Abstract
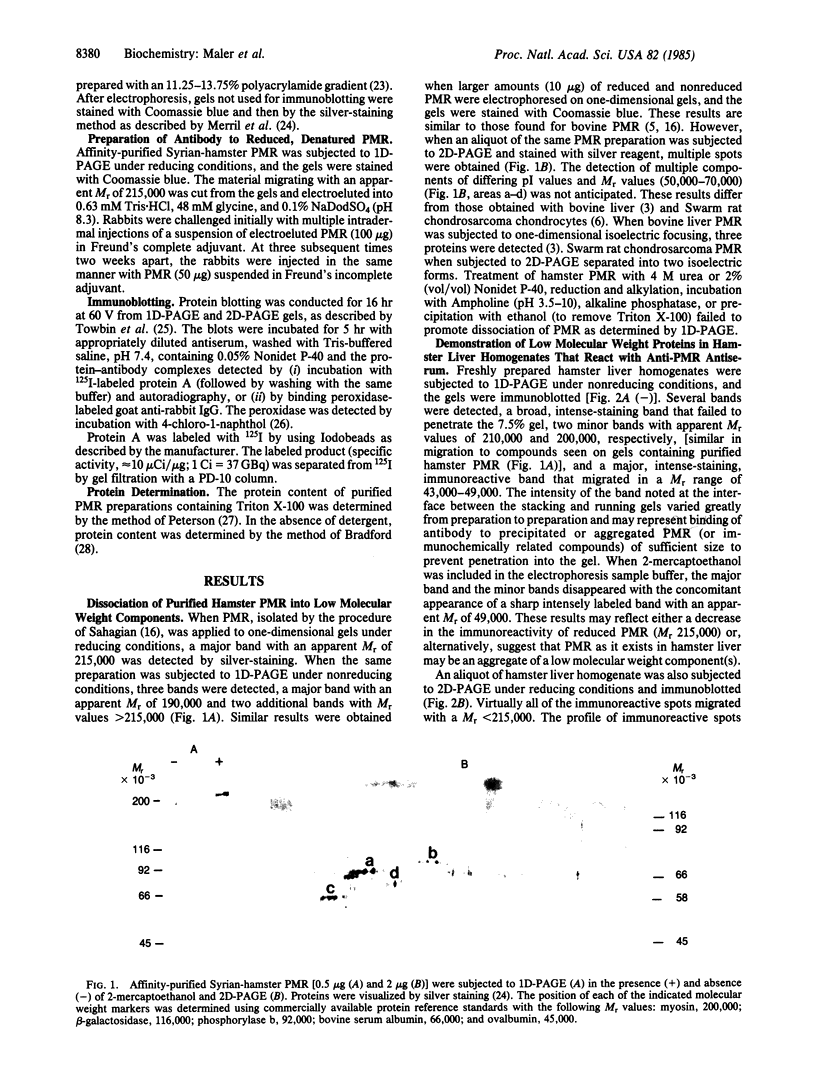
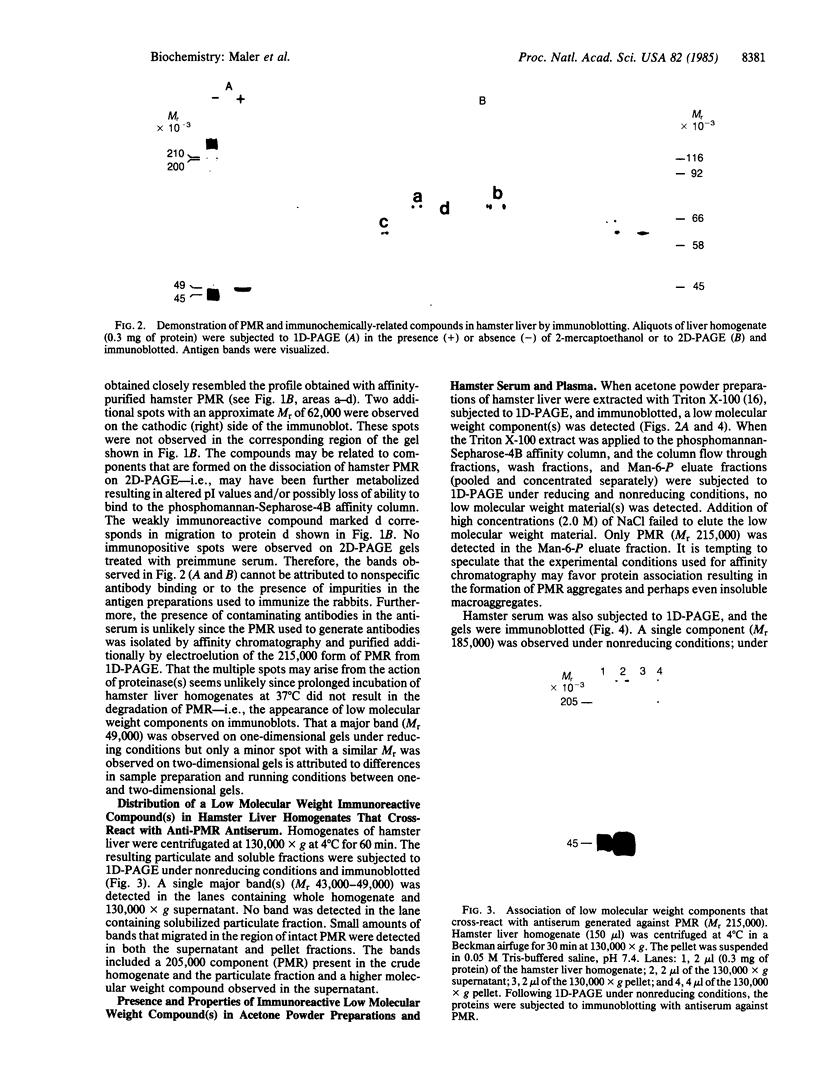
Phosphomannosyl receptor (PMR) isolated from Syrian hamster liver was purified to apparent homogeneity by affinity chromatography and one-dimensional PAGE. On one-dimensional PAGE, the receptor migrated with a Mr approximately equal to 215,000 as detected by a silver-staining reagent or by immunoblotting [utilizing antiPMR generated against purified hamster PMR (Mr 215,000) sequentially purified by affinity chromatography and NaDodSO4/PAGE]. On two-dimensional PAGE the receptor was partially dissociated into low-molecular weight components. The protein distribution on immunoblots of two-dimensional gels of hamster liver homogenates was nearly identical to that observed for purified hamster liver PMR. When liver homogenates were subjected to one-dimensional PAGE and immunoblotted under nonreducing conditions, an intensely labeled band that migrated with an apparent Mr of 43,000-49,000 was observed; under reducing conditions a single band with a Mr of 49,000 was observed. The low molecular weight compound was present in the soluble 130,000 X g supernatant but not in the particulate fraction of liver homogenates. An immunoreactive component of similar molecular weight was also present in hamster serum and plasma. These results suggest that PMR is either an aggregate comprised of small molecular weight components or, alternatively, that a number of small molecular weight components are tightly associated with PMR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares K., Balasubramanian A. S. A binding protein for lysosomal enzymes isolated from brain by phosphomannan-sepharose chromatography. Biochem Biophys Res Commun. 1983 Apr 29;112(2):398–406. doi: 10.1016/0006-291x(83)91477-8. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell C. H., Fine R. E., Squicciarini J., Rome L. H. Coated vesicles from rat liver and calf brain contain cryptic mannose 6-phosphate receptors. J Biol Chem. 1983 Feb 25;258(4):2628–2633. [PubMed] [Google Scholar]
- Campbell C. H., Rome L. H. Coated vesicles from rat liver and calf brain contain lysosomal enzymes bound to mannose 6-phosphate receptors. J Biol Chem. 1983 Nov 10;258(21):13347–13352. [PubMed] [Google Scholar]
- Creek K. E., Sly W. S. Biosynthesis and turnover of the phosphomannosyl receptor in human fibroblasts. Biochem J. 1983 Aug 15;214(2):353–360. doi: 10.1042/bj2140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell. 1982 Nov;31(1):8–10. doi: 10.1016/0092-8674(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Gabel C. A., Kornfeld S. Studies of the biosynthesis of the mannose 6-phosphate receptor in receptor-positive and -deficient cell lines. J Cell Biol. 1983 Dec;97(6):1700–1706. doi: 10.1083/jcb.97.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J., Lowe M., Tsunoo H., Ashwell G. Immunological approaches to the study of membrane receptors. A monoclonal antibody that inhibits the binding of asialoglycoproteins to the rat liver receptor. J Biol Chem. 1982 Nov 10;257(21):12685–12690. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mitchell D. C., Maler T., Jourdian G. W. Detergent dissociation of bovine liver phosphomannosyl binding protein. J Cell Biochem. 1984;24(4):319–330. doi: 10.1002/jcb.240240403. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Saxe J. M., Menge A. C. Inhibition of fertility in rabbits by monoclonal antibodies against sperm. Biol Reprod. 1983 Feb;28(1):249–254. doi: 10.1095/biolreprod28.1.249. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum B. B., Hanash S. M., Yew N., Neel J. V. Two-dimensional electrophoretic analysis of erythrocyte membranes. Clin Chem. 1982 Apr;28(4 Pt 2):925–931. [PubMed] [Google Scholar]
- Sahagian G. G., Distler J. J., Jourdian G. W. Membrane receptor for phosphomannosyl residues. Methods Enzymol. 1982;83:392–396. doi: 10.1016/0076-6879(82)83036-x. [DOI] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Neufeld E. F. Biosynthesis and turnover of the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. J Biol Chem. 1983 Jun 10;258(11):7121–7128. [PubMed] [Google Scholar]
- Sahagian G. G. The mannose 6-phosphate receptor: function, biosynthesis and translocation. Biol Cell. 1984;51(2):207–214. doi: 10.1111/j.1768-322x.1984.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Freeze H. H., Miller A. L., Stahl P. D. Identification of mannose 6-phosphate receptors in rabbit alveolar macrophages. J Biol Chem. 1984 Feb 25;259(4):2257–2261. [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Steiner A. W., Rome L. H. Assay and purification of a solubilized membrane receptor that binds the lysosomal enzyme alpha-L-iduronidase. Arch Biochem Biophys. 1982 Apr 1;214(2):681–687. doi: 10.1016/0003-9861(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K., Basner R., Gieselmann V., Von Figura K. Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413–419. doi: 10.1042/bj1800413. [DOI] [PMC free article] [PubMed] [Google Scholar]