Abstract
Growth factors, such as myostatin (Mstn), play an important role in regulating post-natal myogenesis. In fact, loss of Mstn has been shown to result in increased post-natal muscle growth through enhanced satellite cell functionality; while elevated levels of Mstn result in dramatic skeletal muscle wasting through a mechanism involving reduced protein synthesis and increased ubiquitin-mediated protein degradation. Here we show that miR-27a/b plays an important role in feed back auto-regulation of Mstn and thus regulation of post-natal myogenesis. Sequence analysis of Mstn 3′ UTR showed a single highly conserved miR-27a/b binding site and increased expression of miR-27a/b was correlated with decreased expression of Mstn and vice versa both in vitro and in mice in vivo. Moreover, we also show that Mstn gene expression was regulated by miR-27a/b. Treatment with miR-27a/b-specific AntagomiRs resulted in increased Mstn expression, reduced myoblast proliferation, impaired satellite cell activation and induction of skeletal muscle atrophy that was rescued upon either blockade of, or complete absence of, Mstn. Consistent with this, miR-27a over expression resulted in reduced Mstn expression, skeletal muscle hypertrophy and an increase in the number of activated satellite cells, all features consistent with impaired Mstn function. Loss of Smad3 was associated with increased levels of Mstn, concomitant with decreased miR-27a/b expression, which is consistent with impaired satellite cell function and muscular atrophy previously reported in Smad3-null mice. Interestingly, treatment with Mstn resulted in increased miR-27a/b expression, which was shown to be dependent on the activity of Smad3. These data highlight a novel auto-regulatory mechanism in which Mstn, via Smad3 signaling, regulates miR-27a/b and in turn its own expression. In support, Mstn-mediated inhibition of Mstn 3′ UTR reporter activity was reversed upon miR-27a/b-specific AntagomiR transfection. Therefore, miR-27a/b, through negatively regulating Mstn, plays a role in promoting satellite cell activation, myoblast proliferation and preventing muscle wasting.
Introduction
Myostatin (Mstn) is a secreted growth factor that belongs to the TGF-β super-family. Mstn is produced predominantly in skeletal muscle with lower levels of expression observed in white adipose tissue [1], heart [2] and mammary gland [3]. Analysis has revealed that Mstn is a profound negative regulator of muscle growth; while inactivation or mutation of the Mstn gene leads to increased skeletal muscle mass [1], [4], enhanced myoblast proliferation [5], differentiation [6] and improved skeletal muscle regeneration [7], increased levels of Mstn result in severe cachectic-like muscle wasting [8]–[11].
The expression of Mstn is initially detected at embryonic day 9.5 in developing somites and continues to be detected postnataly in adult skeletal muscle fibers [1]. Furthermore, the mRNA expression of Mstn is developmentally regulated. While relatively abundant expression of Mstn is observed during fetal development, following birth the expression of Mstn rapidly decreases and remains quite low during postnatal development [12]. Previous work has also demonstrated that in adult skeletal muscle the expression of Mstn is greater in fast-twitch when compared to slow-twitch muscles [13], [14] and thus is speculated to play a role in regulating muscle fiber type. Although we know that the expression of Mstn is regulated during myogenesis the exact mechanisms through which the abundance of Mstn is regulated remain to be fully determined. However, work from our lab has revealed that Mstn is transcriptionally regulated by the transcription factor MyoD through E-Box elements contained within the enhancer region of the Mstn gene [15]. In addition, further work has demonstrated that Mstn is able to negatively auto-regulate its own expression through a Smad7-dependent mechanism [16]. In eukaryotes, transcriptional regulation is not the only mechanism through which gene expression is controlled. Most notably, post transcriptional regulation of mRNA stability and translation through the action of microRNAs (miRNAs) plays a major role in regulating mRNA abundance. miRNAs are short single stranded RNAs that can bind specifically to complementary sequences found in 3′ untranslated regions (UTR) of target mRNAs, resulting in either repression of translation or degradation of mRNAs through the RNA-induced silencing complex (RISC) [17], [18]. Previously published work has revealed that Mstn levels are regulated by miRNAs. Specifically, transgenic over expression of miR-208a in the heart has been shown to result in cardiac hypertrophy together with reduced expression of Mstn [19]. Furthermore, over expression of miR-499 leads to reduced Mstn 3′UTR activity, suggesting that Mstn is a target of miR-499 [20]. In addition, essential amino acids have been shown to promote muscle hypertrophy by not only suppressing Mstn levels but by inducing greater expression of miR-499, -208b, -23a, -1, and -206 [21]. Interestingly, a naturally occurring gain of function mutation in the 3′UTR region of the Texel sheep Mstn gene creates a miR-206 site causing translational inhibition of Mstn expression and a double muscling phenotype [22]. More recently, microRNA-27 (miR-27) has been shown to target and inhibit Mstn. Work by Allen and Loh revealed that miR-27a/b is able to reduce Mstn expression and mRNA stability and moreover indicated miR-27a/b may play a role in the increased expression of Mstn observed firstly, in Fast-twitch muscle, when compared to slow-twitch muscle and secondly, in response to Dexamethasone treatment [23]. Similarly, Huang et al revealed that over expression of miR-27a through addition of miR-27a mimics resulted in reduced Mstn mRNA expression and increased myoblast proliferation [24], consistent with known Mstn function.
Here we now show further evidence to support that Mstn gene expression is regulated by the miR-27a/b, as such AntagomiRs against miR-27a/b were able to increase Mstn expression, reduce myoblast proliferation and induce myotubular atrophy. Importantly, AntagomiR-27a/b-mediated myotube atrophy was due to increased Mstn function, as either blockade or complete absence of Mstn rescued the myotubular atrophy. Furthermore, results confirm a role for miR-27a/b in regulating muscle fiber type-specific and tissue-specific expression of Mstn and suggest that miR-27a/b may play a role in regulating Mstn expression and thus function during myogenic differentiation. We further show the utility of miR-27a/b in regulating Mstn expression and activity in vivo and that in Smad3-null mice there is increased expression of Mstn, which is due to reduced endogenous miR-27a/b expression in these mice. Results also reveal for the first time that Mstn up regulates the expression of miR-27a/b via Smad3, which in turn targets and represses Mstn, forming the basis of a novel microRNA-mediated Mstn negative auto-regulatory loop during myogenesis.
Materials and Methods
Ethics Statement
All experiments involving animals were approved by the Nanyang Technological University Institutional Animal Care and Use Committee (NTU-IACUC), Singapore (Approval Number: ARF SBS/NIE-A 0057). All surgery was performed under Ketamine/Xylazine anesthesia and all efforts were made to minimize animal suffering.
Animals
Four-six-week-old C57BL/6J male wild type (WT) mice were obtained from the National University of Singapore Centre for Animal Resources, Singapore. Myostatin-null mice (Mstn −/+) were gifted by Prof. See-Jin Lee (Johns Hopkins University, Baltimore, MD, USA). All mice were housed in groups at a constant temperature (20°C) under a 12 h/12 h artificial light/dark cycle with free access to water. To study the effect of miR-27a blockade in vivo, 20 nM (25 µl total volume; in sterile nuclease free water) of each AntagomiR (AntagomiR-27a or AntagomiR Neg) oligonucleotides [25], was injected into the M. tibialis anterior (TA) muscle of anaesthetized WT mice (n = 3) using a 28-gauge syringe (Hamilton Co., Reno, NV, USA). The contralateral limb of each mouse was injected with negative control AntagomiR (AntagomiR Neg). In vivo transfection of plasmid DNA (pcDNA-miR-27a or pcDNA-miR-neg) was performed by intramuscular injection of 25 µg (25 µl total volume; in sterile PBS) of each plasmid DNA into the TA muscle of anaesthetized mice. The contralateral limb of each mouse was injected with the empty vector (pcDNA-miR-neg) as a control. Electrical pulses (50 Volts/cm, 5 pulses, 200 ms intervals) were then applied with two platinum electrodes placed on either side of the muscle belly, using the ECM 830 Electroporation system (BTX Instrument Division, Harvard Apparatus, Inc. MA, USA). Eight days post-injection, M. tibialis anterior (TA) muscles from both sides of the hind-limb were harvested for histological and molecular analysis. For staining of skeletal muscle sections, TA muscles were covered with OCT compound and then frozen in isopentane cooled with liquid nitrogen. Transverse sections (8 µm) were cut from the mid-belly of the muscle and mounted on slides for hematoxylin and 1% eosin (H&E) staining and for detection of MyoD and Pax7 by immunocytochemistry. Images were captured using the Leica CTR 6500 microscope equipped with the Leica DFC 420 camera and Image Pro Plus software (Media Cybernetics, Bethesda, MD). Muscle fiber size was measured as Cross Sectional Area (CSA) from 500 myofibers per mouse (n = 3).
C2C12 myoblast cell culture
Mouse C2C12 myoblasts, obtained from American Type Culture Collection (Manassas, VA, USA), were maintained as previously described [26]. Assessment of C2C12 myoblast proliferation was performed as previously described [6], [26]. Briefly, C2C12 myoblasts were seeded at a density of 1000 cells/well in 96-well plates in proliferation medium (DMEM, 10% FBS and 1% P/S; Invitrogen, Carlsbad, CA, USA). After an overnight attachment period, myoblasts were transfected with 25 nM each of AntagomiRs specific for miR-27a (AntagomiR-27a), miR-27b (AntagomiR-27b) or negative control AntagomiR (AntagomiR Neg) (Dharmacon Inc, USA) using Lipofectamine 2000 (LF2000; Invitrogen, USA), as per the manufacturer's guidelines. Following a further period of 72 h growth, proliferation was assessed using the methylene blue photometric end-point assay, as previously described [27], where absorbance at 655 nm is directly proportional to final cell number. To assess the effect of miR-27a/b blockade on differentiated myotubes and to study the effect of Mstn blockade on AntagomiR-27a/b-mediated myotube atrophy, C2C12 myoblasts were induced to differentiate on Thermanox coverslips under low-serum conditions (DMEM, 2% Horse Serum) for 24 h. Following this, 24 h differentiated C2C12 myoblasts were transfected, using LF2000, with 50 nM each of AntagomiR-27a, AntagomiR-27b or AntagomiR Neg. Twelve hours following transfection, cells were then treated with either vehicle (Dialysis buffer; DB) or with a soluble form of the Activin receptor Type IIB (sActRIIB) at a final concentration of 3 µg/ml and allowed to differentiate for 72 h. The expression and purification of the sActRIIB Mstn antagonist was performed as previously described [28]. Differentiated Myotubes were then fixed with ethanol∶formaldehyde∶glacial acetic acid (20∶2∶1) and stained with H&E. Images of the cultures were then captured and myotube area assessed. Mstn-overexpressing CHO cells were kindly gifted by Dr. Se-Jin Lee, Johns Hopkins University, USA. Mstn-overexpressing CHO cells were propagated and Mstn protein containing conditioned medium (CMM) was collected as described previously [11]. The final concentration of Mstn protein present in all CMM treatments was 10 ng/ml, as estimated by ELISA (Immundiagnostik, Bensheim, Germany). Conditioned medium collected from control CHO cells (CCM) was used as a control for CMM treatment experiments. For Mstn treatment, C2C12 myoblasts were either grown for 16 h or were differentiated for 48 h before further treatment with either CMM or CCM for 12 h.
Primary myoblast culture
Mouse primary myoblasts were cultured from hind-limb muscles isolated from WT and Mstn−/− mice using a modified method of Partridge TA [29]. Briefly, hind-limb muscles were excised, minced and then digested in 0.2% collagenase type 1A for 90 min. Fibroblasts were removed by pre-plating the cells on uncoated plates for 3 h at 37°C 5% CO2. Primary myoblasts were cultured on 10% Matrigel (BD Biosciences) coated plates and were maintained in proliferation medium, (DMEM, 20% FBS, 10% HS, 1% P/S and 1% Chicken Embryo Extract) at 37°C 5% CO2. Primary myoblasts were induced to differentiate, transfected with either AntagomiR-27a or AntagomiR Neg and fixed and stained with H&E, as described above. Images of the cultures were then captured and myotube area assessed.
Specific Inhibitor of SMAD3 (SIS3) treatment
C2C12 myoblasts were differentiated (as described above) for 48 h followed by a further 24 h in the absence (0.05% DMSO) or presence of the SMAD3-specific inhibitor SIS3 (10 µM; Sigma-Aldrich). Cells were then harvested for total RNA isolation and subsequent quantitative real-time PCR (qPCR) analysis.
Detection of MyoD and Pax7 by immunofluorescence
Muscle sections were fixed in 4% paraformaldehyde for 5 min and then permeabilized in 0.2% PBS-Tween 20. After this, sections were blocked in a solution containing 6% mouse IgG blocking reagent (MOM Immunodetection kit; Vector laboratories, Inc, CA, USA) and 3% bovine serum albumin (BSA) in PBS for 1 h, followed by 5 min in MOM protein diluent with 1.5% BSA in PBS, as per the manufacturer's instruction. Muscle sections were then stained with mouse monoclonal anti-Pax7 (Developmental Studies Hybridoma Bank; DSHB, Iowa City, IA, USA; 1∶1000) primary antibody in 1.5% BSA in PBS overnight at 4°C. Following incubation with horse biotinylated anti-mouse IgG (Vector laboratories, Inc., CA, USA; 1∶500), rabbit polyclonal anti-Laminin (Sigma-Aldrich, Singapore; 1∶1000) and rabbit polyclonal anti-MyoD (Santa Cruz, USA; 1∶40) for 3 h, the sections were then washed and stained with Streptavidin conjugated Alexa Fluor 488 (Invitrogen; 1∶1000) and goat anti-rabbit Alexa Fluor 594 (Invitrogen; 1∶1000) for 30 min. Nuclei were counterstained with 4′6-diamidino-2-phenylindole (DAPI) (Invitrogen) before mounting with prolong gold anti-fade mounting medium (Invitrogen). Pax7+ cells and activated myoblasts (MyoD+) that lie underneath the basal lamina, as detected through Laminin staining, were counted and expressed as the percentage of positive nuclei per 100 myofibers. Images were captured using either the Nikon A1Rsi confocal microscope equipped with Photometrics CoolSNAP HQ2 camera, or the Leica CTR 6500 microscope equipped with Leica DFC 420 camera and Image Pro Plus software (Media Cybernetics, Bethesda, MD).
Plasmids
The 3′-UTR of murine Mstn (1,448 bp) was PCR amplified using the following primers 5′-AAG CTT GCT TTG CAT TAG GTT-3′, 5′-AAG CTT GCC TTT CAA AAA TG-3′ and cloned as a HindIII fragment into the pMIR-REPORT™ miRNA Expression Luciferase Reporter Vector system (Life Technologies). The construct was sequence verified and named Mstn 3′UTR. The predicted miR-27a/b binding site within the Mstn 3′ UTR was mutated using a PCR-based mutagenesis approach with combinations of the primers above and the following megaprimer 5′-CCC CTC AAT TTC GAA GTC ACA GGT TCA AGC ACC ACA GG-3′, as per the protocol by Picard et al 1994 [30]. The mutated Mstn 3′ UTR was then cloned as a HindIII fragment into the pMIR-REPORT™ expression reporter vector, sequence verified to confirm mutation of the miR-27a/b binding region and named Mstn 3′UTR-mut.
The pcDNA 6.2-GW/± EmGFP expression vector containing mature miR-27a (pcDNA-miR-27a) was used for miR-27a over expression studies. The pcDNA 6.2-GW/± EmGFP empty vector (pcDNA-miR-neg) was used as a control.
The miR-27a promoter (miR-27a pro), miR-27b promoter (miR-27b pro) and the mutant miR-27b promoter reporter construct (miR-27b pro-mut) used in this study were kindly gifted by Dr Xiao Yang (State Key Laboratory of Proteomics, Genetic Laboratory of Development and Diseases, Institute of Biotechnology, Beijing, China) and have been described previously [31], [32].
Transfections and Luciferase assay
For co-transfection of reporter plasmids and miR-27a over expressing vectors, C2C12 myoblasts were seeded into 24-well plates at a density of 15,000 cells/cm2 24 h before transfection. Proliferating C2C12 myoblasts were co-transfected with 0.1 µg Mstn 3′UTR or Mstn 3′UTR-mut reporter plasmids and 0.4 µg pcDNA-miR-27a or pcDNA-miR-neg, as a negative control. Transfection was carried out with LF2000 (Invitrogen, USA) according to the manufacturer's protocol. For co-transfection of reporter plasmids and AntagomiRs against miR-27a/b, 50 nM of AntagomiR-27a, AntagomiR-27b or AntagomiR Neg were co-transfected with Mstn 3′UTR or Mstn 3′UTR-mut using LF2000 (Invitrogen, USA) as per the manufacturer's protocol. After 48 h of transfections, cells were lysed, and luciferase assays were performed on protein extracts using the Dual-Luciferase reporter system (Promega, USA), according to the manufacturer's recommendations. To assess for miR-27a and miR-27b promoter reporter activity, miR-27a pro, miR-27b pro and miR-27b pro-mut constructs were electroporated (GenePulsar MXcell, Bio-rad, Hercules, CA, USA) into 1 million C2C12 cells and grown to confluency. The electroporated C2C12 myoblasts were then replated at a density of 15,000 cells/cm2 in 24 well plates and treated with (10 µM) or without (0.05% DMSO) SIS3 in the presence (CMM; 10 ng/ml) or absence (CCM) of Mstn for 24 hrs. Following transfections, cells were lysed, and luciferase assays were performed on protein extracts using the Dual-Luciferase reporter system (Promega, USA), according to the manufacturer's recommendations. Renilla and firefly luciferase signals were detected using the GloMax luminometer (Promega, USA). AntagomiRs (AntagomiR-27a, AntagomiR-27b and AntagomiR Neg) were synthesized by Dharmacon Inc, USA. Synthetic miR-27b mimic and non-targeting miRNA negative control mimic were obtained from Dharmacon. A final concentration of 50 nM each of miR-27b mimic and miRNA negative control were transfected into wild type (WT) and Smad3-null mouse primary myoblasts using LF2000 (Invitrogen) as per the manufacturer's protocol. Following transfection primary myoblasts were induced to differentiate under low-serum conditions (DMEM, 2% Horse Serum) for 72 h and then harvested for total RNA isolation and subsequent qPCR analysis.
RT-PCR and quantitative real-time PCR (qPCR)
Total RNA was extracted using TRIzol reagent according to the manufacturer's protocol (Invitrogen, USA). cDNA was synthesized from 1 µg of total RNA using the iScript cDNA kit (Bio-rad, USA) as per the manufacturer's guidelines. qPCR analysis of precursor-miR-27a/b (Pre-miR-27a/b) and Mstn expression was performed using the CFX96 Real-Time System (Bio-rad). Each qPCR reaction (10 µl) contained 3 µl of diluted cDNA, 5 µl of 2 X SsoFast Evagreen (Bio-rad) and primers at a final concentration of 200 nM. All reactions were performed using the following thermal cycle conditions: 98°C for 3 min followed by 45 cycles of a two step reaction, denaturation at 98°C for 3 sec and annealing at 60°C for 20 sec, followed by a denaturation curve from 60°C to 95°C in 5 sec increments of 0.5°C to ensure amplification specificity. To assess for the expression of mature miR-27a and miR-27b, cDNA was synthesized from extracted RNA using the miScript II RT kit (Cat# 218161; Qiagen), as per the manufacturer's instructions. qPCR was then conducted using miR-27a or miR-27b mature miRNA specific miScript forward primers, referred to as Primer Assays (Cat# MS00001351 and Cat# MS00001358 respectively; Qiagen), miScript universal reverse primer (Qiagen) and miScript SYBR Green PCR Kit (Cat# 218075; Qiagen). The expression of mRNAs and miRNAs were normalized to GAPDH and U6, respectively. The following mouse-specific primers were used for qPCR analysis: precursor-miR-27a/b (pre-miR-27a/b) Forward 5′-GCA GGG CTT AGC TGC TTG-3′, Reverse 5′-GGC GGA ACT TAG CCA CTG T-3′; Mstn Forward 5′-AGT GGA TCT AAA TGA GGG CAG T-3′, Reverse 5′-GTT TCC AGG CGC AGC TTA C-3′; U6 Forward 5′-CTC GCT TCG GCA GCA CA-3′ Reverse 5′-AAC GCT TCA CGA ATT TGC GT-3′; GAPDH Forward 5′-ACA ACT TTG GCA TTG TGG AA-3′, Reverse 5′-GAT GCA GGG ATG ATG TTC TG-3′.
Statistical analysis
Statistical analysis was performed using two-tail Student's-t-test and ANOVA. Data are expressed as mean ±SEM and p<0.05 were considered significant. Experimental replicates are described in relevant figure legends.
Results
miR-27a/b targets and represses Mstn through a miR-27a/b-specific target site in the 3′ UTR of the Mstn gene
In agreement with previously published reports [23], [24], analysis with the TargetScan5.1 (http://www.targetscan.org/) algorithm revealed the presence of a single target site for microRNA-27a/b (miR-27a/b) in the 3′UTR of the murine Mstn gene (Figure 1A). Importantly, TargetScan analysis revealed an 8 mer seed match, defined as a perfect match to positions 2–8 of the mature miRNA followed by an adenine, between the miR-27a and miR-27b seed sequence and the miR-27a/b binding site in the 3′UTR of the murine Mstn gene (Figure 1A). Furthermore the mstn 3′UTR miR-27a/b target site was found to be flanked by AU-rich sequences, which act to boost miRNA efficacy [33].
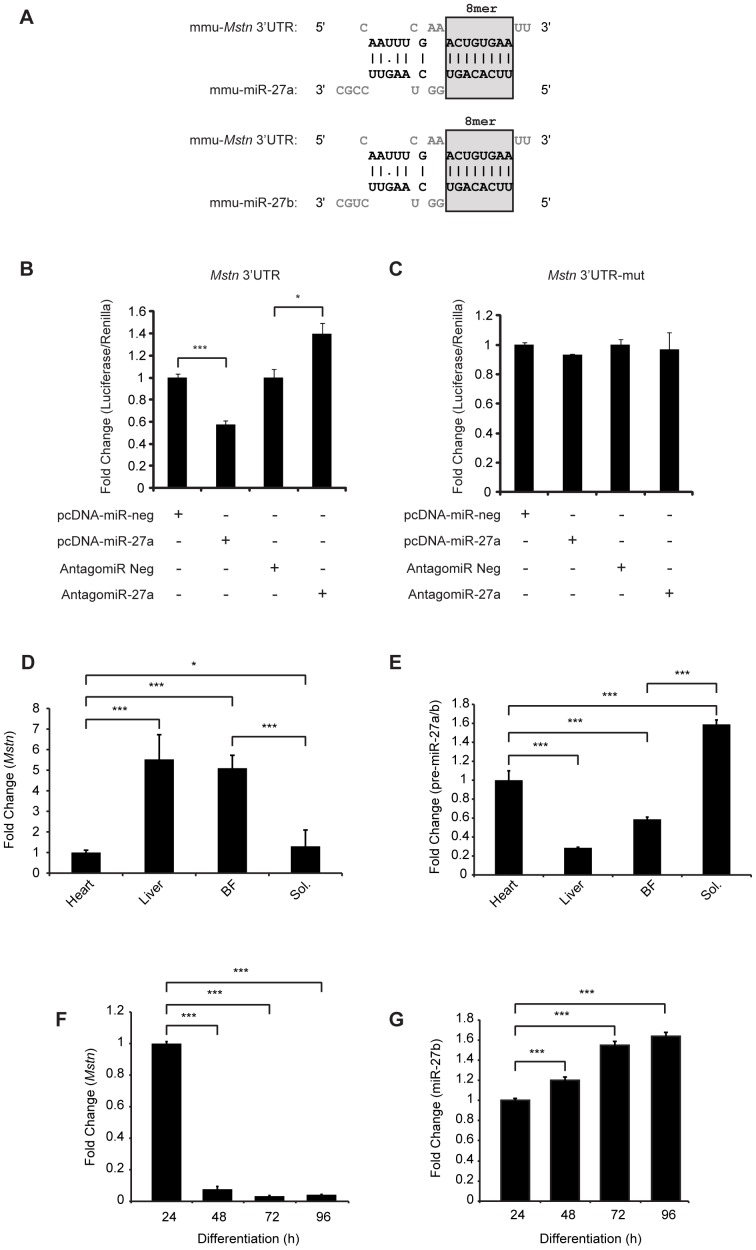
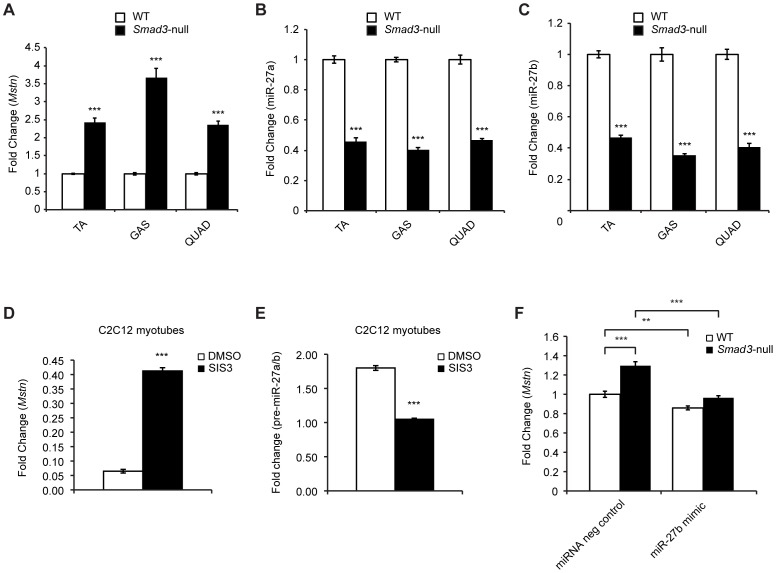
Figure 1. miR-27a/b targets and represses Mstn expression.
(A) In silico analysis, using TargetScan algorithms, showing a 8 mer seed match (grey box) between murine miR-27a (mmu-miR-27a) and miR-27b (mmu-miR-27b) and the miR-27a/b binding site located within the Mstn 3′ UTR sequence (mmu-Mstn 3′UTR). (B) Assessment of pMIR-REPORT™ luciferase activity in C2C12 myoblasts co-transfected with the Mstn 3′UTR reporter construct (Mstn 3′UTR) and either control (pcDNA-miR-neg), miR-27a over expression construct (pcDNA-miR-27a), negative control AntagomiR (AntagomiR Neg) or a miR-27a-specific AntagomiR (AntagomiR-27a) for 48h. (C) Assessment of pMIR-REPORT™ luciferase activity in C2C12 myoblasts co-transfected with the mutant Mstn 3′UTR reporter construct (Mstn 3′UTR-mut), where the miR-27a/b binding site has been mutated, and either control (pcDNA-miR-neg), miR-27a over expression construct (pcDNA-miR-27a), negative control AntagomiR (AntagomiR Neg) or a miR-27a-specific AntagomiR (AntagomiR-27a) for 48h. For all pMIR-REPORT™ transfections, luciferase activity was normalized to Renilla luciferase and expressed as fold change relative to control (pcDNA-miR-neg). Bars represent mean values ± S.E.M (n = 3). p<0.05 (*) and p<0.001(***). qPCR analysis of Mstn mRNA expression (D) and precursor-miR-27a/b (pre-miR-27a/b) expression (E) in Heart, Liver, M. Biceps femoris muscle (BF) and M. Soleus muscle (Sol.) collected from 4-week-old wild type (WT) mice. Bars represent fold change (relative to Heart) ± S.E.M (n = 3) normalized to GAPDH (D) or U6 (E) expression. p<0.05 (*) and p<0.001(***). qPCR analysis of (F) Mstn and (G) miR-27b expression in C2C12 myoblast cultures differentiated across a time course (24 h, 48 h, 72 h and 96 h differentiation). Bars represent fold change (relative to 24 h control) ± S.E.M (n = 3) normalized to either GAPDH (F) or U6 (G) expression. p<0.001(***).
To further study miR-27a/b regulation of Mstn, we cloned the 3′ UTR region of the murine Mstn gene into the pMIR-REPORT™ miRNA Expression Luciferase Reporter Vector and co-transfected together with a miR-27a over expression vector (pcDNA-miR-27a). A significant reduction in Mstn 3′UTR reporter luciferase activity was observed in myoblasts co-transfected with Mstn 3′UTR and pcDNA-miR-27a, when compared with myoblasts co-transfected with Mstn 3′UTR and control (pcDNA-miR-neg) (Figure 1B). To identify if miR-27a/b target site was responsible for the reduced luciferase activity observed, the putative miR-27a/b binding site in the Mstn 3′UTR was mutated. When pcDNA-miR-27a was co-transfected together with the mutant Mstn 3′UTR reporter (Mstn 3′UTR-mut), no significant reduction in luciferase activity was observed (Figure 1C). Furthermore co-transfection of Mstn 3′UTR with a miR-27a-specific AntagomiR (AntagomiR-27a) resulted in significant increase in Mstn 3′UTR reporter luciferase activity, over and above that observed in control AntagomiR Neg transfected cells (Figure 1B). The effect of AntagomiR-27a appeared to be specific to the miR-27a/b site in the Mstn 3′UTR site, since addition of AntagomiR-27a failed to increase luciferase activity in Mstn 3′UTR-mut reporter transfected myoblasts (Figure 1C). These data strongly suggest that miR-27a/b is able to negatively regulate Mstn mRNA and that the miR-27a/b target site found within the Mstn 3′UTR is critical for miR-27a/b regulation of Mstn.
Correlation between Mstn and miR-27a/b expression in vivo and in vitro
While high levels of Mstn are detected in skeletal muscle, lower levels of Mstn are expressed in white adipose tissue, heart and mammary gland [1]–[3]. Furthermore, Mstn expression is higher in fast-twitch muscles, when compared to slow-twitch muscles [13], [14]. To investigate whether or not miR-27a/b plays a role in regulating tissue-specific Mstn expression, we analyzed precursor-miR-27a/b (pre-miR-27a/b) and Mstn expression profiles in various tissues. When compared to liver and biceps femoris (BF) muscle, we noted reduced expression of Mstn and increased expression of pre-miR-27a/b in the heart (Figure 1D and 1E). On the other hand in tissues where relatively higher levels of Mstn were observed, such as liver and BF muscle, lower pre-miR-27a/b expression was detected (Figure 1D and 1E). Consistent with the data published by Allen and Loh [23], we also observed a difference in Mstn and pre-miR-27a/b expression between fast-twitch and slow-twitch muscles. qPCR analysis revealed significantly increased Mstn mRNA expression, concomitant with reduced pre-miR-27a/b expression in the predominantly fast-twitch BF muscle, when compared to the slow-twitch soleus (Sol) muscle (Figure 1D and 1E).
Next we also compared the expression of Mstn and miR-27a/b during differentiation in C2C12 myoblasts. Subsequent qPCR revealed that there was relatively higher expression of Mstn at 24 h differentiation, which sharply declined from 48 h differentiation onwards in C2C12 myoblasts (Figure 1F). However in contrast, we observed a gradual increase in miR-27b expression from 24 h through to 96 h differentiation (Figure 1G). Thus, the expression of Mstn appears to be inversely associated with miR-27a/b expression during C2C12 myoblast differentiation.
AntagomiR-mediated blockade of miR-27a/b leads to enhanced Mstn activity
Mstn, as a negative regulator of skeletal muscle growth, has been previously demonstrated to inhibit myoblast proliferation and moreover induce severe myotubular atrophy in vitro [5], [8], [9]. Therefore, we next assessed the effect of AntagomiR-27a and AntagomiR-27b transfection on Mstn function during myogenesis. As predicted, transfection of AntagomiR-27a or AntagomiR-27b resulted in reduced expression of miR-27a (Figure S1A) and miR-27b (Figure S1B) respectively, together with increased Mstn expression (Figure S1C). Next we assessed C2C12 myoblast proliferation following treatment with conditioned medium collected from Control (AntagomiR Neg), AntagomiR-27a or AntagomiR-27b transfected C2C12 myoblasts. As shown in Figure 2A, we observed a significant decrease in myoblast proliferation in C2C12 myoblasts treated with conditioned medium collected from AtagomiR-27a and AntagomiR-27b transfected cells, when compared to control (AntagomiR Neg) transfected cells (Figure 2A).
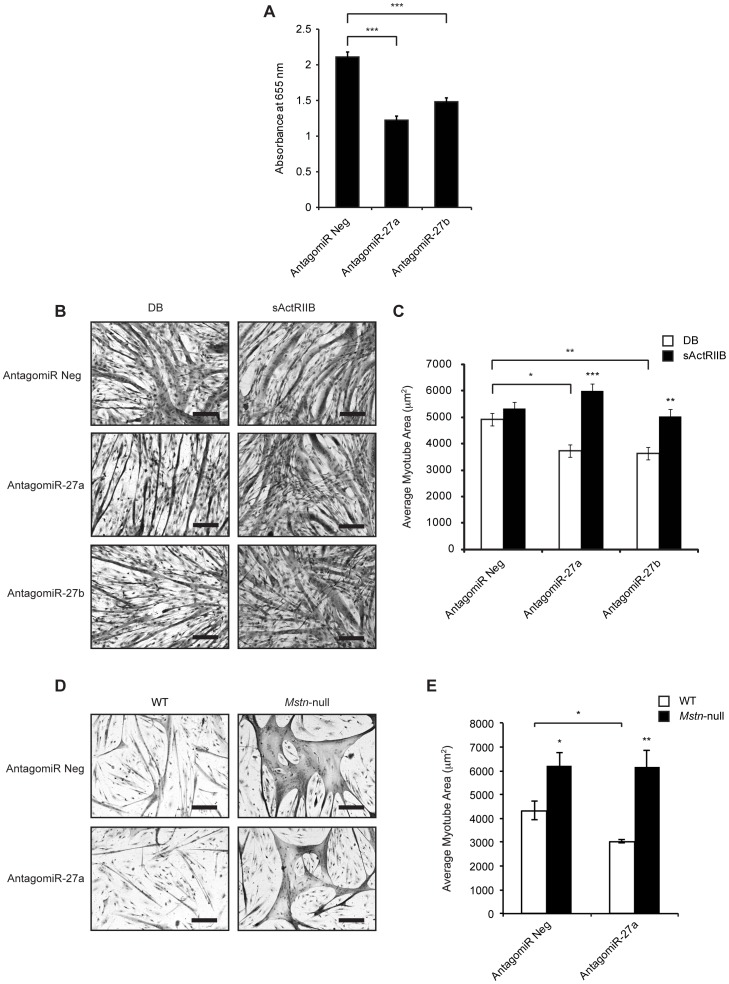
Figure 2. Inhibition of miR-27a and miR-27b results in increased Mstn activity.
(A) Analysis of C2C12 myoblast proliferation in cultures treated with conditioned medium collected from C2C12 myoblasts transfected with either the negative control AntagomiR (AntagomiR Neg), miR-27a-specific AntagomiR (AntagomiR-27a) or miR-27b-specific AntagomiR (AntagomiR-27b) for 72 h, as monitored by methylene blue assay. Values represent mean values ± S.E.M (n = 3). p<0.001 (***). (B) Representative images of H&E stained AntagomiR Neg, AntagomiR-27a or AntagomiR-27b transfected C2C12 myoblasts after 48 h differentiation, followed by a further 72 h differentiation in the absence (Dialysis buffer; DB) or presence of 3 µg/ml sActRIIB. Scale bars = 100 µm. (C) Quantification of average myotube area (µm2) in AntagomiR Neg, AntagomiR-27a or AntagomiR-27b transfected C2C12 myoblasts following 72 h differentiation and treatment without (DB) or with sActRIIB. Average myotube area was calculated from 10 random images per coverslip (n = 3) from three independent experiments. p<0.05 (*), p<0.01 (**) and p<0.001 (***). (D) Representative images of H&E stained AntagomiR Neg or AntagomiR-27a transfected WT and Mstn-null primary myoblasts following 48 h differentiation. Scale bars = 100 µm. (E) Quantification of average myotube area (µm2) in AntagomiR Neg or AntagomiR-27a transfected WT and Mstn-null primary myoblasts following 72 h differentiation. Average myotube area was calculated from 10 random images per coverslip (n = 3). p<0.001 (***).
In addition, transfection of either AntagomiR-27a or AntagomiR-27b into differentiating C2C12 myotubes resulted in noticeable myotubular atrophy when compared to AntagomiR Neg transfected myotubes (Figure 2B). Subsequent quantification revealed a significant 24% and 26% decrease in average myotube area in AntagomiR-27a and AntagomiR-27b transfected myotubes respectively, when compared to AntagomiR Neg transfected myotubes (Figure 2C). Consistent with blockade of miR-27a/b and with the development of myotube atrophy a significant increase in Mstn expression was observed following transfection of C2C12 myotubes with AntagomiR-27a or AntagomiR-27b (Figure S1D).
To confirm whether or not the myotube atrophy observed following AntagomiR-mediated blockade of miR-27a/b was due to enhanced Mstn function, we next assessed myotube area in AntagomiR-27a and AntagomiR-27b transfected C2C12 myotubes cultures treated together with soluble Activin type IIB receptor (sActRIIB) Mstn antagonist. Treatment of AntagomiR-27a and AntagomiR-27b transfected C2C12 myotubes with sActRIIB rescued the myotubular atrophy observed in the AntagomiR only transfected myotubes (Figure 2B). Subsequent quantification revealed an ∼40% and ∼30% increase in average myotube area, which was similar to that observed in AntagomiR Neg transfected myotubes, in sActRIIB treated AntagomiR-27a and AntagomiR-27b transfected myotubes respectively, when compared to respective vehicle control (Dialysis buffer; DB) treated transfected myotubes (Figure 2C).
In agreement with the results above, AntagomiR-mediated reduction of miR-27a expression (Figure S1E) did not result in any appreciable myotube atrophy in Mstn-null mice-derived primary myotube cultures, when compared to AntagomiR Neg transfected primary myotubes (Figure 2D & 2E). However, in contrast, AntagomiR-mediated reduction of miR-27a in primary myotubes cultures isolated from WT mice (Figure S1F) led to elevated Mstn expression (Figure S1G) and observable myotubular atrophy (Figure 2D & 2E), with an ∼32% decrease in average myotube area observed in AntagomiR-27a transfected myotubes, when compared to AntagomiR Neg transfected myotubes (Figure 2E). These results confirm that blockade of miR-27a/b results in myotube atrophy through a mechanism dependent on Mstn.
miR-27a/b regulates myofiber size and SC function through targeting endogenous Mstn expression in skeletal muscle
To investigate whether miR-27a/b regulates endogenous Mstn levels in skeletal muscle, M. tibialis anterior (TA) muscles of WT mice were intramuscularly injected and in vivo electroporated with either the pcDNA-miR-27a over expression construct or control (pcDNA-miR-neg). The expression of Mstn was quantified by qPCR 8 days post-injection and as shown in Figure 3A overexpression of miR-27a in vivo resulted in a significant reduction in Mstn expression in TA muscle. Since Mstn is a potent negative regulator of skeletal muscle mass and satellite cell (SC) function [1], [4], [7], [34], we also stained TA muscle serial sections with H&E (Figure 3B) and quantified myofiber cross sectional area (CSA), as well as the percentage of Pax7+ and MyoD+ cells in TA muscle following miR-27a overexpression. An ∼30% increase in average myofiber CSA was observed in miR-27a overexpressing TA muscle, when compared to the control transfected contralateral TA muscle (Figure 3C). Furthermore we also observed an ∼52% increase in the number of very large myofibers (>2500 µm2) and an ∼60% decrease in the number of very small myofibers (<1500 µm2) upon in vivo overexpression of miR-27a (Figure 3D), which is quite consistent with the increased myofiber CSA and loss of Mstn function. Interestingly, detection of Pax7 and MyoD by immunofluorescence (Figure 3E & S1H) revealed a significant ∼12% and ∼7% increase in the pool of Pax7+ cells and activated myoblasts (MyoD+) respectively in pcDNA-miR-27a transfected TA muscle, when compared with control-transfected muscle (Figure 3E & 3F).
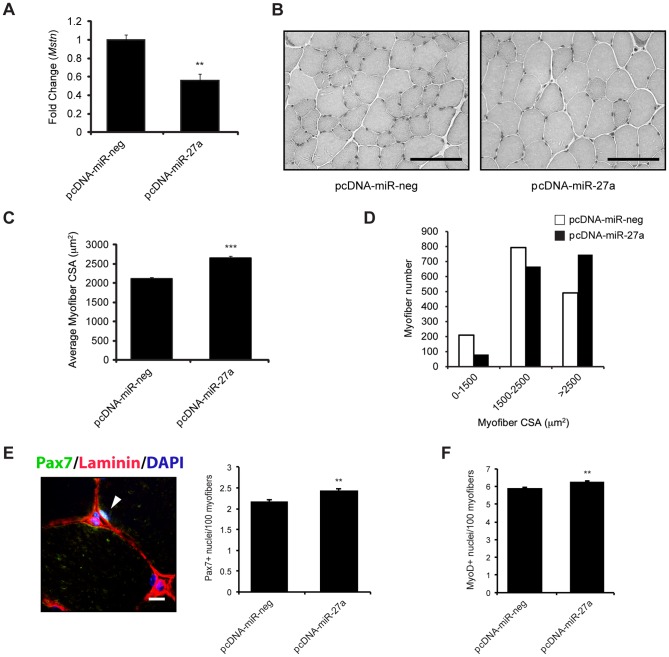
Figure 3. Over expression of miR-27a targets and represses endogenous Mstn expression and function in vivo.
(A) qPCR analysis of Mstn mRNA expression in TA muscle isolated from WT mice (n = 3) 8 days post intramuscular injection and in vivo transfection of either control (pcDNA-miR-neg) or miR-27a (pcDNA-miR-27a) over expression constructs. p<0.01 (**). (B) Representative images of H&E stained pcDNA-miR-neg and pcDNA-miR-27a in vivo transfected TA muscle cross sections from WT mice. Scale bars = 100 µm. (C) Graph showing average myofiber cross sectional area (CSA; µm2) in pcDNA-miR-neg and pcDNA-miR-27a in vivo transfected TA muscle from WT mice. Average myofiber area was calculated from 10 random images per coverslip (n = 3). p<0.001 (***). (D) Frequency distribution of myofiber area (µm2) in pcDNA-miR-neg and pcDNA-miR-27a in vivo transfected TA muscle from WT mice as calculated from 10 random images per coverslip (n = 3). (E) Left: Representative merged immunofluorescence image showing a Pax7+ cell (Green; white arrowhead) in a pcDNA-miR-27a in vivo transfected TA muscle cross section from WT mice. Sections were also stained for Laminin (Red) and nuclei were counterstained with DAPI (Blue). Scale bar = 10 µm. Right: Graph showing the number of Pax7+ cells in pcDNA-miR-neg and pcDNA-miR-27a in vivo transfected TA muscle from WT mice. Bars represent mean number ± S.E.M of Pax7+ cells, per 100 myofibers, from 3 sections each collected from pcDNA-miR-neg and pcDNA-miR-27a transfected WT mice (n = 3). p<0.01 (**). (F) Graph showing the number of MyoD+ cells in pcDNA-miR-neg and pcDNA-miR-27a in vivo transfected TA muscle from WT mice. Bars represent mean number ± S.E.M of MyoD+ cells, per 100 myofibers, from 3 sections each collected from pcDNA-miR-neg and pcDNA-miR-27a transfected WT mice (n = 3). p<0.01 (**).
To further confirm regulation of endogenous Mstn expression by miR-27a, we also injected either an AntagomiR specific for miR-27a (AntagomiR-27a) or a non-silencing negative control AntagomiR (AntagomiR Neg) into TA muscle of WT mice. The expression of Mstn was assessed 8 days post-injection and consistent with reduced miR-27a, Mstn expression was significantly up regulated, albeit modestly, upon AntagomiR-mediated blockade of miR-27a in vivo, when compared to AntagomiR Neg transfected contralateral TA muscle. (Figure 4A). We further stained TA muscle serial sections with H&E (Figure 4B) and although no significant difference was noted in average myofiber CSA (Figure 4C), we did observe an ∼25% increase in the number of very small myofibers (<1500 µm2) and an ∼10% decrease in the number of very large myofibers (>2500 µm2) (Figure 4D), which is quite consistent with the elevated Mstn expression detected. Furthermore, detection of Pax7 and MyoD by immunofluorescence (Figure 4E & S1H) revealed a significant ∼11% and ∼4% decrease in the pool of Pax7+ cells and activated myoblasts (MyoD+) respectively in AntagomiR-27a transfected TA muscle, when compared with AntagomiR Neg-transfected muscle (Figure 4E & 4F).
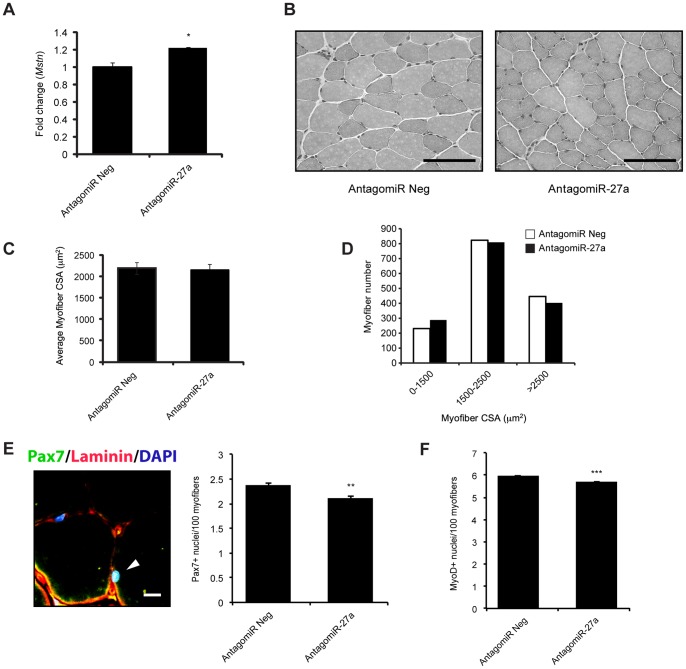
Figure 4. AntagomiR-mediated inhibition of miR-27a enhances endogenous Mstn expression and function in vivo.
(A) qPCR analysis of Mstn mRNA expression in TA muscle isolated from WT mice (n = 3) 8 days post intramuscular injection of either AntagomiR Neg or AntagomiR-27a. p<0.05 (*). (B) Representative images of H&E stained AntagomiR Neg and AntagomiR-27a injected TA muscle from WT mice. Scale bars = 100 µm. (C) Graph showing average myofiber CSA (µm2) in AntagomiR Neg and AntagomiR-27a injected TA muscle from WT mice. Average myofiber area was calculated from 10 random images per coverslip (n = 3). (D) Frequency distribution of myofiber area (µm2) in AntagomiR Neg and AntagomiR-27a injected TA muscle from WT mice as calculated from 10 random images per coverslip (n = 3). (E) Left: Representative merged immunofluorescence image showing a Pax7+ cell (Green; white arrowhead) in an AntagomiR Neg injected TA muscle cross section from WT mice. Sections were also stained for Laminin (Red) and nuclei were counterstained with DAPI (Blue). Scale bar = 10 µm. Right: Graph showing the number of Pax7+ cells in AntagomiR Neg and AntagomiR-27a injected TA muscle from WT mice. Bars represent mean number ± S.E.M of Pax7+ cells, per 100 myofibers, from 3 sections each collected from AntagomiR Neg and AntagomiR-27a injected WT mice (n = 3). p<0.01 (**). (F) Graph showing the number of MyoD+ cells in AntagomiR Neg and AntagomiR-27a injected TA muscle from WT mice. Bars represent mean number ± S.E.M of MyoD+ cells, per 100 myofibers, from 3 sections each collected from AntagomiR Neg and AntagomiR-27a injected WT mice (n = 3). p<0.001 (***).
Increased Mstn expression observed in the absence of Smad3 is due to reduced miR-27a/b expression
Smad3-null mice display severe muscle atrophy, which has been attributed to elevated endogenous levels of Mstn detected in Smad3-null mice [35]. Therefore we next wanted to test whether or not the increased Mstn levels observed in Smad3-null mice was due to reduced miR-27a/b expression. Consistent with previously published data, qPCR analysis revealed a significant increase in Mstn expression in TA, GAS and QUAD muscles isolated from Smad3-null mice, when compared to WT controls (Figure 5A). Importantly, the elevated Mstn expression was associated with a significant decrease in both mature miR-27a and miR-27b expression in all muscle tissues isolated from Smad3-null mice, as compared to WT mice (Figure 5B & 5C). Similarly, C2C12 myotubes treated with Specific Inhibitor of Smad3 (SIS3), a compound previously shown to specifically inhibit Smad3 function via suppressing Smad3 phosphorylation [36], displayed significantly increased Mstn expression concomitant with reduced pre-miR-27a/b (Figure 5D & 5E). To confirm that the reduced expression of miR-27 was responsible for the elevated Mstn expression detected in Smad3-null mice we next assessed Mstn expression between WT and Smad3-null mice primary myoblast cultures following transfection of a miR-27b-specific mimic. As expected Mstn expression was significantly elevated in Smad3-null cultures, when compared to WT cultures (Figure 5F). Importantly, transfection of the miR-27b mimic reduced the expression of Mstn back to levels comparable to that observed in WT controls (Figure 5F), suggesting that reduced miR-27a/b expression may be responsible for the increased levels of Mstn observed in Smad3-null mice.
Figure 5. Increased Mstn expression in Smad3-null mice is due to reduced miR-27a/b expression.
qPCR analysis of (A) Mstn, (B) miR-27a and (C) miR-27b expression in M. Tibialis anterior muscle (TA), M. Gastrocnemius muscle (GAS) and M. Quadriceps muscle (QUAD) isolated from WT and Smad3-null mice. Bars represent fold change (relative to respective WT control) ± S.E.M (n = 3) normalized to either GAPDH (A) or U6 (B & C) expression. p<0.001 (***). (D) qPCR analysis of Mstn expression in 48 h differentiated C2C12 myotubes treated without (0.05% DMSO) or with SIS3 (10 µM) for 24 h. p<0.001 (***). (E) qPCR analysis of pre-miR-27a/b expression in 48 h differentiated C2C12 myotubes treated without (0.05% DMSO) or with SIS3 (10 µM) for 24 h. p<0.001 (***). (F) qPCR analysis of Mstn in 72 h differentiated primary myoblast cultures isolated from WT and Smad3-null mice that were transfected with either non targeting miRNA negative control (miRNA neg control) or miR-27b-specific mimic (miR-27b mimic). Bars represent fold change (relative to WT miRNA Neg control transfected myoblasts) ± S.E.M (n = 3) normalized to GAPDH expression. p<0.01 (**) and p<0.001 (***).
Mstn upregulates miR-27a/b expression through a Smad3-dependent mechanism to negatively auto-regulate its own expression
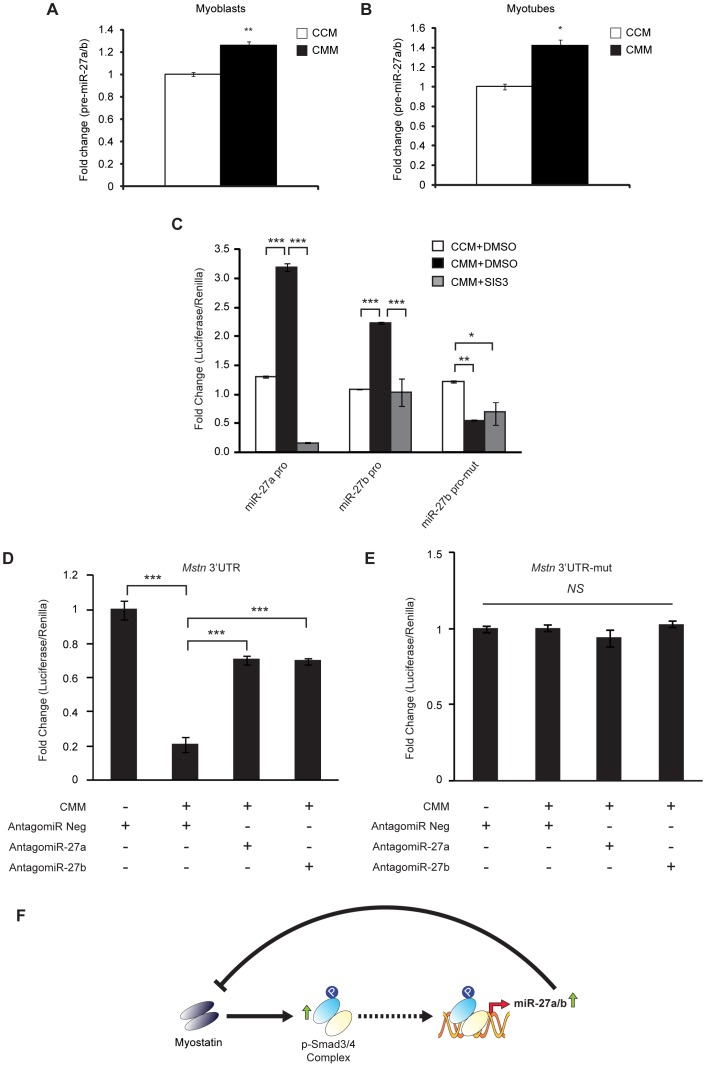
The data presented above suggested to us that Smad3 may play an important role in regulating basal miR-27a/b expression in muscle. Since Mstn is known to activate Smad3, we further hypothesized that Mstn may signal to up regulate the expression of miR-27a/b in muscle. To determine whether Mstn regulates miR-27a/b expression, C2C12 myoblasts and 48 h differentiated myotubes were treated with conditioned medium containing eukaryotic produced CHO-cell secreted Mstn protein (CMM) for 12 h. Pre-miR-27a/b expression was quantified by qPCR and as can be seen in Figure 6A & 6B, the expression of pre-miR-27a/b was significantly increased in both C2C12 myoblasts and myotubes upon treatment with CMM, when compared with cells treated with conditioned medium collected from control CHO cells (CCM) (Figure 6A & 6B). These data confirm that Mstn is able to positively regulate miR-27a/b expression in muscle.
Figure 6. Mstn treatment up regulates miR-27a/b expression via Smad3 to negatively auto-regulate it's own expression.
qPCR analysis of pre-miR-27a/b expression in C2C12 myoblasts (A) and 48 h differentiated C2C12 myotubes (B) following 12 h treatment with conditioned medium from either control CHO cells (CCM) or from CHO-cells designed to produce and secrete Mstn protein (CMM). Bars represent fold change (relative to respective CCM control) ± S.E.M (n = 3) normalized to U6 expression. p<0.05 (*) and p<0.01 (**). (C) Assessment of miR-27a and miR-27b promoter-reporter luciferase activity in C2C12 myoblasts transfected with the miR-27a promoter (miR-27a pro), miR-27b promoter (miR-27b pro) or a mutant miR-27b promoter reporter construct, where the smad binding site has been mutated (miR-27b pro-mut). Transfected C2C12 myoblasts were treated without (CCM) or with CMM in the absence (0.05% DMSO) or presence of SIS3 (10 µM) for 24 h prior to assessment of luciferase activity. All luciferase activity was normalized to Renilla luciferase and expressed as fold change relative to respective controls (CCM+DMSO). Bars represent mean values ± S.E.M (n = 3). p<0.05 (*), p<0.01 (**) and p<0.001 (***). (D) Assessment of pMIR-REPORT™ luciferase activity in C2C12 myoblasts co-transfected with Mstn 3′UTR and either AntagomiR Neg, AntagomiR-27a or AntagomiR-27b in the absence (−) or presence (+) of CMM. Bars represent mean values ± S.E.M (n = 3). p<0.001 (***). (E) Assessment of pMIR-REPORT™ luciferase activity in C2C12 myoblasts co-transfected with Mstn 3′UTR-mut and either AntagomiR Neg, AntagomiR-27a or AntagomiR-27b in the absence (−) or presence (+) of Mstn protein (CMM). Bars represent mean values ± S.E.M (n = 3). All luciferase activity was normalized to Renilla luciferase and expressed as fold change relative to control (CMM - and AntagomiR Neg +). (F) Based on the data presented in this current manuscript we propose that upon Mstn-mediated receptor activation Smad3 up-regulates the expression of miR-27a/b, which in turn leads to reduced Mstn expression and impaired Mstn function, thus forming the basis of a novel negative Mstn auto-regulatory loop in muscle.
To confirm whether Smad3 is involved in Mstn regulation of miR-27a/b, C2C12 myoblasts were transfected with either the miR-27a promoter (miR-27a pro), miR-27b promoter (miR-27b pro) or a mutant miR-27b promoter reporter construct, where the smad binding site has been mutated (miR-27b pro-mut) and subjected to treatment with CMM. Treatment with CMM resulted in a significant increase in promoter-reporter luciferase activity in myoblasts transfected with either the miR-27a or miR-27b promoter constructs (Figure 6C); however, no significant increase in luciferase activity was observed in C2C12 myoblasts transfected with the mutated miR-27b promoter construct following CMM treatment (Figure 6C). Transfected myoblasts were also subjected to treatment with both CMM and SIS3. As shown in Figure 6C, addition of SIS3 was able to partially rescue the increased miR-27a- and miR-27b-promoter-reporter luciferase activity observed following treatment with CMM alone (Figure 6C). Therefore these data confirm that Smad3 plays a critical role in the ability of Mstn to up regulate miR-27a/b expression.
Next we assessed whether or not the increased miR-27a/b, due to CMM treatment, would in turn target and repress Mstn expression. To test this C2C12 myoblasts transfected with the Mstn 3′UTR reporter were subjected to treatment with CMM. As shown in Figure 6D, treatment of Mstn 3′UTR reporter transfected myoblasts with CMM resulted in an ∼50% reduction in Mstn 3′UTR reporter activity. The ability of CMM to reduce Mstn 3′UTR reporter activity appeared to be dependent on miR-27a/b function, as AntagomiR-mediated inhibition of miR-27a/b, as well as mutation of the miR-27a/b binding site in the Mstn 3′UTR, prevented CMM-mediated inhibition of Mstn expression (Figure 6D & 6E). Taken together these data highlight a novel negative auto-regulatory mechanism through which Mstn signals to regulate it's own expression (Figure 6F).
Discussion
In the present study we have further characterized the role of miR-27a/b in regulating Mstn expression and activity. Evidence presented here confirms that Mstn is indeed a target of miR-27a/b both in vitro and in vivo. Consistent with previous reports [23], [24], we show that over expression of miR-27a results in reduced Mstn 3′UTR reporter activity, which is blocked upon mutation of the miR-27a/b binding site in the Mstn 3′ UTR. Furthermore, over expression of miR-27a in vivo led to decreased Mstn expression concomitant with myofiber hypertrophy and increased numbers of Pax7+ cells and activated myoblasts (MyoD+); quite consistent with the fact that loss of Mstn leads to increased muscle mass and enhanced satellite cell number, activation and self-renewal [1], [4], [34]. Previously published work has revealed that hypertrophy of skeletal muscle may occur independent of satellite cell function [37]. Consistent with this, hypertrophy of skeletal muscle induced upon blockade of Mstn, was also shown to occur in the absence of satellite cells [38], [39]. Nevertheless, as satellite cells play a critical role during skeletal muscle regeneration [37] and that loss of Mstn leads to enhanced satellite cell activation, self-renewal and accelerated skeletal muscle regeneration [7], [34] we strongly believe that the increased numbers of Pax7+ and MyoD+ cells observed following over expression of miR-27a is due to loss of Mstn. Recent work from Crist et al has shown that miR-27 is able to down regulate Pax3 protein levels, without affecting the levels of Pax7 [40]. However, we now show that over expression of miR-27 in vivo leads to increased numbers of Pax7+ cells. It is important to highlight that previously published work from our lab revealed that Mstn is a potent negative regulator of Pax7 expression during myogenesis. [26]. Therefore, the increase in Pax7+ cells observed in response to over expression of miR-27 is most likely due to miR-27-mediated inhibition of Mstn as opposed to direct regulation of Pax7 by miR-27. In addition to over expression studies, we now show that blockade of miR-27a, through addition of an AntagomiR specific for miR-27a, results in enhanced Mstn 3′UTR reporter activity. AntagomiR-mediated blockade of miR-27a and miR-27b not only up regulated Mstn expression but also significantly reduced C2C12 myoblast proliferation. These data are consistent with previously published reports demonstrating that excess Mstn inhibits myoblast proliferation [5] and with a recent report, which shows that addition of miR-27a mimics results in decreased Mstn mRNA concomitant with an increase in the number of proliferating C2C12 myoblasts [24]. In addition to controlling myoblast growth excess Mstn has been shown to promote skeletal muscle wasting in vitro and in vivo [10], [11]. In agreement with this, we observed significantly increased expression of Mstn together with pronounced myotubular atrophy upon AntagomiR-mediated inhibition of miR-27a and miR-27b. This effect appeared to be dependent on increased Mstn as sActRIIB-mediated blockade of Mstn rescued the atrophy phenotype and moreover primary myotube cultures isolated from Mstn-null mice were resistant to AntagomiR-induced myotube atrophy. It is important to mention that we noted differences in the effects of AntagomiR-27a and AntagomiR-27b on Mstn expression between proliferating C2C12 myoblasts and differentiated C2C12 myotube cultures (compare Figure S1C to Figure S1D). Although we do observe a significant increase in Mstn expression in both C2C12 myoblasts and myotubes transfected with either AntagomiR-27a or AntagomiR-27b the increase in Mstn was more significant in AntagomiR transfected myotube cultures, when compared to proliferating myoblasts. At this stage we do not know why there are differences in AntagomiR-27a- and AntagomiR-27b-mediated regulation of Mstn between myoblasts and myotubes, however we speculate that the AntagomiR effect might be more persistent in myotube cultures when compared to proliferating myoblasts. Blockade of miR-27a in vivo also resulted in significantly increased Mstn expression, which was associated with decreased myofiber CSA. Unlike the dramatic phenotype observed in vitro, AntagomiR-mediated blockade of miR-27a only resulted in minor muscle atrophy. However, it is important to mention that we only observed a slight increase in Mstn expression in vivo upon AntagomiR injection, which we suggest may account for the subtle atrophy phenotype observed. Nevertheless, we did note a significant reduction in the numbers of Pax7+ cells and activated myoblasts (MyoD+) upon AntagomiR-mediated blockade of miR-27a in vivo, further confirming that miR-27 is able to regulate Mstn. Taken together these data presented here strongly support that miR-27a/b negatively regulates both Mstn expression and function in vitro and in vivo. Importantly, in our current experiments we did not observe any significant difference in the ability of miR-27a or miR-27b to regulate Mstn expression or activity. Given that miR-27a and miR-27b have the same “seed” sequence, UGACACU, which recognizes complementary sequences in the 3′UTRs of target genes, it is not surprising that we found no difference in the ability of miR-27a or miR-27b to regulate Mstn.
To date several studies have shown that Mstn expression is relatively higher in fast twitch muscle fibers, when compared to slow twitch muscle fibers [13], [14]. Recently published evidence suggests that miR-27 may play a role in regulating skeletal muscle fiber type-specific expression of Mstn [23]. Specifically, work from Allen and Loh revealed that miR-27a and miR-27b fast-twitch and slow-twitch muscle-specific expression was complementary to that of Mstn. In the current manuscript we also found a similar trend in miR-27a/b and Mstn expression between fast and slow muscle fiber types. Interestingly, here we further show that miR-27a/b and Mstn expression was inversely associated between different tissues. Specifically, when compared to heart tissue, we noted higher Mstn expression, concomitant with reduced expression of miR-27a/b in liver tissue. It is noteworthy to mention that although expression of Mstn has been detected in Liver previously [41], [42], high expression of Mstn in the Liver, as shown here, has not been previously reported. We speculate that variations in the expression of Mstn detected in Liver tissue between various studies may be due to differences in the sensitivity of the techniques used to assess for Mstn expression. In addition to regulating fiber type- and tissue-specific Mstn expression, we also show that miR-27a/b could potentially regulate Mstn mRNA levels during myogenic differentiation in vitro. As differentiation ensued we noted a decrease in Mstn expression, consistent with previous reports [43], [44], concomitant with a steady increase in miR-27 expression. These data are in agreement with a recently published report by Chen et al, which shows a similar increase in miR-27 expression and associated decrease in Mstn expression during myogenic differentiation [45]. As Mstn is a potent negative regulator of myoblast differentiation [6], we speculate that the elevated miR-27 expression may function to inhibit Mstn expression thus allowing for myogenic differentiation to proceed. Although it is tempting to suggest that epigenetic mechanisms (such as miR-27) could be responsible for regulating Mstn expression during differentiation, we noted that the increase in miR-27 expression during differentiation might not be enough to account for the dramatic drop in Mstn expression observed. These data suggest that additional factors may play a role in inhibiting Mstn during differentiation. One likely candidate, that may be responsible for regulating Mstn expression during differentiation, is Smad7. Consistent with this, previously published work from our lab clearly demonstrates that Smad7 is able to inhibit Mstn expression [16]. Moreover, Kollias et al have previously demonstrated that the expression of Smad7 is elevated during differentiation and that over expression of Smad7 results in enhanced myogenic differentiation and rescue of Mstn-mediated inhibition of differentiation [46]. However, further work will need to be performed to confirm this. A role for miR-27 in regulating myogenic differentiation is not novel, in fact Crist et al recently demonstrated that miR-27b is able to negatively regulate Pax3 protein levels in adult muscle satellite cells to allow for timely entry into the myogenic differentiation program [40]. Therefore, taken together these data suggest that posttranscriptional regulation of Mstn mRNA by miR-27 plays a critical role in controlling timely tissue-specific expression/activity of Mstn during development.
Recently, we have shown that Smad3-null mice have elevated expression of Mstn and not surprisingly pronounced skeletal muscle atrophy [35]. Here we have investigated if miR-27a/b could be responsible for the increased Mstn expression observed in Smad3-null mice; and in agreement with increased Mstn expression we find reduced miR-27a and miR-27b expression in Smad3-null mice. More importantly, mimic-mediated over expression of miR-27b in Smad3-null mice was able to reduce Mstn expression back to levels comparable to WT mice. Therefore we speculate that loss of Smad3 leads to reduced miR-27a/b expression, which in turn increases Mstn mRNA stability and or translation leading to enhanced skeletal muscle wasting. These data, together with the fact that specific inhibitor of Smad3 (SIS3) treatment was able to significantly reduce miR-27a/b expression, suggest that Smad3 plays an important role in regulating endogenous miR-27a/b expression. Further support for Smad3 regulation of miR-27 is seen in published work from Sun et al, which revealed the presence of a Smad binding element in the miR-24-2/miR-23a/miR-27a cluster upstream regulatory sequence and that the Smad binding site was critical for TGF-β1-mediated inhibition of miR-24-2/miR-23a/miR-27a [31].
Interestingly, we now show for the first time that Mstn is able to up regulate the expression of miR-27a/b. Furthermore, Smad3 is critical for Mstn regulation of miR-27a/b as either mutation of the Smad binding site or treatment with SIS3 ablated the Mstn-mediated response. Mstn has been previously shown to negatively feedback to block its own expression/activity, through mechanisms involving inhibition of Mstn proteolytic processing and Smad7-dependent inhibition of Mstn expression [12], [16]. Here we now describe an independent mechanism through which Mstn regulates it's own expression. Specifically, addition of exogenous Mstn resulted in increased miR-27a/b expression, which in turn led to reduced Mstn 3′ UTR activity. This mechanism was dependent on miR-27a/b, as either blockade of miR-27a/b or mutation of the miR-27a/b binding site in the Mstn 3′ UTR prevented Mstn feedback regulation. These data, together with previously published work, suggest that there are independent auto-regulatory mechanisms through which Mstn regulates it's own activity; given the fact that Mstn is a potent negative regulator of skeletal muscle myogeneis, we speculate that such mechanisms are in place to allow for timely regulation of myogenesis.
In summary, we provide further evidence to support a role for miR-27 in regulating Mstn expression. Evidence suggests that miR-27a and miR-27b play an important role in controlling tissue-specific and muscle fiber type-specific expression of Mstn and regulating Mstn function during myogenesis. Furthermore, we now show that miR-27a/b forms the basis of a novel negative auto-regulatory mechanism through which Mstn inhibits it's own expression in muscle.
Supporting Information
AntagomiR-mediated inhibition of miR-27a/b and enhanced expression of Mstn . (A) qPCR analysis of miR-27a expression in C2C12 myoblasts following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). (B) qPCR analysis of miR-27b expression in C2C12 myoblasts following transfection of AntagomiR Neg or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). (C) qPCR analysis of Mstn expression in C2C12 myoblasts following transfection of AntagomiR Neg, AntagomiR-27a or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to GAPDH expression. p<0.05 (*) and p<0.01 (**). (D) qPCR analysis of Mstn expression in differentiated C2C12 myotubes following transfection of AntagomiR Neg, AntagomiR-27a or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to GAPDH expression. p<0.001 (***). (E) qPCR analysis of miR-27a expression in differentiated primary myoblast cultures from Mstn-null mice following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). qPCR analysis of miR-27a (F) and Mstn (G) expression in differentiated primary myoblast cultures from WT mice following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 (F) or GAPDH (G) expression. p<0.001 (***). (H) Upper panel: Representative immunofluorescence images showing Pax7+ cells (Green; white arrowheads) in an in vivo transfected TA muscle cross section from WT mice. Nuclei were counterstained with DAPI (Blue) and a Pax7/DAPI merged image is also shown. Scale bars = 10 µm. Lower panel: Representative immunofluorescence images showing MyoD+ cells (Red; white arrowheads) in an in vivo transfected TA muscle cross section from WT mice. Nuclei were counterstained with DAPI (Blue) and a MyoD/DAPI merged image is also shown. Scale bars = 100 µm.
(TIF)
Acknowledgments
Firstly we would like to thank Prof. Se-Jin Lee (Johns Hopkins University, USA) for gifting the Mstn −/+ mice (C57BL/6 background) and to Prof. Walter Wahli (University of Lausanne, Lausanne, Switzerland) for gifting the Smad3 −/− mice used. Thanks also to Dr Xiao Yang for providing the miR-27a and miR-27b promoter reporter constructs utilized in this present study. Further thanks to Isuru W. Wijesoma for help with confocal microscopy. The Pax7 monoclonal antibody developed by Dr Atsushi Kawakami was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Funding Statement
The work performed in this manuscript was supported by the following grants: Biomedical Research Council (BMRC; M4070097.080) http://www.a-star.edu.sg/AboutASTAR/BiomedicalResearchCouncil/tabid/64/Default.aspx, National Research Foundation (NRF; M4092014.0S4 CRP) http://www.nrf.gov.sg/nrf/default.aspx and intramural research funding (C08031) from Agency for Science, Technology and Research (A*STAR), Singapore http://www.a-star.edu.sg/Default.aspx. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90. [DOI] [PubMed] [Google Scholar]
- 2. Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, et al. (1999) Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol 180: 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Manickam R, Pena RN, Whitelaw CB (2008) Mammary gland differentiation inversely correlates with GDF-8 expression. Molecular reproduction and development 75: 1783–1788. [DOI] [PubMed] [Google Scholar]
- 4. Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7: 910–916. [DOI] [PubMed] [Google Scholar]
- 5. Thomas M, Langley B, Berry C, Sharma M, Kirk S, et al. (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275: 40235–40243. [DOI] [PubMed] [Google Scholar]
- 6. Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, et al. (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277: 49831–49840. [DOI] [PubMed] [Google Scholar]
- 7. McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, et al. (2005) Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118: 3531–3541. [DOI] [PubMed] [Google Scholar]
- 8. Lokireddy S, McFarlane C, Ge X, Zhang H, Sze SK, et al. (2011) Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol Endocrinol 25: 1936–1949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Lokireddy S, Mouly V, Butler-Browne G, Gluckman PD, Sharma M, et al. (2011) Myostatin promotes the wasting of human myoblast cultures through promoting ubiquitin-proteasome pathway-mediated loss of sarcomeric proteins. Am J Physiol Cell Physiol 301: C1316–1324. [DOI] [PubMed] [Google Scholar]
- 10. McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, et al. (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209: 501–514. [DOI] [PubMed] [Google Scholar]
- 11. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, et al. (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488. [DOI] [PubMed] [Google Scholar]
- 12. McFarlane C, Langley B, Thomas M, Hennebry A, Plummer E, et al. (2005) Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev Biol 283: 58–69. [DOI] [PubMed] [Google Scholar]
- 13. Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292: C188–199. [DOI] [PubMed] [Google Scholar]
- 14. Carlson CJ, Booth FW, Gordon SE (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol 277: R601–606. [DOI] [PubMed] [Google Scholar]
- 15. Spiller MP, Kambadur R, Jeanplong F, Thomas M, Martyn JK, et al. (2002) The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol Cell Biol 22: 7066–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, et al. (2006) Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol 206: 264–272. [DOI] [PubMed] [Google Scholar]
- 17. Lee Y, Jeon K, Lee JT, Kim S, Kim VN (2002) MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 21: 4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinez J, Tuschl T (2004) RISC is a 5′ phosphomonoester-producing RNA endonuclease. Genes Dev 18: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, et al. (2009) MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. The Journal of clinical investigation 119: 2772–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bell ML, Buvoli M, Leinwand LA (2010) Uncoupling of expression of an intronic microRNA and its myosin host gene by exon skipping. Molecular and cellular biology 30: 1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drummond MJ, Glynn EL, Fry CS, Dhanani S, Volpi E, et al. (2009) Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. The Journal of nutrition 139: 2279–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, et al. (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38: 813–818. [DOI] [PubMed] [Google Scholar]
- 23. Allen DL, Loh AS (2011) Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. American journal of physiology Cell physiology 300: C124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Z, Chen X, Yu B, He J, Chen D (2012) MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochemical and biophysical research communications 423: 265–269. [DOI] [PubMed] [Google Scholar]
- 25. Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, et al. (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689. [DOI] [PubMed] [Google Scholar]
- 26. McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, et al. (2008) Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res 314: 317–329. [DOI] [PubMed] [Google Scholar]
- 27. Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ (1989) A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci 92: 513–518. [DOI] [PubMed] [Google Scholar]
- 28. Zhang C, McFarlane C, Lokireddy S, Bonala S, Ge X, et al. (2011) Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 54: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 29. Partridge TA (1997) Tissue culture of skeletal muscle. Methods Mol Biol 75: 131–144. [DOI] [PubMed] [Google Scholar]
- 30. Picard V, Ersdal-Badju E, Lu A, Bock SC (1994) A rapid and efficient one-tube PCR-based mutagenesis technique using Pfu DNA polymerase. Nucleic Acids Res 22: 2587–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun Q, Zhang Y, Yang G, Chen X, Cao G, et al. (2008) Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic acids research 36: 2690–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Song Y, Zhang Y, Xiao H, Sun Q, et al. (2012) Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell research 22: 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, et al. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell 27: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ge X, McFarlane C, Vajjala A, Lokireddy S, Ng ZH, et al. (2011) Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res 21: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jinnin M, Ihn H, Tamaki K (2006) Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Molecular pharmacology 69: 597–607. [DOI] [PubMed] [Google Scholar]
- 37. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, et al. (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q, McPherron AC (2012) Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J Physiol 590: 2151–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SJ, Huynh TV, Lee YS, Sebald SM, Wilcox-Adelman SA, et al. (2012) Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A 109: E2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, et al. (2009) Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proceedings of the National Academy of Sciences of the United States of America 106: 13383–13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiao J, Yuan T, Zhou Y, Xie W, Zhao Y, et al. (2011) Analysis of myostatin and its related factors in various porcine tissues. J Anim Sci 89: 3099–3106. [DOI] [PubMed] [Google Scholar]
- 42. Sundaresan NR, Saxena VK, Singh R, Jain P, Singh KP, et al. (2008) Expression profile of myostatin mRNA during the embryonic organogenesis of domestic chicken (Gallus gallus domesticus). Res Vet Sci 85: 86–91. [DOI] [PubMed] [Google Scholar]
- 43. Deveaux V, Picard B, Bouley J, Cassar-Malek I (2003) Location of myostatin expression during bovine myogenesis in vivo and in vitro. Reprod Nutr Dev 43: 527–542. [DOI] [PubMed] [Google Scholar]
- 44. Xi G, Hathaway MR, Dayton WR, White ME (2007) Growth factor messenger ribonucleic acid expression during differentiation of porcine embryonic myogenic cells. Journal of animal science 85: 143–150. [DOI] [PubMed] [Google Scholar]
- 45. Chen X, Huang Z, Chen D, Yang T, Liu G (2013) The role of microRNA-27a in myoblast differentiation. Cell Biol Int [DOI] [PubMed] [Google Scholar]
- 46. Kollias HD, Perry RL, Miyake T, Aziz A, McDermott JC (2006) Smad7 promotes and enhances skeletal muscle differentiation. Mol Cell Biol 26: 6248–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AntagomiR-mediated inhibition of miR-27a/b and enhanced expression of Mstn . (A) qPCR analysis of miR-27a expression in C2C12 myoblasts following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). (B) qPCR analysis of miR-27b expression in C2C12 myoblasts following transfection of AntagomiR Neg or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). (C) qPCR analysis of Mstn expression in C2C12 myoblasts following transfection of AntagomiR Neg, AntagomiR-27a or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to GAPDH expression. p<0.05 (*) and p<0.01 (**). (D) qPCR analysis of Mstn expression in differentiated C2C12 myotubes following transfection of AntagomiR Neg, AntagomiR-27a or AntagomiR-27b. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to GAPDH expression. p<0.001 (***). (E) qPCR analysis of miR-27a expression in differentiated primary myoblast cultures from Mstn-null mice following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 expression. p<0.001 (***). qPCR analysis of miR-27a (F) and Mstn (G) expression in differentiated primary myoblast cultures from WT mice following transfection of AntagomiR Neg or AntagomiR-27a. Bars represent fold change (relative to AntagomiR Neg control) ± S.E.M (n = 3) normalized to U6 (F) or GAPDH (G) expression. p<0.001 (***). (H) Upper panel: Representative immunofluorescence images showing Pax7+ cells (Green; white arrowheads) in an in vivo transfected TA muscle cross section from WT mice. Nuclei were counterstained with DAPI (Blue) and a Pax7/DAPI merged image is also shown. Scale bars = 10 µm. Lower panel: Representative immunofluorescence images showing MyoD+ cells (Red; white arrowheads) in an in vivo transfected TA muscle cross section from WT mice. Nuclei were counterstained with DAPI (Blue) and a MyoD/DAPI merged image is also shown. Scale bars = 100 µm.
(TIF)