Abstract
Hematopoietic stem cells (HSCs) are enriched for aldehyde dehydrogenase (ALDH) activity and ALDH is a selectable marker for human HSCs. However, the function of ALDH in HSC biology is not well understood. We sought to determine the function of ALDH in regulating HSC fate. Pharmacologic inhibition of ALDH with diethylaminobenzaldehyde (DEAB) impeded the differentiation of murine CD34−c-kit+Sca-1+lineage− (34−KSL) HSCs in culture and facilitated a ninefold expansion of cells capable of radioprotecting lethally irradiated mice compared to input 34−KSL cells. Treatment of bone marrow (BM) 34−KSL cells with DEAB caused a fourfold increase in 4-week competitive repopulating units, verifying the amplification of short-term HSCs (ST-HSCs) in response to ALDH inhibition. Targeted siRNA of ALDH1a1 in BM HSCs caused a comparable expansion of radioprotective progenitor cells in culture compared to DEAB treatment, confirming that ALDH1a1 was the target of DEAB inhibition. The addition of all trans retinoic acid blocked DEAB-mediated expansion of ST-HSCs in culture, suggesting that ALDH1a1 regulates HSC differentiation via augmentation of retinoid signaling. Pharmacologic inhibition of ALDH has therapeutic potential as a means to amplify ST-HSCs for transplantation purposes.
Introduction
Hematopoietic stem cell (HSC) transplantation involves the administration of myeloablative radiotherapy and/or chemotherapy followed by infusion of CD34+ hematopoietic stem/progenitor cells [1–3]. Despite a sufficient number of long-term HSCs within the donor graft, a 2–4 week period of pancytopenia typically follows this procedure, during which time patients require antibiotics and transfusion support [1–4]. In the setting of cord blood (CB) transplantation, hematologic recovery can lag for up to 2 months after transplant, putting recipients at risk for life-threatening infectious complications [1–5]. The period of pancytopenia that occurs after transplantation results from the ablative effects of the conditioning on host hematopoiesis coupled with the inherent lag time to the production of donor HSC-derived progeny [6–8]. Since the prolonged period of pancytopenia after CB transplantation accounts for substantial morbidity and mortality, it has been proposed that co-transplantation of committed myeloid progenitor cells or short-term HSCs (ST-HSCs) could accelerate hematologic recovery in these patients and perhaps lessen the mortality of this procedure [6, 7, 9].
Studies in mice have defined the phenotype of long-term HSCs (LT-HSCs) and ST-HSCs [6, 7]. Bone marrow (BM) cells that are depleted of lineage markers, express c-kit+ and Sca-1+ and lack CD34 expression (CD34−c-kit+Sca-1+lineage− [34-KSL] cells) or express Thy 1.1lo, are enriched for LT-HSCs [6, 7], whereas 34+KSL cells are enriched for ST-HSCs, which have a more restricted self-renewal capacity and repopulating ability [6, 7, 10, 11]. It has been shown that the 34+Flt-3-KSL subset is enriched for virtually all ST-HSCs and these cells give rise to 34+Flt-3+KSL cells, which are primarily lymphoid repopulating cells [6]. Although LT-HSCs are required for long-term hematopoietic reconstitution, these cells are substantially less efficient than ST-HSCs at providing radioprotection of lethally irradiated recipients [7, 10, 11]. It has also been shown that transplantation of committed megakaryocyte-erythroid progenitors (MEPs) or common myeloid progenitors (CMPs) can provide radioprotection in lethally irradiated mice in the absence of HSC transplantation [7]. Importantly, transplantation of as few as 500 34+Flt-3-KSL cells was able to radioprotect a fraction of lethally irradiated mice, whereas 50,000 MEPs or CMPs were required to achieve a comparable level of radioprotection [6, 7]. Taken together, these data suggest that co-transplantation of ST-HSCs could be an efficient method to lessen the morbidity and mortality of HSC transplantation.
We recently showed that inhibition of aldehyde dehydrogenase (ALDH) facilitated the expansion of human hematopoietic cells capable of repopulation in NOD/SCID mice, suggesting that ALDH promotes HSC differentiation [12]. In this study, we sought to more precisely determine which cell types within the HSC hierarchy are regulated by ALDH, the ALDH isoform responsible for regulating HSC differentiation and the mechanism through which ALDH regulates HSC differentiation. Since the NOD/SCID assay is limited in discriminating short-term and long-term HSCs, radioprotective cell content, and complete multilineage repopulation [13], we have performed extensive studies in congenic mice to measure the effect of pharmacologic and genetic inhibition of ALDH on HSC content and function. Inhibition of ALDH in the presence of cytokines impeded HSC differentiation in culture and induced a significant expansion of both radioprotective hematopoietic progenitor cells and ST-HSCs compared to input BM 34-KSL cells. Therefore, ALDH plays an important role in regulating the transition of ST-HSCs to committed progenitor cells and inhibition of ALDH has potential as a means to amplify ST-HSCs for therapeutic purposes.
Materials and Methods
Isolation of BM HSCs and ALDH Activity Assay
C57BL6 and congenic B6.SJL-Ptprca Pep3b/BoyJ (B6.SJL) mice (The Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were utilized in experiments approved by the Duke University Animal Care and Use Committee. Whole BM cells were collected from 8–10 week old animals as previously described [14, 15]. BM mononuclear cells (MNC) were isolated via density centrifugation and lineage-marker negative (Lin−) cells were then enriched via magnetic column purification (Miltenyi Biotec, Auburn, CA, Germany, http://www.miltenyibiotec.com), according to the manufacturer’s guidelines. Multiparameter flow cytometry was conducted to isolate purified CD34−c-kit+Sca-1+lin− (34-KSL) or KSL subsets as previously described [14, 15].
ALDH activity assays of murine and human cells were performed using the Aldefluor kit (Stem Cell Technologies, Vancouver, BC, Canada, http://www.stemcell.com), according to the manufacturer’s recommended procedure, followed by immunophenotyping with PE-Sca-1, APC-c-kit, and an APC-anti-lineage marker cocktail. All antibodies were purchased from Becton Dickinson (BD Biosciences, San Diego, http://www.bdbiosciences.com), unless otherwise noted. Data were acquired using a FACScalibur or FACScanto II flow cytometer (BD Biosciences).
In Vitro Cultures of BM HSCs
Purified murine BM 34-KSL or KSL cells were seeded at 0.2–1.0 × 104 cells per milliliter in culture with Iscove’s modified Dulbecco’s medium + 10% fetal bovine serum (FBS) + 1% penicillin/streptomycin (pcn/strp), containing 120 ng/ml murine stem cell factor (SCF), 50 ng/ml murine fms-like tyrosine kinase-3 (Flt-3) ligand, and 20 ng/ml murine thrombopoietin (“TSF”; R&D Systems Inc., Minneapolis, http://www.rndsystems.com), with or without 100 μM diethylaminobenzaldehyde (DEAB) (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) for 7 days at 37°C in 5% CO2. Retinaldehyde (retinal; Sigma-Aldrich) was added at 1 μM to certain cultures. All trans retinoic acid (ATRA) (Sigma-Aldrich) was added to a subset of cultures of BM 34-KSL cells with TSF + DEAB. At day 7, viable cell counts were obtained and immunophenotypic analysis for KSL subsets was performed as previously described [14, 15]. Methods for immunophenotypic analyses and cell cycle analysis [16] are provided in the supporting information.
In Vivo Radioprotection and Competitive Repopulating Assays
For the lethal radioprotection assay, mice received 950-cGy total body irradiation (TBI) and subsequently were transplanted with either 10, 30, or 100 BM 34-KSL donor cells or their progeny after culture with TSF alone or TSF + DEAB. Kaplan-Meier analysis of the survival data was analyzed using Prism 4 software (GraphPad Software Inc., San Diego, http://www.graphpad.com).
For the competitive repopulating assays (CRU), BM 34-KSL cells from B6.SJL mice (CD45.1+) were sorted into 96-well U-bottom plates (BD Biosciences). Day 0 34-KSL cells were either injected into recipient animals or placed into cultures containing TSF with or without DEAB, as described above. Recipient C57BL6 animals (CD45.2+) were irradiated with 950-cGy TBI using a Cs137 irradiator and then injected, via tail vein, with 10, 30, or 100 34-KSL cells or their progeny after culture with TSF alone or TSF + DEAB. 1 × 105 nonirradiated CD45.2 MNCs were co-injected into recipient mice as competitor cells. Donor-derived hematologic reconstitution was monitored in the peripheral blood by flow cytometry, as previously described [15, 17], at weeks 4, 8, 12, and 30 after transplant. Animals were considered to be engrafted if donor CD45.1+ cells were present at ≥1% for all lineages [15, 17]. Radioprotective cell frequency and CRU calculations were performed using L-Calc software (Stem Cell Technologies) [15, 17, 18].
siRNA of ALDH1a1 in HSCs
KSL cells, which were sorted via FACS, from C57BL/6J mice were cultured in 96-well U-bottom plates containing Accell Media (Thermo Fisher Scientific, part of Thermo Fisher Scientific, Chicago, http://www.thermo.com) with 120 ng/ml SCF, 50 ng/ml Flt-3 ligand, 20 ng/ml thrombopoietin, and 1 μM ALDH1a1-specific or nontargeting siRNA construct (Thermo Fisher Scientific, Waltham, MA) for 96 hours, according to the manufacturer’s recommended protocol. At 96 hours, 10% FBS was added and the cultures were carried to day 7. To assess ALDH1a1 expression, total RNA from day 0 BM KSL cells or their siRNA-treated progeny was isolated, reverse-transcribed, and polymerase chain reaction (PCR) amplification reactions were performed as previously described [12]. To measure the effect of siRNA of ALDH1a1 on BM radioprotective cell frequency, C57BL6 mice were irradiated with 950-cGy TBI and subsequently transplanted via tail vein injection with day 0 BM KSL cells or the progeny of BM KSL cells treated with ALDH1a1 siRNA or nontargeting construct. Overall survival was monitored through day +40.
Statistical Analysis
Student’s t tests were performed for comparisons of all results from in vitro cultures. Kaplan-Meier estimates were performed to compare the overall survival between groups of mice transplanted with radioprotective cells and siRNA-treated cells [14, 15]. The limiting dilution method of Taswell was applied and Poisson statistical analysis was performed to estimate the CRU frequencies between groups of mice [12, 19, 20].
Results
Treatment with DEAB Inhibits ALDH Activity and Blocks Retinoid Signaling in HSCs
DEAB is a competitive antagonist of ALDH [21–24]. To confirm that DEAB inhibited ALDH activity in BM HSCs, we examined ALDH activity using the Aldefluor reagent within day 0 BM KSL cells versus the progeny of BM KSL cells treated for 7 days with cytokines (thromobopoietin, SCF, Flt-3 ligand; “TSF”) or the progeny of BM KSL cells treated with TSF + 100 μM DEAB (Fig. 1A, 1B). We were unable to detect a distinct ALDH+ population within the BM KSL subset or within the progeny of TSF or TSF + DEAB cultures at 24 hours, day 3, or day 7 (Fig. 1B). Therefore, we analyzed human cord blood (CB) CD34+CD38-lin− HSCs to determine whether DEAB treatment decreased ALDH activity in human HSCs. The majority (mean 72.9%) of day 0 CD34+CD38-lin− cells were ALDH bright, as were 41% of the TSF-treated progeny. However, only 2.5% of the progeny of TSF + DEAB cultures demonstrated ALDH activity, verifying that DEAB treatment inhibited ALDH activity in human HSCs during culture (Fig. 1B, 1C). Interestingly, the expression of ALDH1a1 also decreased >50% in response to TSF + DEAB compared to TSF alone (supporting information Fig. S1).
Figure 1.
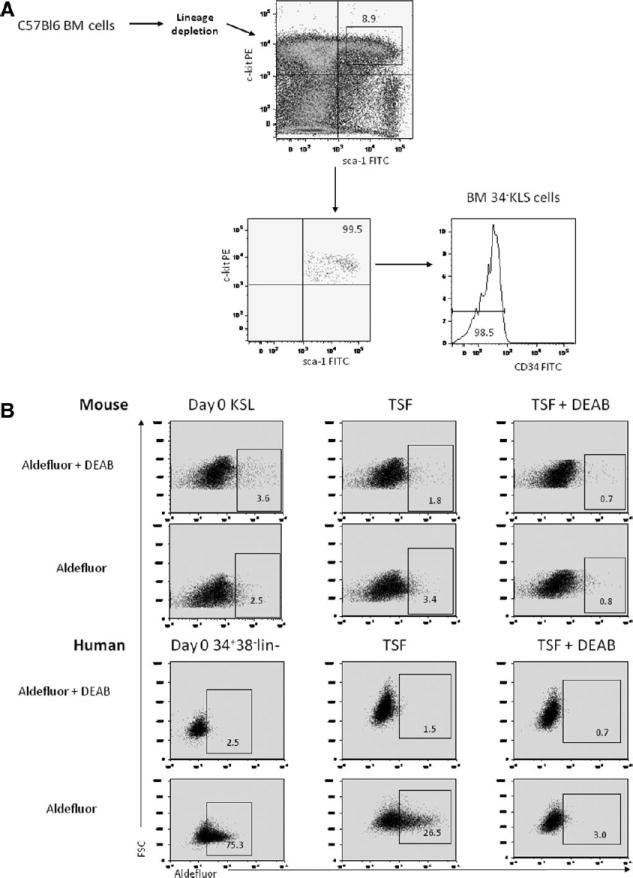
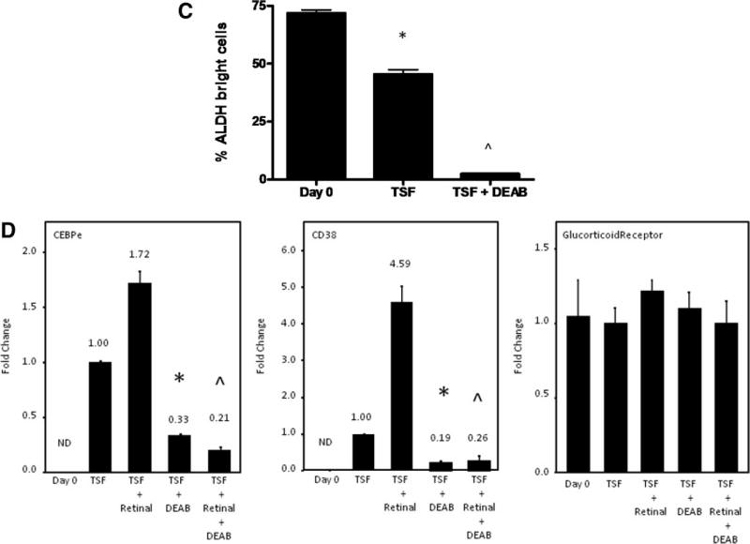
DEAB inhibits ALDH enzyme activity and blocks retinoid signaling in HSCs. (A): FACS-based strategy for isolation of BM CD34-KSL cells is shown. Whole BM cells from adult C57Bl6 mice were lineage-depleted and subsequently stained with antibodies to c-kit, Sca-1, and CD34 and the CD34-KSL population was collected by FACS. (B): Representative flow cytometric analysis using the Aldefluor reagent was performed on murine BM KSL cells at day 0 and after 7 days of culture with TSF versus TSF + DEAB (top). Identical analysis was performed on human CB CD34+CD38-lin− cells under the same conditions (bottom). (C): Treatment of BM 34-KSL cells with TSF + DEAB caused a significant reduction in ALDH-positive cells in culture (means ± SD, n = 3; *, p = .02 vs. day 0;∧, p = .01 vs. day 0, p = .02 vs. TSF). (D): The expression of CEPBε and CD38, RAR-dependent genes, is shown in murine BM 34-KSL cells at day 0 and after culture with TSF, TSF + DEAB, TSF + retinal, and TSF + retinal + DEAB. Treatment with DEAB blocked cytokine-induced (TSF, SCF, Flt-3 ligand) and retinal-induced expression of CEBPε (*, p < .001; ∧, p < .001) and CD38 (*, p < .001; ∧, p < .001) in BM HSCs. Neither retinal nor DEAB affected the expression of the glucocorticoid receptor, a non-RAR-dependent gene (n = 3, means ± SD). Numbers represent the fold change relative to expression in the TSF group. Abbreviations: 34-KSL, CD34−c-kit+Sca-1+lineage−; ALDH, aldehyde dehydrogenase; BM, bone marrow; CB, cord blood; DEAB, diethylaminobenzaldehyde; Flt-3, fms-like tyrosine kinase-3; HSCs, hematopoietic stem cells; ND, not detected; RAR, retinoic acid receptor; SCF, stem cell factor; TSF, thrombopoietin, stem cell factor, Flt-3 ligand.
Microarray analyses have suggested that several isoforms of ALDH may be expressed by HSCs, including ALDH1a1, ALDH2, ALDH1a7, ALDH3a2, and ALDH9a1 [25–27]. Using quantitative reverse transcriptase (qRT)-PCR analysis, we found that ALDH1a1, which catalyzes retinoic acid biosynthesis, and ALDH2, ALDH1a7, ALDH3a2 and ALDH9a1 were expressed by murine BM 34-KSL cells (supporting information Fig. S2). ALDH1a2, ALDH1a3, and ALDH8a1, which also catalyze retinoic acid synthesis, were not expressed. Since ALDH1a1 has retinaldehyde (retinal) metabolizing activity and the other expressed isoforms have little (ALDH2) or no retinal metabolizing activity (ALDH1a7, ALDH3a2, and ALDH9a1) [25, 28], we treated BM 34-KSL cells with retinal + DEAB to determine if ALDH1a1 was a primary target of DEAB action. We measured expression of CEBPε and CD38, which are retinoic acid receptor (RAR)- target genes [29–31], in the BM 34-KSL cells since the expression of CEBPε and CD38 increases in response to retinoic acid signaling. CEBPε expression increased in response to TSF and further increased in response to retinal (Fig. 1D). In contrast, when DEAB was added to BM 34-KSL cell cultures with TSF or TSF + retinal, CEBPε expression was significantly reduced (Fig. 1D). The expression of CD38 also increased significantly in response to TSF + retinal. When DEAB was added to TSF + retinal, CD38 expression was reduced 18-fold (Fig. 1D). Conversely, expression of the glucocorticoid receptor, which is not RAR-dependent, did not change significantly in BM 34-KSL cells in response to retinal or retinal + DEAB. Taken together, these data confirmed that DEAB treatment antagonized retinoid signaling in HSCs and suggested that ALDH1a1, which has retinal-metabolizing activity, was a primary target of DEAB action. Of note, the progeny of HSCs treated with TSF + DEAB also demonstrated significantly reduced expression of Mac-1 (myeloid) and B220 (B cell) differentiation markers compared to the progeny of TSF alone (supporting information Fig. S3). These results confirmed the inhibitory effect of DEAB on HSC differentiation in culture but also raised the possibility that other differentiation pathways independent of ALDH and RAR were antagonized by DEAB.
Inhibition of ALDH Maintains Phenotypic and Functional Progenitor Cells in Culture
To determine the effect of ALDH inhibition on HSC content in culture, BM 34-KSL cells were cultured with TSF with or without 100 μM DEAB for 7 days. Treatment of 34-KSL cells with TSF caused a significant decline in lineage negative cells at day 7 (mean 42.6%), whereas the progeny of TSF + DEAB contained more than twofold increased percentage of lineage negative cells compared to that of TSF alone (97.1%; Fig. 2A, 2B). Moreover, treatment with TSF + DEAB maintained a more than sevenfold higher percentage of KSL cells in culture as compared to culture with TSF alone (mean 14.2% vs. 2.0%; Fig. 2A, 2B). However, culture with TSF alone caused a 360-fold expansion of total cells whereas culture with TSF + DEAB supported a 22.6-fold expansion (Fig. 2B). Therefore, the total number of KSL cells increased 2.6-fold in TSF cultures compared to TSF + DEAB cultures at day 7 (Fig. 2B). Of note, the progeny of TSF + DEAB cultures contained 16-fold increased numbers of colony-forming cells as compared to the progeny of TSF alone, demonstrating that inhibition of ALDH impeded the differentiation of functional BM progenitor cells in culture (Fig. 2C).
Figure 2.
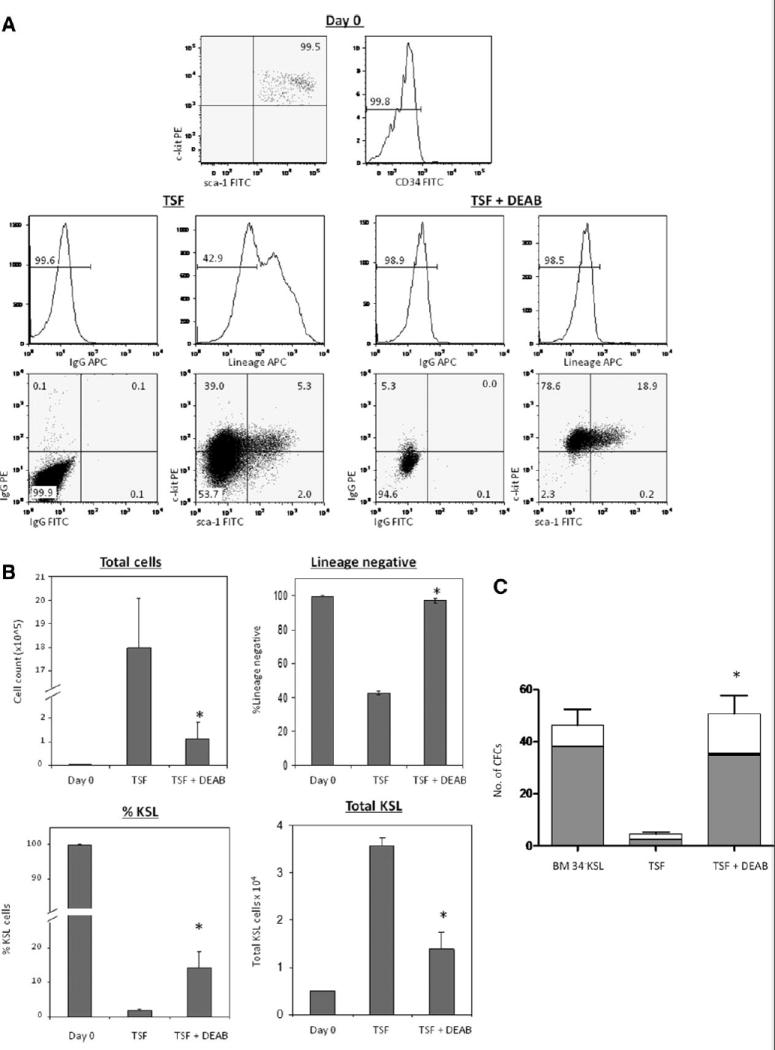
Inhibition of ALDH increases the frequency of phenotypic and functional BM progenitor cells in culture. (A): Representative flow cytometric analyses are shown of day 0 BM 34-KSL cells (top) and the progeny of 34-KSL cells after 7-day culture with TSF alone (left) and TSF + DEAB (right). Note that the progeny of TSF + DEAB remain lineage negative at day +7, with increased KSL content compared to the progeny of TSF alone. (B): The addition of DEAB to cultures of 34-KSL cells caused a significant reduction in total cell expansion compared to TSF alone (upper left, *, p = .001), but yielded a significant increase in the percentage of lineage negative progenitor cells (upper right, *, p < .001) and percentage of KSL cells compared to treatment with TSF alone (lower left, *, p = .01) (n = 3–5, means ± SD). The absolute number of KSL cells was 2.6-fold increased in TSF cultures compared to TSF + DEAB (lower right, *, p = .001) (n = 3–5, means ± SD). (C): Inhibition of ALDH preserves CFC content in cultures of BM 34-KSL cells. CFC assays were performed using day 0 BM 34-KSL cells or their progeny after 7-day culture with TSF alone or TSF + DEAB. The mean number of CFU total was 16-fold increased in the TSF + DEAB cultures compared to TSF alone. *, p = .009 for difference between CFCs in TSF + DEAB versus TSF alone (n = 3, means ± SD). Gray bar indicates CFU-GM, black bar indicates BFU-E, and white bar indicates CFU-Mix. Abbreviations: ALDH, aldehyde dehydrogenase; BFU-E, burst forming unit-erythroid; BM, bone marrow; 34-KSL, CD34−c-kit+Sca-1+lineage−; CFC, colony forming cell; CFU, colony-forming unit; CFU-GM, colony forming unit-granulocyte monocyte; CFU-Mix, colony forming unit-mix; DEAB, diethylaminobenzaldehyde; TSF, thrombopoietin.
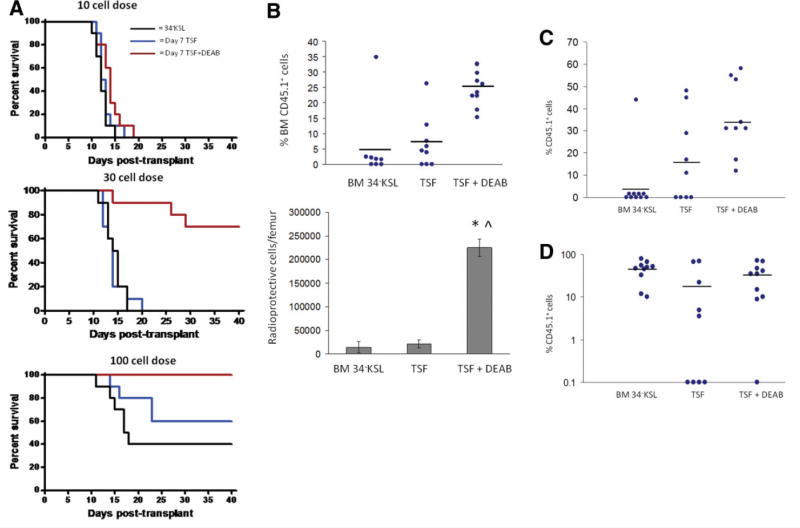
Inhibition of ALDH Facilitates the Expansion of Radioprotective Cells
We next sought to determine whether inhibition of ALDH could increase the number of BM stem/progenitor cells with in vivo radioprotective capacity. We transplanted B6.SJL (CD45.1+) day 0 BM 34-KSL cells (10, 30, or 100 cells) or their progeny after 7-day culture with TSF alone or TSF + DEAB, into lethally irradiated, congenic C57BL6 (CD45.2+) recipient mice. At the 10-cell dose, no mice survived beyond day +19 in any of the three groups (Fig. 3A). At the 30-cell dose, no mice transplanted with day 0 BM 34-KSL cells or the progeny of 34-KSL cells cultured with TSF alone survived past day +20. In contrast, 70% of the mice transplanted with the progeny of TSF + DEAB survived beyond day +40 (p < .0001 and p < .0001 vs. day 0 and TSF groups). At the 100-cell dose, 40% of mice transplanted with day 0 34-KSL cells and 60% of mice transplanted with the progeny of TSF alone survived after lethal irradiation, compared to 100% of animals transplanted with DEAB-cultured progeny (p = .004, p = .03; Fig. 3A). Applying Poisson statistics, we estimated the radioprotective cell frequency within the day 0 BM 34-KSL population to be 1 in 297 cells [95% confidence interval (CI): 1:113–1:781], whereas it was 1 in 179 [CI: 1:82–1:391] for the progeny of 34-KSL cells cultured with TSF alone. In contrast, the radioprotective cell frequency within the progeny of 34-KSL cells cultured with TSF + DEAB was 1 in 33 cells [CI: 1:19–1:57], representing a nine- and sixfold increase in radioprotective cell frequency compared to day 0 34-KSL cells and the progeny of cultures with TSF alone (Table 1). Importantly, the progeny of TSF cultures contained 15.9-fold more total cells than TSF + DEAB-cultured progeny. Therefore, when normalized to the total cell numbers transplanted into recipient mice, the progeny of TSF + DEAB had a 96-fold (15.9 × 6-fold) increased radioprotective cell frequency compared to the progeny of TSF alone. If normalized to total KSL progenitor cells infused per mouse, the progeny of TSF + DEAB had a 14-fold increased frequency of radioprotective cells compared to TSF alone.
Figure 3.
Inhibition of ALDH expands ST-HSCs with radioprotective capacity. (A): Kaplan-Meier analysis of the survival of lethally irradiated (950-cGy total body irradiation (TBI)) adult C57BL6 mice transplanted with 10, 30, or 100 BM 34-KSL cells from B6.SJL donors (black line) or the progeny of the same dose of BM 34-KSL cells after 7-day culture with TSF alone (blue line) or TSF + DEAB (red line) is shown (30-cell dose: p < .0001 and p < .0001 for TSF + DEAB group vs. day 0 and TSF groups; 100-cell dose: p = .004, p = .03 for TSF + DEAB vs. day 0 and TSF groups; n = 10 mice per dose per condition). (B): (Top) Lethally irradiated mice transplanted with the progeny of BM 34-KSL cells cultured with TSF + DEAB have higher levels of donor CD45.1+ cell engraftment (blue dots) in the BM at day +14 than mice transplanted with day 0 BM 34-KSL cells or their progeny after culture with TSF alone (mean 25.0% ± 1.9 donor CD45.1+ cells vs. 5.4% ± 4.9% vs. 6.8% ± 1.7% at day +14; p = .0008 and p < .0001 vs. day 0 and TSF group; n = 10 mice per condition; horizontal bars represent mean engraftment). (Bottom) Mice transplanted with the progeny of BM 34-KSL cells treated with TSF + DEAB have significantly increased numbers of donor CD45.1+ radioprotective cells per femur (% CD45.1+ cells × total BM cells) at day +14 compared to mice transplanted with day 0 BM 34-KSL cells or the progeny of TSF cultures (mean 224,862 CD45.1+ cells per femur vs. 14,562 cells per femur vs. 21,577 cells per femur; *, p < .0001 vs. day 0; ∧, p < .0001 vs. TSF group; means ± SD, n = 8–10 per group). (C): Inhibition of ALDH causes an expansion of short-term CRU. A scatter plot is shown of engraftment of donor CD45.1+ cells (blue dots) at 4 weeks in the PB of lethally irradiated CD45.2+ recipient mice after competitive repopulating assay (100-cell dose) with day 0 BM 34-KSL cells or their progeny after 7-day culture with TSF or TSF + DEAB. Horizontal bars represent mean levels of engraftment. (D): Inhibition of ALDH does not facilitate the expansion of LT-HSCs. A representative scatter plot is shown demonstrating peripheral blood (PB) donor CD45.1+ cell engraftment at 12 weeks in lethally irradiated CD45.2+ mice after transplantation of 100 donor BM34-KSL cells or their progeny after 7-day culture with TSF or TSF + DEAB. Horizontal bars represent mean levels of engraftment. Abbreviations: ALDH, aldehyde dehydrogenase; BM, bone marrow; 34-KSL, CD34−c-kit+Sca-1+lineage−; CRU, competitive repopulating assay; DEAB, diethylaminobenzaldehyde; HSCs, hematopoietic stem cells; LT-HSCs, hematopoietic stem cells; ST-HSCs, short-term hematopoietic stem cells; TSF, thrombopoietin.
Table 1.
Inhibition of ALDH1 increases the radioprotective cell frequency
| Condition | Cell dose | No. surviving | radioprotective cell (RPC) estimate | 95% CI |
|---|---|---|---|---|
| 34−KSL | 10 | 0:10 | 1 in 297 | 113–781 |
| 30 | 0:10 | |||
| 100 | 4:10 | |||
| TSF | 10 | 0:10 | 1 in 179 | 82–391 |
| 30 | 0:10 | |||
| 100 | 6:10 | |||
| TSF + DEAB | 10 | 0:10 | 1 in 33 | 19–57 |
| 30 | 7:10 | |||
| 100 | 10:10 |
FACS-sorted bone marrow 34-KSL cells were transplanted into lethally irradiated C57BI6 mice (950-cGy total body irradiation (TBI)) via tail vein injection of 10, 30, or 100 cells (n = 10 mice per dose). The identical numbers of 34-KSL cells were placed in culture with TSF, stem cell factor, and Flt-3 ligand or TSF + 100 μM DEAB x 7 days and their progeny were transplanted into lethally irradiated mice as described in Materials and Methods. A limiting dilution analysis demonstrated that the radioprotective cell content was ninefold increased in the progeny of TSF + DEAB cultures compared to input 34-KSL cells and sixfold increased compared to the progeny of culture with TSF alone.
Abbreviations: 34-KSL, CD34−c-kit+ Sca-1+ lineage−; CI, confidence interval; DEAB, diethylaminobenzaldehyde; TSF, thrombopoietin.
To directly demonstrate the increased radioprotective cell frequency in the progeny of TSF + DEAB cultures versus the progeny of TSF alone, we measured donor CD45.1+ cell engraftment in the BM of lethally irradiated mice at day +14 after transplantation of a limiting dose (100 cells) of BM 34-KSL cells or their progeny after culture with TSF alone or TSF + DEAB (Fig. 3B). Mice transplanted with the progeny of TSF + DEAB cultures had 4.6-fold increased donor CD45.1+ cell engraftment in the BM at day 14 compared to mice transplanted with day 0 BM 34-KSL cells and 3.8-fold increased engraftment compared to mice transplanted with the progeny of TSF alone (mean 25.0% donor CD45.1+ cells vs. 5.4% vs. 6.8% at day +14; p = .0008 and p < .0001 vs. day 0 and TSF group; Fig. 3B). Since mice transplanted with the progeny of TSF + DEAB had 3.6- and 2.3-fold increased total BM cells at day +14, this translated into a 15.4-fold increase in the number of donor CD45.1+ radioprotective cells per femur in mice transplanted with the progeny of TSF + DEAB cultures versus mice transplanted with day 0 BM 34-KSL cells and a 10.4-fold increase compared to mice transplanted with the progeny of TSF alone (mean 224,862 CD45.1+ cells per femur vs. 14,562 cells per femur vs. 21,577 cells per femur; p < .0001 and p < .0001 vs. day 0 and TSF group; Fig. 3B).
Inhibition of ALDH Expands ST-HSCs with Competitive Repopulating Capacity
Since inhibition of ALDH increased the number of BM radioprotective cells in culture, we sought to determine if this was caused by an amplification of short-term HSCs (ST-HSCs) with competitive repopulating capacity. BM 34-KSL cells from B6.SJL mice (CD45.1+) or their progeny after culture with TSF alone or TSF + DEAB were transplanted at limiting doses in a competitive repopulating assay into lethally irradiated CD45.2+ C57BL6 mice. At 4 weeks after transplant, mice transplanted with the progeny of 100 BM 34-KSL cells cultured with TSF + DEAB demonstrated significantly increased CD45.1+ cell multilineage engraftment (mean 34.0% CD45.1+) compared to mice transplanted with the same dose of day 0 34-KSL cells (mean 4.4%) or their progeny after culture with TSF alone (mean 16.7%) (Fig. 3C). Transplantation of mice over a range of limiting doses (10–200 cells) coupled with Poisson statistical analysis demonstrated the frequency of 4-week CRU within day 0 BM 34-KSL cells to be 1 in 147 cells (95% CI: 1:91–1:238) and 1 in 156 cells for the progeny of TSF cultures (95% CI: 1:95–1:258) (supporting information Table S1). Conversely, the CRU frequency within the progeny of TSF + DEAB cultures was 1 in 39 cells (95% CI: 1:25–1:62). Therefore, the progeny of TSF + DEAB cultures contained 3.8- and 4-fold increased numbers of ST-HSCs compared to day 0 34-KSL cells and the progeny of 34-KSL cells cultured with TSF alone, respectively.
To measure the effect of ALDH inhibition on long-term HSC (LT-HSC) content in culture, we also performed CRU analysis at 12 and 30 weeks after transplantation (Fig. 3D). Mice transplanted with day 0 BM 34-KSL cells displayed increasing donor 45.1+ cell repopulation over time and the CRU estimates were 1 in 17 and 1 in 20 cells at 12 and 30 weeks, respectively (supporting information Table S2). The CRU estimates within the progeny of TSF + DEAB culture were 1 in 56 and 1 in 67 cells at 12 and 30 weeks, respectively. Similarly, the CRU estimates for the progeny of TSF alone were 1 in 61 and 1 in 70 cells at 12 and 30 weeks, respectively. These data demonstrate that inhibition of ALDH activity does not amplify LT-HSCs in culture compared to day 0 BM 34-KSL cells.
As a complement to competitive repopulating assays in mice, we also examined the effect of DEAB treatment on the short-term engraftment potential of human CB CD34+CD38-lin− cells in a NOD/SCID transplantation assay. Mice transplanted with 2.5 × 103 CB CD34+38-lin− HSCs demonstrated human CD45+ cell engraftment at 4 weeks in 3 of 12 mice (25%; mean 0.9% huCD45+ BM cells). Similarly, mice transplanted with the progeny of the identical dose of CD34+38-lin− cells after culture with TSF demonstrated engraftment in 2 of 10 mice (20%; mean 0.7% huCD45+ cells). In contrast, mice transplanted with the progeny of CB CD34+CD38-lin− cells after culture with TSF + DEAB demonstrated human CD45+ cell engraftment in 5 of 10 mice (50%; mean 1.4% huCD45+ cells). These results suggested that treatment with DEAB enhanced the short-term repopulating activity of human HSCs. This observation is also consistent with our prior observation that inhibition of ALDH caused an expansion of human LT-HSCs as measured in primary and secondary transplanted NOD/SCID mice [12].
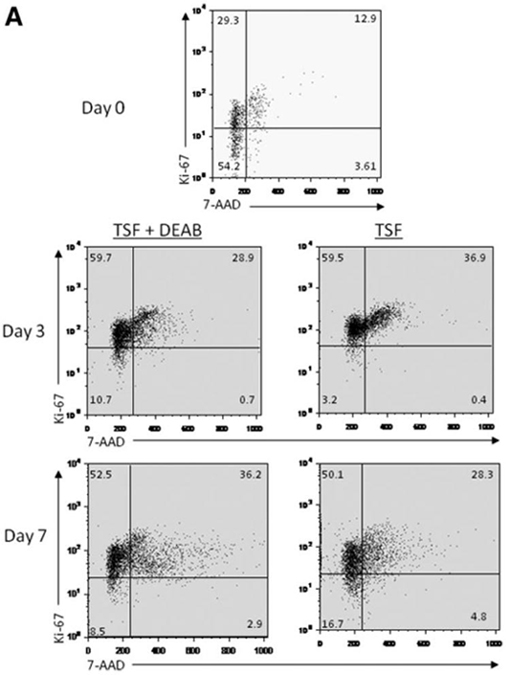
Inhibition of ALDH Delays Cell Cycle Progression of HSCs in Culture
The repopulating capacity of HSCs has been shown to decline in association with transit through cell cycle in vitro [32, 33]. We sought to determine whether inhibition of ALDH increased BM stem/progenitor cell repopulating capacity via modulation of cell cycle progression of HSCs in culture. Day 0 BM KSL cells were mostly quiescent (50.4% in G0, 26.2% in G1). After 3 days in culture, the progeny of TSF cultures were primarily in cycle, whereas the progeny of TSF + DEAB showed a modest increase in the percentage of cells in G0 and a decrease in cells in the G2/S/M phase (Fig. 4A; p = .004 for G0 comparison, p = .02 for G2/S/M comparison). After 7 days in culture, the progeny of TSF + DEAB culture demonstrated an increase in cell cycling compared to the progeny of TSF alone (Fig. 4A; p = .01 and p = .02 for G0 and G2/S/M phase, respectively), perhaps reflecting replicative senescence in TSF-treated cells at day 7 as compared to the progeny of TSF + DEAB. The mean frequency of day 0 KSL cells and their progeny after culture with TSF and TSF + DEAB in each phase of the cell cycle is shown in supporting information Fig. S5.
Figure 4.
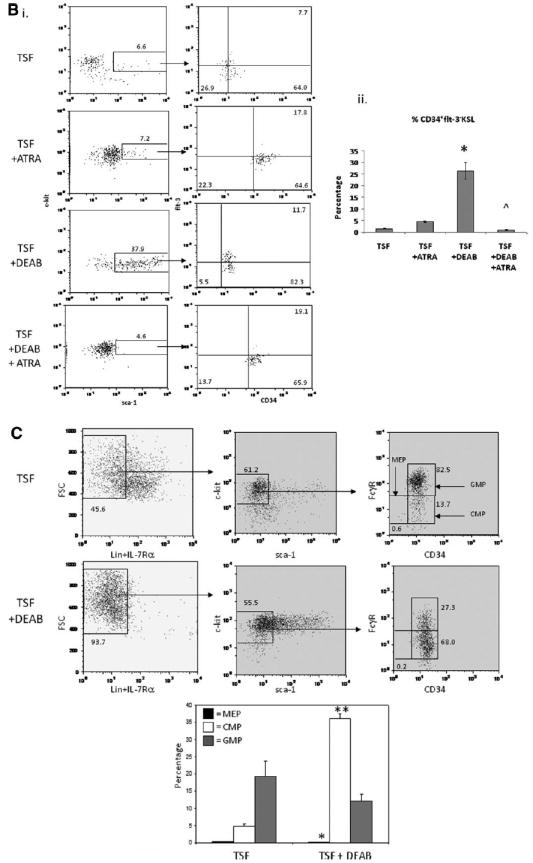
Antagonism of ALDH inhibits cell cycle progression of HSCs and increases the frequency of 34+Flt-3-KSL cells in culture compared to cytokines alone. (A): A representative cell cycle analysis of 34-KSL cells and their progeny after 7-day cultures with TSF alone or TSF + DEAB is shown. The majority (mean 83.5%) of day 0 34-KSL cells were in G0/G1. At day +3, the progeny of TSF + DEAB cultures demonstrated a modest increase in cells in G0 and decreased numbers in G2/S/M phase, consistent with overall inhibition of cell cycle progression compared to TSF alone. At day +7, the progeny of TSF cultures demonstrated increased numbers of cells in G0 compared to TSF + DEAB. (B): (i) Representative FACS analyses of 34+Flt-3-KSL cells in culture of BM 34-KSL cells with TSF, TSF + 1 μM ATRA, TSF + DEAB, or TSF + DEAB + ATRA are shown. (ii) Treatment with TSF + DEAB supported an increase in the frequency of 34+Flt-3-KSL cells in culture compared to culture with TSF alone (*, p = .003, n = 3, mean ± SD). The addition of ATRA reversed the effects of DEAB on ST-HSC expansion in culture (∧p = .003, n = 3; mean ± SD). (C): Representative FACS analyses of CMP and MEP content are shown for BM 34-KSL cells after culture with TSF (top) or TSF + DEAB (middle). The progeny of TSF + DEAB cultures contained an increased frequency of CMPs compared to the progeny of TSF alone (mean 36.1% vs. 4.8%, **, p < .001) and a decreased frequency of MEPs (mean 0.1% vs. 0.3%, respectively; *, p = .01) (n = 6; means ± SD) (bottom). Abbreviations: ALDH, aldehyde dehydrogenase; ATRA, All trans retinoic acid; 34-KSL, CD34−c-kit+Sca-1+lineage−; CMP, common myeloid progenitor; DEAB, diethylaminobenzaldehyde; Flt-3, fms-like tyrosine kinase-3; HSCs, hematopoietic stem cells; MEP, megakaryocyte-erythroid progenitor; TSF, thrombopoietin.
Inhibition of ALDH Increases the Frequency of CD34+Flt-3-KSL Cells in Culture and Can Be Reversed with ATRA
BM 34+Flt-3-KSL cells have been shown to be highly enriched for ST-HSCs [6]. Since inhibition of ALDH supported an increase in short-term CRUs and radioprotective cells in culture, we sought to determine if DEAB treatment caused an increase in the number of 34+Flt-3-KSL cells in culture compared to cytokines alone. At day 7 of culture of BM 34-KSL HSCs with TSF alone, only 1.6% ± 0.2 of the cultured progeny remained 34+Flt-3-KSL cells, whereas 26.4% ± 3.5 of the progeny after culture with TSF + DEAB were 34+Flt-3-KSL cells (Fig. 4B, p = .003). These data demonstrate that inhibition of ALDH facilitates the expansion of ST-HSCs in culture. Interestingly, the addition of 1 μM ATRA to TSF + DEAB cultures essentially reversed the effect of DEAB treatment, yielding >20-fold less 34+Flt-3-KSL cells in culture (mean 1.1% ± 0.2, p = .003). This result suggested that inhibition of ALDH impeded HSC differentiation via antagonism of retinoid signaling.
Since committed CMPs and MEPs have also been shown to possess radioprotective capacity [7], we compared the frequency of IL-7Rα-Lin-Sca-1-c-kit+Fcγ RloCD34+ cells (CMPs) with IL-7Rα-Lin-Sca-1-c-kit+Fcγ RloCD34− cells (MEPs) within the progeny of TSF cultures versus TSF + DEAB cultures [7]. Interestingly, the progeny of TSF + DEAB cultures contained a significantly increased frequency of CMPs and a significant decrease in MEPs compared to the progeny of TSF alone (Fig. 4C; p < .001 and p = .01, respectively). Taken together, these data demonstrate that inhibition of ALDH causes a generalized inhibition of myeloid differentiation of HSCs in response to cytokines alone.
siRNA of ALDH1a1 Increases BM Radioprotective Progenitor Cell Content
We show here that several isoforms of ALDH are expressed in HSCs, including ALDH1a1, ALDH2, ALDH3a2, ALDH1a7, and ALDH9a1 [25–27]. Since treatment with DEAB blocked retinoid signaling in HSCs and ALDH1a1 has retinoic acid biosynthetic activity, we hypothesized that ALDH1a1 might be the primary mechanistic target of DEAB. We therefore treated BM KSL cells with an siRNA specifically targeting ALDH1a1 in BM HSCs to determine if this would cause comparable expansion of radioprotective ST-HSCs as observed with DEAB. Treatment of BM KSL cells with ALDH1a1-siRNA caused an 80% decrease in ALDH1a1 expression compared to nontargeting siRNA-treated BM KSL cells at day 7 of culture (Fig. 5; p = .006). Mice transplanted with the progeny of BM KSL cells treated with ALDH1a1-siRNA demonstrated significantly increased survival at day +40 compared to mice transplanted with day 0 BM KSL cells (Fig. 5, 60 % vs. 0%, p = .02) and increased survival compared to mice transplanted with BM KSL cells treated with nontargeting siRNA (60% vs. 0%, p = .007). These results demonstrated that silencing of ALDH1a1 was as potent as DEAB treatment toward amplifying radioprotective cells in culture and suggested that ALDH1a1 was the mechanistic target of DEAB action.
Figure 5.
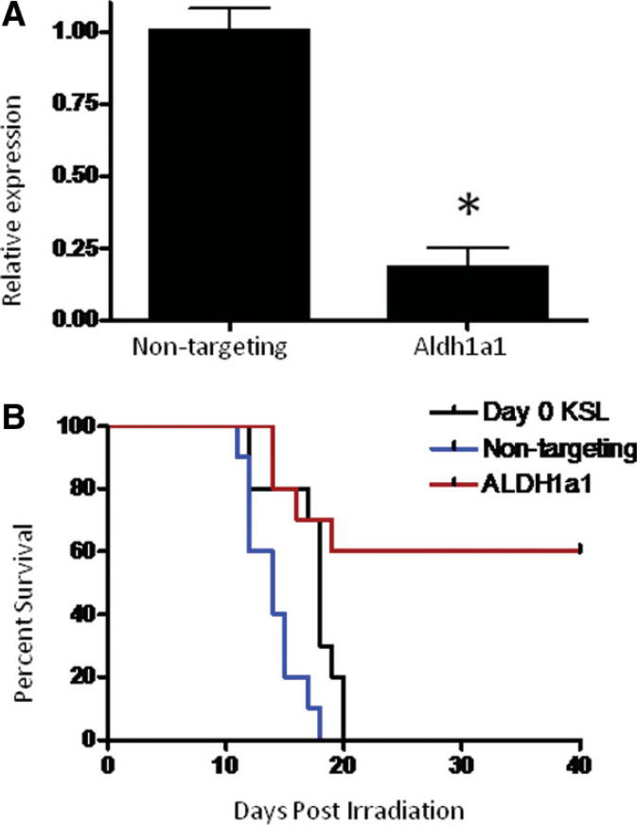
siRNA-mediated knockdown of ALDH1a1 increases BM radioprotective cell capacity. (A): Quantitative reverse transcriptase- polymerase chain reaction (RT-PCR) analysis of ALDH1a1 expression in BM KSL cells treated with either nontargeting or ALDH1a1-specific siRNA constructs. ALDH1a1 expression was reduced by approximately 80% (*, p = .006) at day 7 as compared to nontargeting control (n = 3, means ± SD). (B): Kaplan-Meier analysis of survival of lethally irradiated (950 cGy) adult C57BL6 mice transplanted with 500 day 0 BM KSL cells (black line) or their progeny after 7-day culture with TSF, and either nontargeting (blue line) or ALDH1a1-specific siRNA (red line) (p = .02 and p = .007 for TSF + DEAB vs. day 0 and TSF groups, respectively; n = 10 mice per group). Abbreviations: ALDH, aldehyde dehydrogenase; BM, bone marrow; 34-KSL, CD34−c-kit+Sca-1+lineage−; DEAB, diethylaminobenzaldehyde; HSCs, hematopoietic stem cells; TSF, thrombopoietin, stem cell factor, Flt-3 ligand.
Discussion
Myeloablative conditioning prior to HSC transplantation results in a period of pancytopenia during which patients are highly susceptible to infectious complications. This is particularly true in the setting of adult CB transplantation in which hematologic recovery can be delayed for up to 2 months [1–4]. Previous studies have demonstrated that LT-HSCs are inefficient in providing the rapid hematologic recovery that is required for radioprotection of lethally irradiated recipients [10]. Conversely, ST-HSCs, which can generate rapid but transient repopulation, are more efficient at providing early hematopoietic reconstitution and radioprotection of lethally irradiated recipients [6]. Therefore, transplantation of ST-HSCs could, in principle, serve to lessen the morbidity and mortality which occurs in patients early post-HSC transplantation. In this study, we demonstrate that pharmacologic inhibition of ALDH induces a 9-fold expansion of radioprotective progenitor cells and produces a 15.4-fold increase in donor cell engraftment in lethally irradiated mice during the critical nadir phase (day 14) after myeloablative irradiation compared to mice transplanted with unmanipulated HSCs. Transplantation of the progeny of only 30 BM 34-KSL cells cultured with DEAB (absolute cell doses, 330–1140 cells per mouse) was radioprotective in 70% of lethally irradiated mice, indicating a high radioprotective efficiency within this population. For comparison, it was previously shown that transplantation of 50,000 CMPs or MEPs was required to radioprotect 50% of lethally irradiated mice [7] and a dose of 500 34+Flt-3-KSL cells was required to radioprotect 46% of lethally irradiated mice [6]. Competitive repopulating assays and detailed phenotypic analyses demonstrated that inhibition of ALDH caused a 4-fold expansion of short-term CRUs and a 17-fold increase in 34+Flt-3-KSL cells in this population compared to cytokine treatment alone. Taken together, these results demonstrate that ALDH is an inducible regulator of HSC differentiation and inhibition of ALDH facilitates the expansion of ST-HSCs in culture.
Although these results suggested that ALDH regulates HSC differentiation in response to cytokines, we sought to determine which isoform of ALDH was the target of DEAB and whether DEAB affected other pathways which impact HSC differentiation. We verified that treatment with DEAB causes a significant reduction in ALDH enzyme activity and gene expression in HSCs which temporally corresponds to the amplification of radioprotective cells and ST-HSCs in culture. Consistent with prior studies by Pearce and Levi [25, 34], we were unable to discriminate an ALDH-bright population by Aldefluor analysis of murine HSCs, but confirmed the effects of DEAB on ALDH activity in human CD34+CD38-lin− HSCs [22–24]. Importantly, although we found that several isoforms of ALDH are expressed by BM HSCs, we also determined that siRNA-mediated silencing of ALDH1a1 in BM KSL cells reproduced the effect of DEAB treatment, causing a significant increase in BM radioprotective cell activity compared to input BM KSL cells or the progeny of nontargeting siRNA-treated BM KSL cells. Taken together, these results suggest that DEAB-mediated expansion of BM radioprotective cells and ST-HSCs is mediated through inhibition of ALDH and that ALDH1a1 is the primary isoform involved in this process. Of note, Levi et al. recently showed that homozygous genetic deletion of ALDH1a1 caused no alteration in HSC content or function in mice [25]. The differences in these observations can be resolved by consideration that homozygous deletion of a gene in vivo allows for other pathways to compensate for loss of gene function over time. Conversely, acute pharmacologic inhibition or genetic silencing of a specific target, as shown here via DEAB treatment and ALDH1a1 siRNA, may reveal a gene function (e.g., regulation of HSC differentiation) prior to the upregulation of compensatory pathways.
Since ALDH is required for the intracellular production of retinoic acids [35], we hypothesized further that ALDH mediates HSC differentiation via augmentation of retinoid signaling and that inhibition of retinoid-mediated signaling plays a causative role in the expansion of ST-HSCs, which we have observed. It has been shown previously that retinoid signaling promotes the differentiation of primary myeloid progenitor cells to mature granulocytes [36, 37] and introduction of a dominant negative retinoic acid receptor-α (RARα) construct into a hematopoietic progenitor cell line suppresses myeloid differentiation [38]. Similarly, we have previously shown that pharmacologic antagonism of the RAR/Retinoid X Receptor (RXR) heterodimer promotes the maintenance of human NOD/SCID repopulating cells in culture [39]. ATRA administration is also used therapeutically to induce the terminal differentiation of acute promyelocytic leukemia cells, which bear dominant negative activity at RARα [40]. Conversely, it has been shown that the addition of ATRA to cytokine-containing cultures of murine BM KSL cells enhances the maintenance of pre-CFU-S cells and long-term repopulating cells compared to cytokine cultures alone [41, 42]. It was subsequently shown that these effects of ATRA were mediated through RARγ, rather than RARα, and that the addition of ATRA also enhanced the serial transplantability of KSL cells compared to input KSL cells or their progeny after cytokine treatment [43]. Taken together, these studies suggest that the effects of retinoic acid are dependent upon the developmental stage of the hematopoietic stem/progenitor cell. Retinoic acid appears to maintain the repopulating activity of primitive HSCs [41–43], whereas it promotes the myeloid differentiation of multipotent progenitor cells (MPPs) [41]. Our results are not inconsistent with these prior observations. We show that inhibition of ALDH decreases RAR-mediated signaling and increases the number of ST-HSCs and radioprotective cells in culture, which is consistent with antagonism of RAR-mediated differentiation of ST-HSCs and MPPs. Furthermore, we demonstrate that the addition of ATRA to cultures of BM 34-KSL cells with DEAB overrides the effect of DEAB toward maintaining ST-HSCs in culture and promotes differentiation and lineage commitment. Taken together, our results implicate the ALDH/RAR axis in regulating the myeloid differentiation of ST-HSCs. It remains possible that DEAB treatment inhibits HSC differentiation via alternative pathways independent of effects on ALDH or RAR. However, this appears less likely since siRNA-mediated silencing of ALDH1a1 caused a comparable increase in BM radioprotective cell activity.
It has been previously demonstrated that ex vivo culture of HSCs with proliferation-inducing cytokines and the transition of HSCs from G1 to G2/S/M phase are associated with a loss of repopulating capacity [32]. Here, we show that pharmacologic inhibition of ALDH with DEAB slowed the G0/G1 transition in HSCs, resulting in a modestly higher frequency of HSCs in G0 and less cells in G2/S/M phase compared to HSCs treated with TSF alone. This inhibition of cell cycle progression in HSCs, although modest, may have contributed to the significant increase in repopulating ST-HSCs observed in DEAB-treated BM 34-KSL cells as compared to the progeny of cytokines alone. Since several tumor-specific cancer stem cells have high expression of ALDH [44, 45], these results also have implications for cancer research. ALDH-bright tumor cells have been shown to have a higher proliferative and repopulating potential than ALDH-negative tumor cells [44, 45]. Our study suggests that pharmacologic inhibition of ALDH should be explored as a strategy to decrease the growth and proliferation of cancer stem cells.
In summary, we show here that ALDH has a precise function in regulating the differentiation of ST-HSCs, inhibition of ALDH causes significant amplification of ST-HSCs with radioprotective capacity, and these effects are mediated via inhibition of retinoid signaling and HSC cell cycle transition. siRNA-mediated silencing of ALDH1a1 in BM HSCs maintained radioprotective cells in culture at a level comparable to DEAB treatment, suggesting that ALDH1a1 is the primary target of DEAB. ALDH inhibition represents a translatable strategy to expand ST-HSCs to augment hematopoietic engraftment in patients undergoing stem cell transplantation.
Supplementary Material
Acknowledgments
The authors acknowledge the Duke Human Vaccine Institute Flow Cytometry Core Facility for assistance with cell sorting. This work was supported, in part, by a grant from the National Institute of Allergy and Infectious Diseases No. AI067798 (J.P.C.).
Footnotes
Author contributions: G.M. and J.L.R.: collection of data, data analysis, manuscript writing, final approval of manuscript; G.M. and J.L.R.: contributed equally to this work; R.S.: collection of data, data analysis, conception and design; A.S., H.H., P.D., and P.D.: collection of data; S.M.: collection of data, data analysis; R.S. and N.C.: conception and design, data analysis, manuscript writing; D.M.: conception and design, provision of materials, manuscript writing; J.C.: conception and design, financial support, data analysis, manuscript writing, final approval of manuscript.
References
- 1.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Lowenberg B. Role of allogeneic stem cell transplantation in current treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2005:151–155. doi: 10.1182/asheducation-2005.1.151. [DOI] [PubMed] [Google Scholar]
- 3.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Meijer E, Dekker AW, Lokhorst HM, et al. Low incidence of infectious complications after nonmyeloablative compared with myeloablative allogeneic stem cell transplantation. Transpl Infect Dis. 2004;6:171–178. doi: 10.1111/j.1399-3062.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 5.Barker JN. Umbilical Cord Blood (UCB) Transplantation: An Alternative to the Use of Unrelated Volunteer Donors? Hematology Am Soc Hematol Educ Program. 2007:55–61. doi: 10.1182/asheducation-2007.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(−) Sca1(+)kit(+)CD34(+)Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 7.Na Nakorn T, Traver D, Weissman IL, et al. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plett PA, Frankovitz SM, Orschell-Traycoff CM. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 2002;100:3545–3552. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- 9.BitMansour A, Burns SM, Traver D, et al. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood. 2002;100:4660–4667. doi: 10.1182/blood-2002-05-1552. [DOI] [PubMed] [Google Scholar]
- 10.Osawa M, Hanada K, Hamada H, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 11.Uchida N, Aguila HL, Fleming WH, et al. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1. 1lo Lin-Sca-1+ hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- 12.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn PA, Thomasson BM, Wood BL, et al. Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood. 2003;102:4329–4335. doi: 10.1182/blood-2003-01-0082. [DOI] [PubMed] [Google Scholar]
- 14.Chute J, Muramoto G, Salter A, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salter A, Meadows S, Muramoto G, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan CT, Yamasaki G, Minamoto D. High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol. 1996;24:1347–1355. [PubMed] [Google Scholar]
- 17.Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnefoix T, Bonnefoix P, Verdiel P, et al. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J Immunol Methods. 1996;194:113–119. doi: 10.1016/0022-1759(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 19.Chute J, Saini A, Chute D, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 20.Chute J, Muramoto G, Fung J, et al. Soluble factors elaborated by human brain endothelial cells induce the concomitant expansion of purified human BM CD34+CD38− cells and SCID repopulating cells. Blood. 2005;105:576–583. doi: 10.1182/blood-2004-04-1467. [DOI] [PubMed] [Google Scholar]
- 21.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 22.Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storms RW, Green PD, Safford KM, et al. Distinct hematopoietic progenitor compartments are delineated by the expression of aldehyde dehydrogenase and CD34. Blood. 2005;106:95–102. doi: 10.1182/blood-2004-09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 25.Levi BP, Yilmaz O, Deuster G, et al. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood. 2009;113:1670–1680. doi: 10.1182/blood-2008-05-156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsberg E, Prohaska S, Katzman S, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanova N, Dimos J, Schaniel C, et al. A stem cell moleclar signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 28.Duester G. Genetic dissection of retinoid dehydrogenases. Chem Biol Interact. 2001;130–132:469–480. doi: 10.1016/s0009-2797(00)00292-1. [DOI] [PubMed] [Google Scholar]
- 29.Parrella E, Gianni M, Cecconi V, et al. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the camp and the retinoic acid signaling pathways. J Biol Chem. 2004;279:42026–42040. doi: 10.1074/jbc.M406530200. [DOI] [PubMed] [Google Scholar]
- 30.Park D, Chumarakov A, Vuong P, et al. CCAAT/enhancer binding protein epsilon is a potential retinoid target gene in acute promyelocytic leukemia treatment. J Clin Invest. 1999;103:1399–1408. doi: 10.1172/JCI2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walkley CR, Purton LE, Snelling HJ, et al. Identification of the molecular requirements for an RAR alpha-mediated cell cycle arrest during granulocytic differentiation. Blood. 2004;103:1286–1295. doi: 10.1182/blood-2003-07-2391. [DOI] [PubMed] [Google Scholar]
- 32.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 33.Traycoff CM, Orazi A, Ladd AC, et al. Proliferation-induced decline of primitive hematopoietic progenitor cell activity is coupled with an increase in apoptosis of ex vivo expanded CD34+ cells. Exp Hematol. 1998;26:53–62. [PubMed] [Google Scholar]
- 34.Pearce DJ, Bonnet D. The combined use of Hoechst efflux ability and aldehyde dehydrogenase activity to identify murine and human hematopoietic stem cells. Exp Hematol. 2007;35:1437–1446. doi: 10.1016/j.exphem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Haselbeck RJ, Hoffmann I, Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 37.Kastner P, Chan S. Function of RARalpha during the maturation of neutrophils. Oncogene. 2001;20:7178–7185. doi: 10.1038/sj.onc.1204757. [DOI] [PubMed] [Google Scholar]
- 38.Tsai S, Bartelmez S, Heyman R, et al. A mutated retinoic acid receptor-alpha exhibiting dominant-negative activity alters the lineage development of a multipotent hematopoietic cell line. Genes Dev. 1992;6:2258–2269. doi: 10.1101/gad.6.12a.2258. [DOI] [PubMed] [Google Scholar]
- 39.Safi R, Muramoto G, Salter A, et al. Pharmacological manipulation of the RAR/RXR signaling pathway maintains the repopulating capacity of hematopoietic stem cells in culture. Mol Endocrinol. 2009;23:188–201. doi: 10.1210/me.2008-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 41.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid delays the differentiation of primitive hematopoietic precursors (lin-c-kit+Sca-1(+)) while enhancing the terminal maturation of committed granulocyte/monocyte progenitors. Blood. 1999;94:483–495. [PubMed] [Google Scholar]
- 42.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood. 2000;95:470–477. [PubMed] [Google Scholar]
- 43.Purton LE, Dworkin S, Olsen GH, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginestier C, Hur M, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang E, Hynes M, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.