Abstract
Background
Associations between angiotensin-converting enzyme (ACE) gene insertion/deletion (I/D) polymorphisms and chronic kidney disease (CKD) have been extensively studied, with most studies reporting that individuals with the D allele have a higher risk. Although some factors, such as ethnicity, may moderate the association between ACE I/D polymorphisms and CKD risk, gender-dependent effects on the CKD risk remain controversial.
Objectives
This study investigated the gender-dependent effects of ACE I/D polymorphisms on CKD risk.
Data sources
PubMed, the Cochrane library, and EMBASE were searched for studies published before January 2013.
Study eligibility criteria, participants, and interventions
Cross-sectional surveys and case–control studies analyzing ACE I/D polymorphisms and CKD were included. They were required to match the following criteria: age >18 years, absence of rare diseases, and Asian or Caucasian ethnicity.
Study appraisal and synthesis methods
The effect of carrying the D allele on CKD risk was assessed by meta-analysis and meta-regression using random-effects models.
Results
Ethnicity [odds ratio (OR): 1.24; 95% confidence interval (CI): 1.08–1.42] and hypertension (OR: 1.55; 95% CI: 1.04–2.32) had significant moderate effects on the association between ACE I/D polymorphisms and CKD risk, but they were not significant in the diabetic nephropathy subgroup. Males had higher OR for the association between ACE I/D polymorphisms and CKD risk than females in Asians but not Caucasians, regardless of adjustment for hypertension (p<0.05). In subgroup analyses, this result was significant in the nondiabetic nephropathy group. Compared with the I allele, the D allele had the highest risk (OR: 3.75; 95% CI: 1.84–7.65) for CKD in hypertensive Asian males.
Conclusions and implications of key findings
The ACE I/D polymorphisms may incur the highest risk for increasing CKD in hypertensive Asian males.
Introduction
The prevalence of chronic kidney disease (CKD) is approximately 10% in several countries [1]–[3]. CKD patients have high risk for cardiovascular disease and death [4]. Genetic factors, including ethnicity [5] and family history of disease [6], , play a key role in CKD pathogenesis. Thus, it is desirable to identify candidate genes and evaluate their effects.
The renin–angiotensin system (RAS) regulates blood pressure and electrolyte balance [8]. Owing to the key role of angiotensin-converting enzyme (ACE) in RAS, ACE polymorphisms have frequently been investigated. One of the most important ACE polymorphisms is a 287-bp insertion/deletion in intron 16 (ACE I/D), and a previous study revealed a significant effect of this polymorphism on ACE gene expression [9]. Most studies have found that carriers of the D allele had a higher risk of CKD or end-stage renal disease (ESRD) than those of the I allele [10]–[12].
Because previous studies have found that gender and the DD genotype had an additive effect on blood ACE levels [13], we hypothesized that gender differences affect the relative risk of ACE I/D polymorphisms for CKD. Numerous studies of CKD or ESRD have reported appreciable, but not significant, gender-dependent effects of ACE I/D polymorphisms [14]–[19], but the populations used in these studies were of different ethnicities. Studies of Caucasian subjects have indicated additive effects of the D allele in females [14]–[16], but studies of Asian subjects have shown different results [17]–[19]. Although many previous meta-analysis studies investigating ACE I/D polymorphisms and CKD have been reported, but no studies have considered moderate effects of gender in our knowledge [10]–[12], [20]–[22]. This study focused on general population without genetic abnormality or rare disorder, and we wanted to compare the risk of CKD in people with major allele (I allele) or minor allele (D allele) on ACE I/D. In addition, gender-dependent effects of ACE I/D polymorphisms on CKD risk was investigated.
Methods
Search Methods and Criteria for Considering Studies
The PRISMA 2009 Checklist was reported in Table S1. This study focused on the general population without genetic predisposing factors, and aimed to compare CKD risk between individuals carrying the major (I) and minor (D) alleles of ACE I/D. To identify relevant studies, English-language articles in MEDLINE, Cochrane Library and EMBASE were searched using relevant text words and medical subject headings that included all spellings of ACE I/D and CKD (the detailed search strategy is shown in Table S2). All articles published from the dates of inception of these medical databases to January 2013 were included. We manually scanned the reference lists of identified trials and review articles to avoid missing any other relevant studies [10]–[12], [20]–[22].
All related studies assessing the association between ACE I/D polymorphisms and CKD risk were considered for inclusion. The criteria for inclusion of a study were as follows: (1) cross-sectional surveys or case–control studies; (2) CKD defined according to the National Kidney Foundation: kidney damage by clinical diagnosis or a glomerular filtration rate <60 ml/min/1.73 m2; (3) presence of a control group with normal kidney function; (4) study population age >18 years; (5) Asian or Caucasian ethnicity of the populations, and (6) articles providing detailed distribution of ACE genotypes. Studies investigating the relationships between genetic polymorphisms and other kidney diseases (lupus nephritis, polycystic kidney disease, endemic nephropathy, or reflux nephropathy) were excluded.
Data Extraction and Quality Assessment
Two reviewers (Chin Lin and Sui-Lung Su) independently extracted the data and assessed risk of bias. We recorded the first author’s name, year of publication, ethnicity of the study population, kidney function of the cases, definition of the case group and its population characteristics (mean age, proportion of male subjects, body mass index, diabetes mellitus prevalence, hypertension prevalence, and ACE I/D genotype distribution). Ethnicity of the study population was categorized by study area. Subjects in the Arabian peninsula were classified as Caucasian because Arabs were the main race, and subjects in other regions of Asia (excluding Russia) were classified as Asian. Diabetes mellitus and hypertension were defined by plasma glucose level of >126 mg/dL and systolic blood pressure of >140 mmHg. If the article did not report the prevalence of diabetes mellitus and hypertension or the definition did not match, we assumed a normal distribution of plasma glucose level and systolic blood pressure for calculation.
Estimating moderate effects is difficult in meta-analysis using case–control studies. Researchers prefer that studies provide stratified data or matching data, but previous studies have seldom reported these. Fortunately, the characteristics of case groups may be used to estimate moderate effects under the following two conditions: (1) outcomes were rare events, (2) the major independent variable and moderators were independent events.
For example, when the major independent variable is exposure, with values “yes” or “no,” and the moderator is gender, with values “male” or “female,” the variables p 1, p 2, p 3, and p 4 are the outcome prevalence of women without exposure, men without exposure, women with exposure, and men with exposure. The variable p 5 is the proportion of individuals with exposure in the whole population; p 6 is the proportion of men in the population without exposure; and p 7 is the proportion of men in the population with exposure.
When researchers wish to conduct a case–control study, they must survey the exposure proportion in the case and control groups to estimate the odds ratio (OR). The exposure proportions using a stratified view and ORs are as follows:
Exposure proportion among case women:
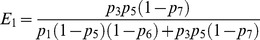
Exposure proportion among case men:
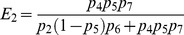
Exposure proportion among control women:
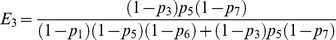
Exposure proportion among control men:
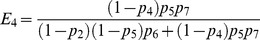
OR in women: 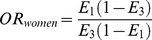
OR in men: 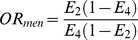
Crude OR (simple combined):
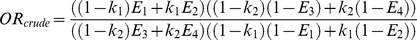 .
.
k 1 = proportion of males in the case group.
k 2 = proportion of males in the control group.
Two factors (k 1 and k 2) may affect the crude OR. However, when p 1, p 2, p 3, and p 4 are very rare and there is no association between the major independent variable and the moderator (p 6 = p 7),
If moderate effects are present (OR women ≠ OR men), the proportion of males in the case group (k 1) is the only factor that can affect the crude OR. In addition, the relationship between k 1 and log of odds ratio approximate the first order polynomial when above assumption were proper. We may accordingly use the characteristics of the case group to estimate the moderate effects in this study under the above assumptions.
Risk of bias was assessed by the following procedures suggested by the Newcastle–Ottawa Quality Assessment Scale [23] (shown in Table S3). This tool assesses studies with a focus on the following factors: (1) selection of study population, (2) comparability between the case and control groups, and (3) the exposure assessed. Each study received a score between 0 and 9. We investigated the relationship between the quality of studies and the estimation of risk.
Statistical Analysis
Variables are presented as means, proportions, or numbers as appropriate. Our meta-analysis examined the association between ACE I/D polymorphisms and CKD risk for each study by odds ratios (ORs) with 95% confidence intervals (CIs).
The τ2 statistic estimated by the DerSimonian–Laird method was used for the assessment of heterogeneity, and a random-effects model based on the Mantel–Haenszel method was applied. Allele type, genotype, and dominant/recessive models were used to calculate the association between genetic polymorphism and CKD risk. This report displays results from the allele type model, unless estimates using a different model were obviously different. Egger’s regression was used to test symmetry of pooled results. Prespecified subgroup analyses included the causes of CKD.
A moderate effect was defined as ratio between ORs in a stratified analysis. For example, if OR for the association between ACE I/D polymorphisms and CKD risk is 6 in the Asian group and 3 in the Caucasian group, the moderate effect of ethnicity will be 6/3 = 2. Possible moderators (ethnicity, age, gender, body mass index, diabetes mellitus, and hypertension) and study characteristic (quality score, study design, and kidney functions of cases) were tested by meta-regression. In multivariable analyses, we adjusted ethnicity because most previous studies have found significant moderate effects [10]–[12]. To assess the effect of ethnicity on gender-dependent effects, we investigated the interaction between gender and ethnicity. The interaction between other moderators and gender were also tested.
This study considered a p-value of <0.05 as significant for all analyses. Statistical analyses were carried out with R, version 2.15.0, using the “metafor” [24] and “meta” [25] packages.
Results
Screening Process
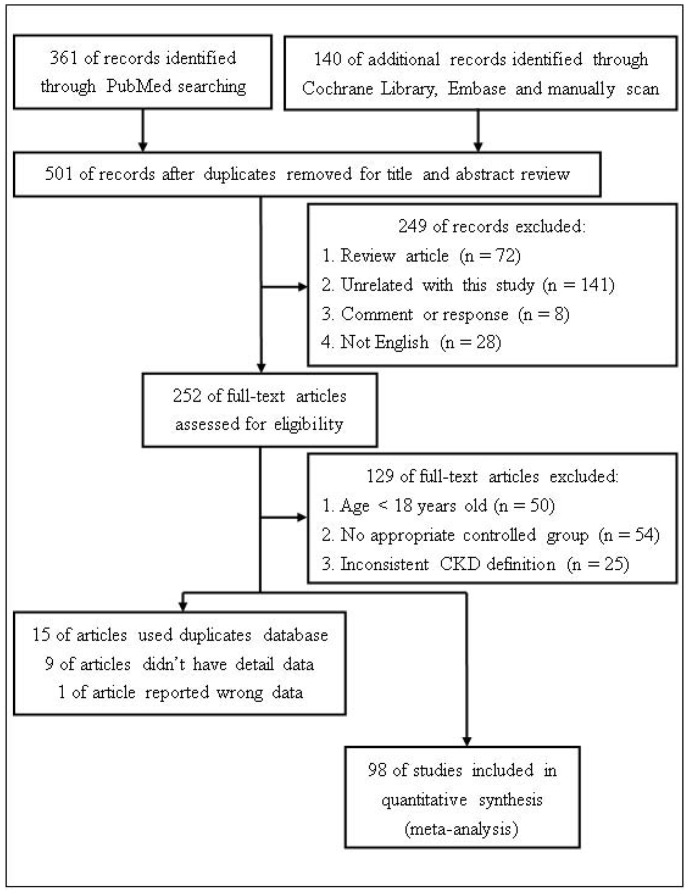
Our search strategy returned 501 papers (the identification process is shown in Figure 1). We excluded 249 papers after a preliminary search of titles and abstracts. An additional 129 papers were excluded after full-text articles were assessed, leaving 123 articles that matched our criteria. Of these, 15 used duplicate databases, 9 lacked detailed data, and 1 likely reported wrong data. In 98 of the studies finally included [14]–[19], [26]–[117], 2 (Zsom et al. [30] and Lee et al. [26]) reported results of stratification, but studies by Zsom et al. [30] used a single control group. Accordingly, 99 populations were included in this meta-analysis, and their detailed data are shown in Tables 1 and 2.
Figure 1. Flow diagram of identification process for eligible studies in this study.
n: number of studies were deleted for aforementioned reasons.
Table 1. Characteristics of published studies included in this meta-analysis.
| First author & year | Ethnicity | Studydesign | CKDtype | Kidneyfunctionof case | Definition of case |
| Shaikh, 2012 [27] | Caucasian | CC | DN | non-ESRD | UAE >300 mg/day11 |
| Rahimi, 2012 [28] | Caucasian | CS | DN | non-ESRD | ACR >30 mg/g |
| El-Baz, 2012 [29] | Caucasian | CS | DN | non-ESRD | ACR >30 mg/g |
| Zsom(1), 2011 [30] | Caucasian | CC | non-DN | non-ESRD | biopsy, ultrasound diagnosed & eGFR <60 ml/min/1.73 m2 |
| Zsom(2), 2011 [30] | Caucasian | CC | DN | non-ESRD | proteinuria & eGFR <60 ml/min/1.73 m2 |
| Al-Harbi, 2011 [31] | Caucasian | CC | DN | non-ESRD | no description |
| Jung, 2011 [32] | Asian | CC | GN | non-ESRD | biopsy diagnosed |
| Ali, 2011 [33] | Asian | CC | Comb | ESRD | doctor diagnosed |
| Huang, 2010 [34] | Asian | CC | GN | ESRD | biopsy diagnosed |
| Jayapalan, 2010 [35] | Asian | CS | DN | non-ESRD | ACR >30 mg/g or RRT |
| Mansoor, 2010 [14] | Caucasian | CS | DN | non-ESRD | albuminuria or RRT |
| Naresh, 2009 [36] | Asian | CC | DN | non-ESRD | no description |
| Ezzidi, 2009 [37] | Caucasian | CS | DN | non-ESRD | ACR >30 mg/g or eGFR <90 ml/min/1.73 m2 |
| Nikzamir, 2009 [38] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Anbazhagan, 2009 [39] | Asian | CC | Comb | ESRD | RRT |
| Ahluwalia, 2009 [40] | Asian | CC | DN | non-ESRD | UAE >200 µg/min, ACR >300 mg/g orRRT |
| Palomo-Piñón, 2009 [16] | Caucasian | CS | DN | non-ESRD | ACR >30 mg/g |
| Möllsten, 2008 [41] | Caucasian | CS | DN | non-ESRD | ACR >20 mg/g |
| Eroglu, 2008 [42] | Caucasian | CC | DN | non-ESRD | 30 mg/day<UAE <300 mg/day |
| Arfa, 2008 [43] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Tripathi, 2008 [44] | Asian | CC | non-DN | ESRD | CCr <15 mL/min/1.73 m2 & ultrasound diagnosedor CT |
| Movva, 2007 [45] | Asian | CC | DN | ESRD | SCr >1.5 mg/dL and UAE >30 mg/day |
| Uddin, 2007 [46] | Asian | CC | DN | ESRD | porteinuria |
| Buraczynska, 2006 [47] | Caucasian | CC | Comb | ESRD | RRT |
| So, 2006 [48] | Asian | CS | DN | non-ESRD | ACR >30 mg/g |
| Shestakova, 2006 [49] | Caucasian | CC | DN | non-ESRD | ACR >300 mg/g |
| Ng, 2006 [50] | Caucasian | CS | DN | non-ESRD | urinalyses positive, ACR >250 mg/g (men) or >355 mg/g (women) |
| Prasad, 2006 [51] | Asian | CC | DN | non-ESRD | SCr >3 mg/dL?ACR >200 mg/g |
| Degirmenci, 2005 [52] | Caucasian | CS | DN | ESRD | UAE >30 mg/day |
| van der Sman-de Beer, 2005 [53] | Caucasian | CC | Comb | non-ESRD | RRT |
| Park, 2005 [18] | Asian | CC | DN | ESRD | RRT |
| Canani, 2005 [54] | Caucasian | CS | DN | non-ESRD | UAE >20 µg/min |
| Fabris, 2005 [55] | Caucasian | CC | HN | non-ESRD | SCr >1.5 mg/dL |
| Lau, 2004 [56] | Asian | CC | GN | ESRD | biopsy diagnosed |
| Suzuki, 2004 [57] | Asian | CC | GN | non-ESRD | biopsy diagnosed |
| Stratta, 2004 [58] | Caucasian | CC | GN | non-ESRD | biopsy diagnosed |
| Lochynska, 2003 [59] | Caucasian | CC | GN | non-ESRD | biopsy diagnosed |
| Papp, 2003 [60] | Caucasian | CC | GN | ESRD | RRT |
| Aucella, 2003 [61] | Caucasian | CC | Comb | non-ESRD | RRT |
| Okuno, 2003 [62] | Asian | CS | DN | non-ESRD | UAE >10 µg/min |
| Ortiz, 2003 [63] | Caucasian | CC | non-DN | non-ESRD | CCr <50 mL/min/1.73 m2 |
| Hadjadj, 2003 [15] | Caucasian | CC | DN | non-ESRD | urinary albumin concentration >20 mg/L |
| Wang, 2003 [64] | Asian | CC | Comb | ESRD | RRT |
| Dixit, 2002 [65] | Caucasian | CC | GN | ESRD | biopsy diagnosed |
| Lee(1), 2002 [26] | Asian | CS | non-DN | non-ESRD | UAE >20 µg/min or ACR >20 mg/g |
| Lee(2), 2002 [26] | Asian | CS | DN | non-ESRD | UAE >20 µg/min or ACR >20 mg/g |
| Losito, 2002 [66] | Caucasian | CC | Comb | ESRD | RRT |
| Yoon, 2002 [67] | Asian | CC | GN | non-ESRD | biopsy diagnosed |
| Fradin, 2002 [68] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Drouet, 2002 [69] | Caucasian | CC | GN | non-ESRD | biopsy diagnosed |
| Nicod, 2002 [70] | Caucasian | CC | Comb | non-ESRD | RRT |
| Araz, 2001 [71] | Caucasian | CS | DN | non-ESRD | ACR >30 mg/g |
| Azar, 2001 [72] | Caucasian | CC | DN | non-ESRD | UAE >30 mg/day |
| Lovati, 2001 [73] | Caucasian | CC | Comb | ESRD | RRT |
| Wang, 2001 [74] | Caucasian | CS | Comb | non-ESRD | CCr <60 mL/min/1.73 m2 |
| Taniwaki, 2001 [75] | Asian | CS | DN | non-ESRD | UAE >30 mg/day |
| Viswanathan, 2001 [76] | Asian | CC | DN | non-ESRD | proteinuria >500 mg/dL |
| Hadjadj, 2001 [77] | Caucasian | CS | DN | non-ESRD | urinary albumin concentration >20 mg/L |
| Wu, 2000 [78] | Asian | CC | DN | non-ESRD | no description |
| Hsieh, 2000 [79] | Asian | CC | DN | non-ESRD | proteinuria >500 mg/dL |
| van Ittersum, 2000 [80] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Tomino, 1999 [19] | Asian | CS | DN | non-ESRD | UAE >20 µg/min or ACR >30 mg/g |
| Solini, 1999 [81] | Caucasian | CS | DN | non-ESRD | Albuminuria |
| De Cosmo, 1999 [82] | Caucasian | CC | DN | non-ESRD | UAE >30 mg/day |
| Miura, 1999 [83] | Asian | CS | DN | non-ESRD | UAE >10 µg/min |
| Kuramoto, 1999 [84] | Asian | CS | DN | non-ESRD | UAE >15 µg/min |
| Huang, 1998 [85] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Walder, 1998 [86] | Caucasian | CC | DN | non-ESRD | UAE >30 mg/day |
| Freire, 1998 [87] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Grzeszczak, 1998 [88] | Caucasian | CS | DN | non-ESRD | ACR >1.9 mg/mol (men) or >2.8 mg/mol (women) |
| Young, 1998 [89] | Asian | CS | DN | non-ESRD | UAE >30 mg/day |
| Penno, 1998 [90] | Caucasian | CS | DN | non-ESRD | UAE >20 µg/min |
| Fernández-Llama, 1998 [91] | Caucasian | CS | HN | non-ESRD | UAE >20 µg/min |
| Frost, 1998 [92] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Pei, 1997 [93] | Caucasian | CS | GN | non-ESRD | biopsy diagnosed |
| Marre, 1997 [94] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Barnas, 1997 [95] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Ringel, 1997 [96] | Caucasian | CC | DN | non-ESRD | UAE >30 mg/day |
| Schmidt, 1997 [97] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Kawada, 1997 [98] | Asian | CC | Comb | ESRD | RRT |
| Kario, 1997 [99] | Asian | CS | HN | non-ESRD | UAE >15 µg/min |
| Nakajima, 1996 [17] | Asian | CS | DN | non-ESRD | ACR >30 mg/g |
| Chowdhury, 1996 [100] | Caucasian | CS | DN | non-ESRD | Albuminuria |
| McLaughlin, 1996 [101] | Caucasian | CC | Comb | non-ESRD | RRT or doctor diagnosed |
| Oh, 1996 [102] | Asian | CS | DN | non-ESRD | UAE >20 µg/min |
| Schmidt, 1996 [103] | Caucasian | CC | Comb | ESRD | RRT |
| Doi, 1996 [104] | Asian | CS | DN | non-ESRD | ACR >30 mg/g |
| Ohno, 1996 [105] | Asian | CS | DN | non-ESRD | ACR >10 mg/g |
| Mizuiri, 1995 [106] | Asian | CC | DN | non-ESRD | UAE >20 µg/min or RRT |
| Yorioka, 1995 [107] | Asian | CC | GN | non-ESRD | biopsy diagnosed |
| Fujisawa, 1995 [108] | Asian | CS | DN | non-ESRD | Albuminuria or RRT |
| Panagiotopoulos, 1995 [109] | Caucasian | CS | DN | non-ESRD | UAE >20 µg/min |
| Tarnow, 1995 [110] | Caucasian | CC | DN | non-ESRD | UAE >300 mg/day or biopsy diagnosed |
| Schmidt, 1995 [111] | Caucasian | CC | GN | non-ESRD | biopsy diagnosed |
| Yoshida, 1995 [112] | Asian | CC | GN | non-ESRD | biopsy diagnosed |
| Dudley, 1995 [113] | Caucasian | CC | DN | non-ESRD | patients with urine in top tertile of the median UAE |
| Harden, 1995 [114] | Caucasian | CC | GN | non-ESRD | biopsy diagnosed |
| Doria, 1994 [115] | Caucasian | CC | DN | non-ESRD | UAE >30 µg/min |
| Marre, 1994 [116] | Caucasian | CS | DN | non-ESRD | UAE >30 mg/day |
| Powrie, 1994 [117] | Caucasian | CS | DN | non-ESRD | ACR >3 mg/mmol |
CC: case control study; CS: cross-sectional survey; DN: diabetic nephropathy; non-DN: non diabetic nephropathy; GN: glomerulonephritis; HN: hypertensive nephropathy; Comb: combined; ESRD: only ESRD patients; non-ESRD: not only ESRD patients; UAE: urinary albumin excretion rate; ACR: Albumin creatinine ratio; eGFR: estimated glomerular filtration rate; CCr: creatinine clearance; RRT: renal replacement therapy; CT: computed tomography; SCr: serum creatinine.
Table 2. Quality score and description of studies’ population in included studies.
| First author & year | Quality score | Age (years) | Male (%) | BMI (kg/m2) | DM (%) | HT (%) | Case group | Control group | ||||
| II | ID | DD | II | ID | DD | |||||||
| Shaikh, 2012 [27] | 7 | 58 | 54 | 27 | 100 | 86 | 18 | 98 | 52 | 123 | 148 | 25 |
| Rahimi, 2012 [28] | 7 | 56 | 40 | 27 | 100 | 63 | 19 | 66 | 55 | 14 | 32 | 26 |
| El-Baz, 2012 [29] | 6 | 59 | 53 | 100 | 4 | 58 | 40 | 5 | 72 | 23 | ||
| Zsom(1), 2011 [30] | 4 | 64 | 55 | 39 | 88 | 68 | 44 | 110 | 46 | |||
| Zsom(2), 2011 [30] | 4 | 70 | 61 | 100 | 20 | 60 | 34 | 44 | 110 | 46 | ||
| Al-Harbi, 2011 [31] | 4 | 58 | 48 | 30 | 100 | 86 | 12 | 39 | 59 | 25 | 75 | 96 |
| Jung, 2011 [32] | 7 | 34 | 57 | 61 | 78 | 142 | 41 | 98 | 164 | 38 | ||
| Ali, 2011 [33] | 5 | 55 | 55 | 25 | 16 | 41 | 47 | 125 | 18 | 86 | 90 | 14 |
| Huang, 2010 [34] | 3 | 40 | 50 | 54 | 10 | 29 | 8 | 58 | 52 | 10 | ||
| Jayapalan, 2010 [35] | 7 | 60 | 38 | 100 | 42 | 77 | 77 | 21 | 31 | 31 | 19 | |
| Mansoor, 2010 [14] | 6 | 53 | 31 | 27 | 100 | 29 | 27 | 45 | 12 | 65 | 102 | 33 |
| Naresh, 2009 [36] | 5 | 54 | 53 | 29 | 100 | 95 | 4 | 11 | 15 | 12 | 11 | 7 |
| Ezzidi, 2009 [37] | 7 | 60 | 46 | 28 | 100 | 50 | 88 | 260 | 167 | 152 | 196 | 54 |
| Nikzamir, 2009 [38] | 7 | 59 | 56 | 26 | 100 | 54 | 31 | 84 | 64 | 42 | 75 | 28 |
| Anbazhagan, 2009 [39] | 7 | 49 | 73 | 28 | 75 | 33 | 58 | 27 | 23 | 53 | 22 | |
| Ahluwalia, 2009 [40] | 7 | 58 | 66 | 24 | 100 | 59 | 44 | 64 | 132 | 49 | 117 | 89 |
| Palomo-Piñón, 2009 [16] | 7 | 60 | 48 | 27 | 100 | 26 | 87 | 105 | 43 | 85 | 91 | 24 |
| Möllsten, 2008 [41] | 7 | 47 | 49 | 100 | 52 | 25 | 69 | 27 | 36 | 113 | 48 | |
| Eroglu, 2008 [42] | 7 | 58 | 41 | 29 | 100 | 38 | 13 | 17 | 16 | 13 | 24 | 19 |
| Arfa, 2008 [43] | 7 | 62 | 43 | 29 | 100 | 68 | 9 | 41 | 40 | 6 | 24 | 21 |
| Tripathi, 2008 [44] | 6 | 36 | 88 | 0 | 69 | 53 | 72 | 55 | 379 | 148 | 42 | |
| Movva, 2007 [45] | 6 | 57 | 70 | 100 | 47 | 88 | 39 | 74 | 74 | 27 | ||
| Uddin, 2007 [46] | 4 | 51 | 54 | 100 | 78 | 12 | 22 | 24 | 24 | 28 | 14 | |
| Buraczynska, 2006 [47] | 8 | 51 | 56 | 19 | 78 | 174 | 346 | 228 | 112 | 268 | 140 | |
| So, 2006 [48] | 6 | 100 | 407 | 364 | 93 | 549 | 526 | 150 | ||||
| Shestakova, 2006 [49] | 6 | 26 | 48 | 23 | 100 | 26 | 15 | 35 | 13 | 24 | 30 | 12 |
| Ng, 2006 [50] | 7 | 61 | 61 | 32 | 100 | 48 | 47 | 148 | 96 | 32 | 83 | 52 |
| Prasad, 2006 [51] | 7 | 57 | 33 | 100 | 63 | 67 | 74 | 55 | 76 | 97 | 52 | |
| Degirmenci, 2005 [52] | 4 | 100 | 6 | 25 | 12 | 19 | 47 | 30 | ||||
| van der Sman-de Beer, 2005 [53] | 6 | 59 | 61 | 26 | 17 | 65 | 110 | 227 | 116 | 112 | 235 | 125 |
| Park, 2005 [18] | 5 | 60 | 58 | 23 | 100 | 83 | 27 | 49 | 27 | 30 | 51 | 7 |
| Canani, 2005 [54] | 6 | 100 | 66 | 181 | 126 | 120 | 308 | 181 | ||||
| Fabris, 2005 [55] | 7 | 60 | 78 | 26 | 0 | 100 | 13 | 32 | 41 | 34 | 83 | 55 |
| Lau, 2004 [56] | 5 | 43 | 48 | 53 | 48 | 17 | 47 | 43 | 4 | |||
| Suzuki, 2004 [57] | 5 | 36 | 56 | 42 | 54 | 21 | 111 | 106 | 53 | |||
| Stratta, 2004 [58] | 5 | 50 | 67 | 39 | 23 | 50 | 44 | 29 | 76 | 66 | ||
| Lochynska, 2003 [59] | 5 | 42 | 74 | 0 | 0 | 13 | 32 | 5 | 20 | 45 | 35 | |
| Papp, 2003 [60] | 4 | 49 | 46 | 65 | 11 | 25 | 14 | 34 | 83 | 33 | ||
| Aucella, 2003 [61] | 5 | 62 | 54 | 16 | 58 | 57 | 201 | 203 | 170 | 576 | 561 | |
| Okuno, 2003 [62] | 7 | 78 | 50 | 20 | 100 | 31 | 1 | 8 | 3 | 21 | 12 | 5 |
| Ortiz, 2003 [63] | 6 | 56 | 59 | 22 | 0 | 100 | 9 | 71 | 81 | 17 | 66 | 46 |
| Hadjadj, 2003 [15] | 9 | 66 | 73 | 29 | 100 | 72 | 552 | 1468 | 1119 | 115 | 282 | 208 |
| Wang, 2003 [64] | 5 | 55 | 51 | 23 | 30 | 66 | 106 | 104 | 36 | 71 | 88 | 24 |
| Dixit, 2002 [65] | 7 | 24 | 55 | 12 | 26 | 9 | 3 | 24 | 13 | |||
| Lee(1), 2002 [26] | 8 | 0 | 20 | 45 | 12 | 330 | 277 | 66 | ||||
| Lee(2), 2002 [26] | 8 | 100 | 117 | 137 | 40 | 208 | 170 | 39 | ||||
| Losito, 2002 [66] | 6 | 67 | 61 | 25 | 14 | 50 | 27 | 81 | 52 | 22 | 84 | 63 |
| Yoon, 2002 [67] | 5 | 35 | 56 | 0 | 35 | 44 | 116 | 31 | 70 | 128 | 25 | |
| Fradin, 2002 [68] | 7 | 57 | 53 | 32 | 100 | 33 | 18 | 61 | 38 | 20 | 54 | 44 |
| Drouet, 2002 [69] | 6 | 44 | 77 | 16 | 46 | 63 | 13 | 28 | 36 | |||
| Nicod, 2002 [70] | 5 | 54 | 57 | 25 | 63 | 156 | 41 | 54 | 153 | 53 | ||
| Araz, 2001 [71] | 7 | 56 | 41 | 28 | 100 | 63 | 18 | 64 | 34 | 23 | 57 | 43 |
| Azar, 2001 [72] | 6 | 23 | 46 | 100 | 2 | 27 | 23 | 2 | 7 | 1 | ||
| Lovati, 2001 [73] | 7 | 54 | 57 | 25 | 12 | 51 | 63 | 156 | 41 | 69 | 195 | 63 |
| Wang, 2001 [74] | 8 | 28 | 99 | 41 | 313 | 662 | 311 | |||||
| Taniwaki, 2001 [75] | 7 | 61 | 59 | 23 | 100 | 54 | 32 | 40 | 14 | 31 | 26 | 12 |
| Viswanathan, 2001 [76] | 7 | 57 | 66 | 26 | 100 | 64 | 17 | 45 | 24 | 10 | 8 | 5 |
| Hadjadj, 2001 [77] | 7 | 40 | 59 | 23 | 100 | 53 | 8 | 34 | 17 | 46 | 116 | 89 |
| Wu, 2000 [78] | 3 | 60 | 55 | 100 | 22 | 31 | 18 | 19 | 20 | 2 | ||
| Hsieh, 2000 [79] | 5 | 61 | 49 | 23 | 100 | 60 | 80 | 59 | 40 | 86 | 50 | 21 |
| van Ittersum, 2000 [80] | 6 | 55 | 59 | 100 | 80 | 23 | 33 | 13 | 53 | 86 | 49 | |
| Tomino, 1999 [19] | 7 | 61 | 63 | 100 | 312 | 337 | 96 | 163 | 189 | 55 | ||
| Solini, 1999 [81] | 8 | 60 | 58 | 30 | 100 | 61 | 33 | 66 | 58 | 27 | 83 | 62 |
| De Cosmo, 1999 [82] | 7 | 43 | 61 | 100 | 42 | 23 | 76 | 73 | 18 | 53 | 65 | |
| Miura, 1999 [83] | 7 | 36 | 34 | 100 | 21 | 36 | 49 | 13 | 35 | 58 | 10 | |
| Kuramoto, 1999 [84] | 7 | 58 | 61 | 23 | 100 | 56 | 8 | 16 | 9 | 13 | 13 | 3 |
| Huang, 1998 [85] | 6 | 100 | 4 | 12 | 8 | 7 | 29 | 23 | ||||
| Walder, 1998 [86] | 7 | 42 | 71 | 100 | 86 | 12 | 25 | 18 | 12 | 16 | 16 | |
| Freire, 1998 [87] | 6 | 28 | 48 | 100 | 17 | 12 | 32 | 33 | 10 | 45 | 34 | |
| Grzeszczak, 1998 [88] | 7 | 62 | 29 | 100 | 68 | 103 | 230 | 129 | 63 | 118 | 73 | |
| Young, 1998 [89] | 7 | 56 | 34 | 25 | 100 | 73 | 23 | 30 | 3 | 26 | 20 | 8 |
| Penno, 1998 [90] | 5 | 100 | 15 | 38 | 23 | 62 | 258 | 114 | ||||
| Fernández-Llama, 1998 [91] | 5 | 0 | 100 | 1 | 6 | 7 | 5 | 38 | 18 | |||
| Frost, 1998 [92] | 5 | 100 | 10 | 7 | 16 | 21 | 48 | 46 | ||||
| Pei, 1997 [93] | 6 | 49 | 68 | 32 | 81 | 55 | 21 | 49 | 30 | |||
| Marre, 1997 [94] | 8 | 43 | 57 | 24 | 100 | 57 | 50 | 168 | 119 | 40 | 69 | 48 |
| Barnas, 1997 [95] | 6 | 47 | 70 | 100 | 48 | 9 | 27 | 14 | 15 | 21 | 4 | |
| Ringel, 1997 [96] | 8 | 51 | 54 | 26 | 100 | 43 | 64 | 152 | 79 | 78 | 199 | 92 |
| Schmidt, 1997 [97] | 7 | 65 | 51 | 29 | 100 | 74 | 61 | 129 | 121 | 62 | 154 | 131 |
| Kawada, 1997 [98] | 5 | 59 | 62 | 22 | 89 | 89 | 30 | 84 | 96 | 28 | ||
| Kario, 1997 [99] | 6 | 72 | 41 | 25 | 0 | 100 | 42 | 62 | 29 | 86 | 98 | 16 |
| Nakajima, 1996 [17] | 6 | 56 | 64 | 100 | 37 | 50 | 14 | 18 | 19 | 4 | ||
| Chowdhury, 1996 [100] | 6 | 39 | 55 | 100 | 97 | 40 | 124 | 78 | 32 | 79 | 55 | |
| McLaughlin, 1996 [101] | 4 | 42 | 57 | 114 | 366 | 312 | 75 | 203 | 93 | |||
| Oh, 1996 [102] | 6 | 35 | 42 | 19 | 100 | 12 | 9 | 10 | 11 | 10 | 7 | |
| Schmidt, 1996 [103] | 5 | 55 | 58 | 63 | 27 | 51 | 28 | 21 | 38 | 36 | ||
| Doi, 1996 [104] | 7 | 62 | 51 | 22 | 100 | 55 | 50 | 85 | 29 | 56 | 56 | 12 |
| Ohno, 1996 [105] | 7 | 61 | 53 | 23 | 100 | 56 | 26 | 38 | 15 | 33 | 15 | 9 |
| Mizuiri, 1995 [106] | 6 | 54 | 100 | 90 | 11 | 51 | 19 | 11 | 11 | 9 | ||
| Yorioka, 1995 [107] | 5 | 33 | 44 | 6 | 27 | 13 | 8 | 46 | 47 | 10 | ||
| Fujisawa, 1995 [108] | 4 | 100 | 24 | 23 | 7 | 17 | 12 | 6 | ||||
| Panagiotopoulos, 1995 [109] | 5 | 62 | 66 | 100 | 10 | 25 | 15 | 29 | 44 | 42 | ||
| Tarnow, 1995 [110] | 8 | 41 | 61 | 24 | 100 | 68 | 40 | 95 | 63 | 46 | 77 | 67 |
| Schmidt, 1995 [111] | 6 | 45 | 75 | 59 | 44 | 81 | 79 | 40 | 117 | 77 | ||
| Yoshida, 1995 [112] | 7 | 39 | 64 | 15 | 20 | 17 | 16 | 19 | 24 | 3 | ||
| Dudley, 1995 [113] | 7 | 53 | 67 | 29 | 100 | 58 | 29 | 82 | 47 | 35 | 87 | 36 |
| Harden, 1995 [114] | 5 | 35 | 19 | 41 | 40 | 17 | 42 | 39 | ||||
| Doria, 1994 [115] | 5 | 29 | 100 | 15 | 35 | 24 | 20 | 41 | 16 | |||
| Marre, 1994 [116] | 6 | 39 | 60 | 23 | 100 | 4 | 35 | 23 | 15 | 28 | 19 | |
| Powrie, 1994 [117] | 6 | 35 | 50 | 20 | 100 | 4 | 8 | 7 | 24 | 37 | 24 | |
Quality score: result of quality assessed in each study (detailed data were shown in supplementary file); Age: mean age; Male: probability of male; BMI: mean body mass index; DM: prevalence of diabetes mellitus; HT: prevalence of hypertension; II: number of II genotype carries; ID: number of ID genotype carries; DD: number of DD genotype carries.
Preliminary Pooled Analyses
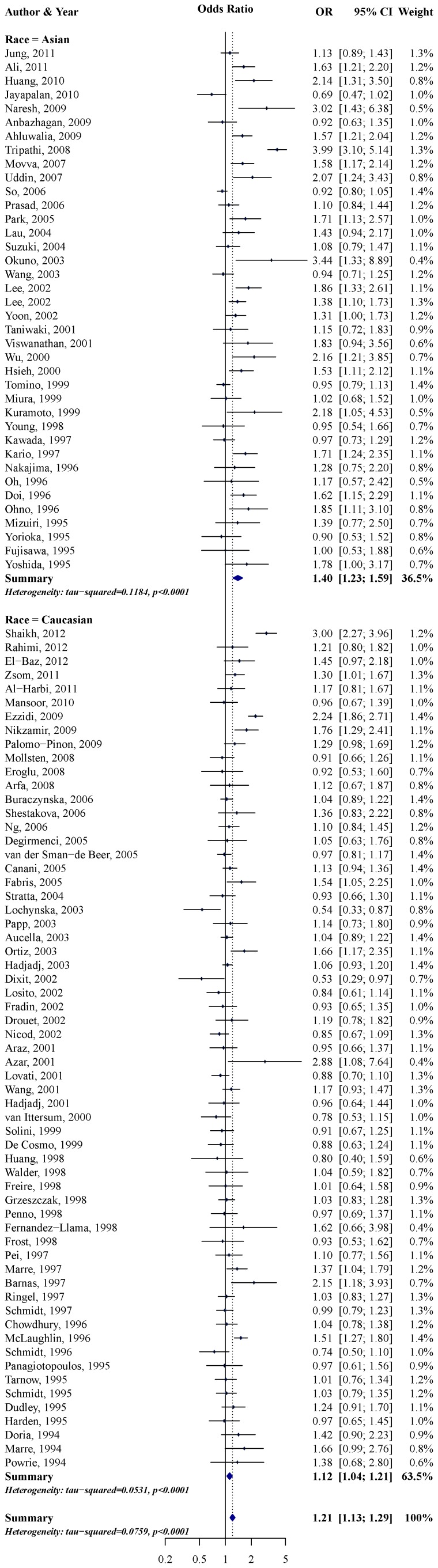
Our meta-analysis showed that a significantly increased CKD risk was associated with the D allele compared with the I allele in each subgroup. Figure 2 shows that D allele carriers had an OR of 1.21 for risk of all-cause CKD compared with I allele carriers, and that these ORs were dissimilar in different ethnicities (p = 0.002). OR for CKD in Asian individuals carrying the D allele compared with those carrying the I allele was 1.40 (95% CI: 1.23–1.59), and in Caucasian individuals was 1.12 (95% CI: 1.04–1.21). A summary of the other results is shown in Table 3 and are very similar to those in Figure 2. In the allele type, genotype, and dominant/recessive models, individuals carrying the D allele showed higher CKD risk, and ORs were higher in Asians than in Caucasians. The heterogeneities were higher in Asian populations than in Caucasian populations in each subgroup.
Figure 2. Forest plot of the association between ACE I/D and all-cause CKD using allele type model.
Table 3. Odds ratio of ACE I/D and all-cause CKD, diabetic nephropathy, non-diabetic nephropathy using assumption of allele type, genotype, dominant and recessive model.
| Model | Ethnicity | All-cause CKD | Diabetic nephropathy | Non-diabetic nephropathy | |||||||||
| n | OR | 95% CI | τ2 | n | OR | 95% CI | τ2 | n | OR | 95% CI | τ2 | ||
| Allele type (D vs. I) | All studies | 99 | 1.21 | (1.13, 1.29) | 0.076 | 65 | 1.23 | (1.14, 1.33) | 0.063 | 22 | 1.29 | (1.06, 1.56) | 0.166 |
| Asian | 38 | 1.40 | (1.23, 1.59) | 0.118 | 24 | 1.36 | (1.18, 1.56) | 0.070 | 10 | 1.59 | (1.17, 2.17) | 0.212 | |
| Caucasian | 61 | 1.12 | (1.04, 1.21) | 0.053 | 41 | 1.17 | (1.06, 1.29) | 0.064 | 12 | 1.08 | (0.91, 1.29) | 0.054 | |
| Genotype-1 (DD vs. II) | All studies | 99 | 1.44 | (1.26, 1.64) | 0.277 | 65 | 1.48 | (1.26, 1.74) | 0.249 | 22 | 1.67 | (1.16, 2.41) | 0.556 |
| Asian | 38 | 1.87 | (1.46, 2.38) | 0.377 | 24 | 1.66 | (1.27, 2.18) | 0.230 | 10 | 2.77 | (1.62, 4.75) | 0.567 | |
| Caucasian | 61 | 1.25 | (1.07, 1.46) | 0.224 | 41 | 1.39 | (1.14, 1.71) | 0.275 | 12 | 1.11 | (0.77, 1.60) | 0.211 | |
| Genotype-2 (ID vs. II) | All studies | 99 | 1.20 | (1.10, 1.32) | 0.098 | 65 | 1.26 | (1.13, 1.40) | 0.082 | 22 | 1.17 | (0.92, 1.49) | 0.206 |
| Asian | 38 | 1.34 | (1.14, 1.57) | 0.152 | 24 | 1.31 | (1.09, 1.57) | 0.088 | 10 | 1.43 | (0.99, 2.05) | 0.259 | |
| Caucasian | 61 | 1.13 | (1.02, 1.25) | 0.062 | 41 | 1.23 | (1.07, 1.42) | 0.084 | 12 | 0.91 | (0.74, 1.13) | 0.570 | |
| Dominant(DD+ID vs. II) | All studies | 99 | 1.28 | (1.16, 1.41) | 0.132 | 65 | 1.33 | (1.19, 1.50) | 0.110 | 22 | 1.30 | (1.00, 1.69) | 0.271 |
| Asian | 38 | 1.48 | (1.25, 1.74) | 0.180 | 24 | 1.42 | (1.19, 1.70) | 0.095 | 10 | 1.66 | (1.12, 2.45) | 0.317 | |
| Caucasian | 61 | 1.17 | (1.05, 1.31) | 0.100 | 41 | 1.28 | (1.10, 1.49) | 0.130 | 12 | 1.01 | (0.80, 1.27) | 0.031 | |
| Recessive(DD vs. ID+II) | All studies | 99 | 1.27 | (1.15, 1.39) | 0.090 | 65 | 1.26 | (1.13, 1.42) | 0.112 | 22 | 1.53 | (1.18, 1.99) | 0.262 |
| Asian | 38 | 1.56 | (1.28, 1.90) | 0.227 | 24 | 1.42 | (1.12, 1.79) | 0.169 | 10 | 2.22 | (1.42, 3.48) | 0.369 | |
| Caucasian | 61 | 1.16 | (1.04, 1.28) | 0.090 | 41 | 1.21 | (1.06, 1.37) | 0.097 | 12 | 1.20 | (0.92, 1.57) | 0.118 | |
Identifying Moderators of the Association between ACE I/D Polymorphisms and CKD Risk
Table 4 shows the assessment results of moderate effect on all-cause CKD using the allele type model. Compared with I allele carriers, D allele carriers had a higher OR for CKD risk in Asians than in Caucasians (OR of moderate effect: 1.24; 95% CI: 1.08–1.42). Hypertension also was a moderator (OR of moderate effect: 1.55; 95% CI: 1.04–2.32) and still had a significant moderate effect after adjusting ethnicity (OR of moderate effect: 1.57; 95% CI: 1.07–2.31). No additional moderators were significant after adjustment for ethnicity and hypertension (data not shown). In the final model of these analyses, τ2 declined by 15.5% (crude: 0.097; after adjustment: 0.082) and p of Egger’s regression test was not significant (p = 0.258). We did not find a significant gender-dependent effect.
Table 4. Moderator effects of allele type model (D vs. I) on all-cause CKD.
| Unadjusted | Adjusted ethnicity | ||||
| n | OR | 95% CI | OR | 95% CI | |
| Ethnicity (Caucasian is ref.) | 99 | 1.24* | (1.08, 1.42) | ||
| Study design (CS is ref.) | 99 | 1.05 | (0.92, 1.20) | 1.03 | (0.90, 1.18) |
| Quality score (per 1 score) | 99 | 0.98 | (0.93, 1.04) | 0.99 | (0.94, 1.05) |
| Kidney function of case (non-ESRD is ref.) | 99 | 1.03 | (0.87, 1.23) | 0.97 | (0.81, 1.16) |
| Age (per 10 years) | 88 | 1.02 | (0.95, 1.09) | 1.01 | (0.95, 1.08) |
| Male (per 100%) | 84 | 1.48 | (0.72, 3.01) | 1.63 | (0.81, 3.28) |
| BMI (per 5 kg/m2) | 45 | 0.86 | (0.73, 1.03) | 0.95 | (0.78, 1.16) |
| DM (per 100%) | 81 | 0.96 | (0.78, 1.18) | 0.99 | (0.81, 1.22) |
| Hypertension (per 100%) | 68 | 1.55* | (1.04, 2.32) | 1.57* | (1.07, 2.31) |
Depend variable: log odds ratio of ACE I/D and all-cause CKD using allele type model.
n: number of studies; OR: odds ratio for moderate effect; 95% CI: 95% confidence interval.
CS: cross-sectional study; non-ESRD: not only ESRD patients; Age: mean age; Male: probability of male; BMI: mean body mass index; DM: prevalence of diabetes mellitus; Hypertension: prevalence of hypertension.
: p<0.05.
In subgroup analyses of diabetic nephropathy, study design (OR for moderate effect: 1.21; 95% CI: 1.02–1.42) and body mass index (OR of moderate effect: 0.84; 95% CI: 0.70–1.00) had significant moderate effects on ACE I/D polymorphisms and diabetic nephropathy. After adjustment for ethnicity, the moderate effects of study design (OR of moderate effect: 1.19; 95% CI: 1.01–1.41) was still significant but that of body mass index (OR for moderate effect: 0.91; 95% CI: 0.73–1.13) was not (data not shown). The results of the nondiabetic nephropathy subgroup were similar to those for all-cause CKD, with ethnicity (OR of moderate effect: 1.69; 95% CI: 1.07–2.68) and hypertension (OR of moderate effect: 2.42; 95% CI: 1.19–4.93) the only two significant moderators in multivariable analyses (data not shown).
Estimated Gender-dependent Effects
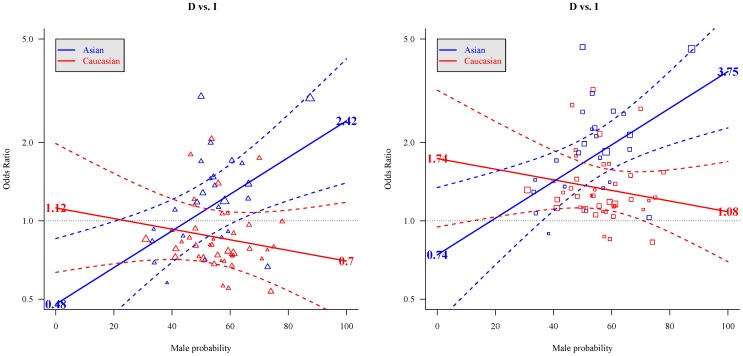
Table 5 shows the interaction of ethnicity and gender. We found no significant interactions between the other variables and gender. Coefficients of interaction were significant for hypertension (p before adjustment for hypertension = 0.015; p after adjustment for hypertension = 0.003). No other moderators were significant when added to the final model. A proportion of 32.8% of heterogeneity (crude τ2 = 0.100; after adjustment, τ2 = 0.068) was caused by different ethnicity, gender probability, and prevalence of hypertension in the study population, and p of Egger’s regression test was not significant (p = 0.217). Figure 3 shows OR of ACE I/D polymorphisms and CKD risk in different combination of ethnicity, gender, and hypertension status based on Model 2 in Table 5. A gender-dependent effect analysis showed the strongest association between the ACE I/D polymorphisms and CKD risk in Asian males with hypertension (OR: 3.75; 95% CI: 1.84–7.65) or without (OR: 2.42; 95% CI: 1.40–4.20).
Table 5. Three way interaction of Asian, male and ACE D allele on all-cause CKD, diabetic nephropathy and non-diabetic nephropathy.
| All-cause CKD | Diabetic nephropathy | Non-diabetic nephropathy | ||||||
| Model 1 | Model 2 | |||||||
| β | se | β | se | β | se | β | se | |
| Intercept | 0.297 | 0.275 | 0.115 | 0.291 | 0.090 | 0.369 | −0.213 | 0.746 |
| Race (Caucasian is ref.) | −0.697 | 0.391 | −0.853* | 0.402 | −0.817 | 0.569 | −1.080 | 0.816 |
| Male (per 100%) € | −0.312 | 0.476 | −0.470 | 0.501 | −0.122 | 0.691 | −0.349 | 1.050 |
| Race×Male | 1.662* | 0.686 | 2.094* | 0.715 | 2.064 | 1.082 | 2.666* | 1.231 |
| Hypertension (per 100%) € | 0.437* | 0.192 | 0.229 | 0.294 | 0.857* | 0.245 | ||
| τ2 | 0.075 | 0.068 | 0.081 | 0.036* | ||||
| Egger’s test | p = 0.097 | p = 0.217 | p = 0.385 | p = 0.032 | ||||
Depend variable: log odds ratio of ACE I/D and CKD using allele type model.
β: coefficients in meta-regression; se: standard error of β.
Model 1: Hypertension was not included in independent variables.
Model 2: Hypertension was included in independent variables.
Egger’s test: p-value of Egger’s regression test.
€: references of parameters were 0%.
: p<0.05.
Figure 3. Gender-dependent effects of ACE I/D and all-cause CKD in each population.
Left figure was showed the OR in people without hypertension; right figure was showed the odds ratio in patient with hypertension. Red triangle and square: individual studies in Caucasian; blue triangle and square: individual studies in Asian. Solid line: unbiased estimator of odds ratio; dashed line: 95% confidence interval of odds ratios. Estimated value of log odds ratio in individual studies without hypertension used following formula: observed log odds ratio – hypertension prevalence of study population×0.437 (according to Model 2 in Table 5) Estimated value of log odds ratio in individual studies with hypertension used following formula: observed log odds ratio+(1– hypertension prevalence of study population)×0.437 (according to Model 2 in Table 5).
Interaction of ethnicity and gender was borderline significant (p = 0.056) in the diabetic nephropathy subgroup, but was significant (p = 0.030) in the nondiabetic nephropathy subgroup. Although the result of symmetry assessment was significant in nondiabetic nephropathy subgroup (p of Egger’s regression test = 0.032), it was noteworthy that 78.3% of heterogeneity (crude τ2∶0.166; after adjustment, τ2∶0.036) were caused by ethnicity, gender, and hypertension.
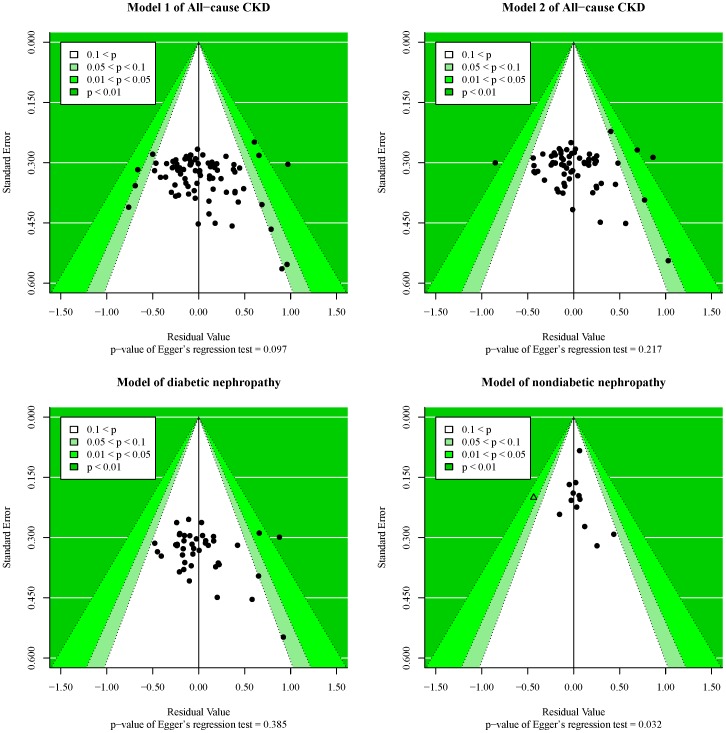
The symmetry of final models was shown in Figure 4. Funnel plots presented the association between residual and standard error based on results of Table 5, and each point represents a study. Egger’s regression test indicated no evidence of publication bias among studies included into the final model this meta-analysis and diabetic nephropathy subgroup. The model in nondiabetic nephropathy subgroup was asymmetric, and it might be due to the study reported by Jung et al. [32]. We did sensitivity analyses leaving the article out (data not shown). The result of symmetry assessment was not significant (p of Egger’s regression test = 0.245), and the coefficients in this model were still significant (p of interaction effect of ethnicity and gender = 0.002; p of moderate effect of hypertension <0.001). In addition, the τ2 was 0 in this sensitivity model.
Figure 4. Funnel plot of three way interaction model in each subgroup.
The model in nondiabetic nephropathy subgroup was asymmetric. The triangle in that plot was study reported by Jung et[32], and the p value of the student residual was less 0.05. After excluding this study, the p value of Egger’s regression test was not significant (p = 0.245) and the moderate effect of interaction and hypertension were more significantly (p of interaction: 0.0304→0.0023; p of hypertension: 0.0005→<0.0001).
Discussion
This study showed that CKD risk was higher in D allele carriers than in I allele carriers, and there was no strong evidence that analyses using different model assumptions might produce dissimilar results. Heterogeneity was higher in the Asian population than in the Caucasian population. Interaction between ACE I/D polymorphisms and hypertension exerted an additive effect on CKD risk. A gender-dependent effect of ACE I/D polymorphisms on CKD risk was clearly apparent in Asians but not in Caucasians.
The DD genotype showed higher gene expression and serum ACE levels than the ID genotype, followed by the II genotype [9], [118]. High blood ACE levels may increase blood angiotensin II levels [8], and individuals with higher angiotensin II levels may have a higher CKD risk [119], [120]. Previous studies showed that the association between ACE I/D polymorphisms and CKD risk might not be dominant or recessive [8], [9], [118]. Previous meta-analysis studies showed the supported results, they reported that DD genotype had higher risk of CKD than ID genotype, followed by the II genotype. We also observed the apparent linear association between numbers of D allele and odds ratios compared the II genotype in genotype analyses [10]–[12], [20]–[22]. The assumption of the allele type model in this association might be more reasonable, and it may thus be true that individuals carrying the D allele have a higher CKD risk.
Hypertension in some patients is due to a dysfunction of RAS such as abnormal secretion of renin, causing increased blood angiotensin I levels [121]. D allele carriers had higher ACE levels than I allele carriers [118], leading to more efficient conversion of angiotensin I to angiotensin II, resulting in CKD [119], [120]. The mechanism may be an additive effect of hypertension and the D allele. An additive effect was significant in the nondiabetic group but not in the diabetic nephropathy subgroups. The blood levels of advanced glycation end products (AGE) diabetic patients may be high, possibly causing blood pressure increases [122]. We accordingly hypothesize that the probability of hypertension because of a dysfunction of RAS was higher in the nondiabetic nephropathy subgroup than in the diabetic nephropathy subgroup. Thus, the interaction between ACE I/D polymorphisms and hypertension was significant only in the nondiabetic nephropathy subgroup. This hypothesis may require further studies for confirmation.
We found a significant gender-dependent effect of ACE I/D polymorphisms on CKD risk in Asians. In previous studies in Asians, the ORs of the additive effect on the DD genotype of males were 2.94 and 1.41 in Japanese [17] and Koreans [18], respectively. Another study in Japan also reported a positive additive effect of the DD genotype of males [19]. Studies of Caucasians reported contrary results, with an interaction OR of 0.42 in Pakistan [14]. Another two studies in France [15] and Mexico [16] also showed an additive effect between the DD genotype and female gender but not male gender. Although the interaction tests in these studies were not significant, we could observe dissimilar gender-dependent effect in different ethnicity. Previous studies have also reported a different gender-dependent effect of ACE I/D polymorphisms on blood ACE levels in Asians and Caucasians. In a study conducted in China [13], differences in blood ACE levels between DD genotype and other genotypes among men were significantly greater than those in women. On the other hand, a study conducted in Germany [123] reported the opposite result.
Androgens may play a key role in this additive effect. A study has shown that in intact male rats and ovariectomized female rats that received testosterone for 5 weeks, the androgen may have contributed to the decrease in pressure natriuresis [124]. In an animal study, ACE activity was higher in male mice than in female mice, and this gender difference disappeared after gonadectomy [125]. In previous reports, sensitivity to androgens was stated to be higher in Caucasians than in Asians [126]. Blood androgen levels in Caucasians and Asians showed no significant differences [127], [128]. On the basis of previous studies, we hypothesized that the dissimilar gender-dependent effect of ACE I/D polymorphisms on CKD risk in Caucasians and Asians might be accounted for by dissimilar sensitivity to androgens. The gender difference of male sex hormone utilization was higher in Asians than in Caucasians. Therefore, that the additive effect of the D allele and male gender was also higher in Asians than in Caucasians.
In subgroup analyses, the above additive effect was borderline significant in the diabetic nephropathy subgroup, but there was no evidence that diabetic mellitus might contribute to this additive effect. Although the additive effect also could explain why two populations with different ethnicities had different heterogeneity before adjustment for any moderators, the calculated risk ratio of ACE I/D polymorphisms on CKD risk may have been affected by the gender-dependent effect in Asians.
Our study had three limitations. First, we relied on tabular data rather than on individual patient data, possibly leading to an inflated standard error in pooled analyses. However, we still observed a significant gender-dependent effect difference in different ethnicities. Second, estimates of diabetes mellitus and hypertension prevalence did not factor in the effects of therapy for them. Some subjects having higher blood glucose and blood pressure may have taken drugs, leading to normal biochemical values in reports. Third, we may have missed unpublished data for the nondiabetic nephropathy subgroup. But the results of this subgroup were similar to the results of previous studies and we still observed a significant result excluding the greatest impact of symmetry study; therefore, there is no evidence to question their reliability.
In conclusion, CKD risk was higher with the D allele than with the I allele. Asian ethnicity and hypertension had positive moderate effects, and their effects were more likely to be higher in patients with nondiabetic nephropathy. A gender-dependent effect of ACE I/D polymorphisms on CKD risk was confirmed in Asians; the D allele showed 3.75-fold greater risk for CKD than the I allele in hypertensive Asian males. These results suggest that Asian males should be offered testing for defects in ACE I/D polymorphisms, especially if they are hypertensive. We suggest that physicians should provide specific protection to D-allele carriers, for example by administering ACE inhibitors to hypertensive patients.
Supporting Information
PRISMA 2009 Checklist.
(DOC)
Search strategies. Web sites and uniform resource locator: MEDLINE: http://www.ncbi.nlm.nih.gov/pubmed Cochrane Library: http://www.thecochranelibrary.com Embase: https://www.embase.com
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, et al. (2007) Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 2. Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, et al. (2009) Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 13: 621–630. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Wang F, Wang L, Wang W, Liu B, et al. (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379: 815–822. [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 5. Li S, McAlpine DD, Liu J, Li S, Collins AJ (2004) Differences between blacks and whites in the incidence of end-stage renal disease and associated risk factors. Adv Ren Replace Ther 11: 5–13. [DOI] [PubMed] [Google Scholar]
- 6. Tsai JC, Chen SC, Hwang SJ, Chang JM, Lin MY, et al. (2010) Prevalence and risk factors for CKD in spouses and relatives of hemodialysis patients. Am J Kidney Dis 55: 856–866. [DOI] [PubMed] [Google Scholar]
- 7. McClellan WM, Warnock DG, Judd S, Muntner P, Patzer RE, et al. (2012) Association of family history of ESRD, prevalent albuminuria, and reduced GFR with incident ESRD. Am J Kidney Dis 59: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remuzzi G, Perico N, Macia M, Ruggenenti P (2005) The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl: S57–65. [DOI] [PubMed]
- 9. Mizuiri S, Hemmi H, Kumanomidou H, Iwamoto M, Miyagi M, et al. (2001) Angiotensin-converting enzyme (ACE) I/D genotype and renal ACE gene expression. Kidney Int 60: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 10. Qin YH, Zhou TB, Su LN, Lei FY, Huang WF, et al. (2011) Association between ACE polymorphism and risk of IgA nephropathy: a meta-analysis. J Renin Angiotensin Aldosterone Syst 12: 215–223. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Fang Q, Yu N, Zhao D, Zhang Y, et al. (2012) Association between genetic polymorphism of the angiotensin-converting enzyme and diabetic nephropathy: a meta-analysis comprising 26,580 subjects. J Renin Angiotensin Aldosterone Syst 13: 161–174. [DOI] [PubMed] [Google Scholar]
- 12.Zhou TB, Yin SS, Qin YH (2012) Association between angiotensin-converting enzyme insertion/deletion gene polymorphism and end-stage renal disease susceptibility. J Renin Angiotensin Aldosterone Syst [Epub ahead of print]. [DOI] [PubMed]
- 13.Zhang YF, Cheng Q, Tang NL, Chu TT, Tomlinson B, et al. (2013) Gender difference of serum angiotensin-converting enzyme (ACE) activity in DD genotype of ACE insertion/deletion polymorphism in elderly Chinese. J Renin Angiotensin Aldosterone Syst. [DOI] [PubMed]
- 14. Mansoor Q, Bilal N, Qureshi S, Qureshi O, Javaid A, et al. (2010) Gender based disparities in ACE I/D polymorphism associated with progression of diabetic nephropathy in Pakistani patients with type 2 diabetes mellitus. International Journal of Diabetes and Metabolism 18: 67–71. [Google Scholar]
- 15. Hadjadj S, Gallois Y, Alhenc-Gelas F, Chatellier G, Marre M, et al. (2003) Angiotensin-I-converting enzyme insertion/deletion polymorphism and high urinary albumin concentration in French Type 2 diabetes patients. Diabet Med 20: 677–682. [DOI] [PubMed] [Google Scholar]
- 16. Palomo-Pinon S, Gutierrez-Rodriguez ME, Diaz-Flores M, Sanchez-Barrera R, Valladares-Salgado A, et al. (2009) DD genotype of angiotensin-converting enzyme in type 2 diabetes mellitus with renal disease in Mexican Mestizos. Nephrology (Carlton) 14: 235–239. [DOI] [PubMed] [Google Scholar]
- 17. Nakajima S, Baba T, Yajima Y (1996) Is ACE gene polymorphism a useful marker for diabetic albuminuria in Japanese NIDDM patients? Diabetes Care 19: 1420–1422. [DOI] [PubMed] [Google Scholar]
- 18. Park HC, Choi SR, Kim BS, Lee TH, Kang BS, et al. (2005) Polymorphism of the ACE Gene in dialysis patients: overexpression of DD genotype in type 2 diabetic end-stage renal failure patients. Yonsei Med J 46: 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomino Y, Makita Y, Shike T, Gohda T, Haneda M, et al. (1999) Relationship between polymorphism in the angiotensinogen, angiotensin-converting enzyme or angiotensin II receptor and renal progression in Japanese NIDDM patients. Nephron 82: 139–144. [DOI] [PubMed] [Google Scholar]
- 20. Zhou TB, Qin YH, Su LN, Lei FY, Huang WF, et al. (2011) The association between angiotensin-converting enzyme insertion/deletion gene variant and risk of focal segmental glomerulosclerosis: a systematic review and meta-analysis. J Renin Angiotensin Aldosterone Syst 12: 624–633. [DOI] [PubMed] [Google Scholar]
- 21.Zhou TB, Yin SS, Liang R (2012) A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and end-stage renal disease risk in IgA nephropathy patients. J Renin Angiotensin Aldosterone Syst [Epub ahead of print]. [DOI] [PubMed]
- 22. Yu ZY, Chen LS, Zhang LC, Zhou TB (2012) Meta-analysis of the relationship between ACE I/D gene polymorphism and end-stage renal disease in patients with diabetic nephropathy. Nephrology (Carlton) 17: 480–487. [DOI] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. (2002) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomizedstudies in meta-analysis. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2013 Aug 23.
- 24. Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36: 1–48. [Google Scholar]
- 25.Schwarzer G (2012) meta: Meta-Analysis with R.
- 26. Lee YJ, Tsai JC (2002) ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care 25: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 27. Shaikh R, Shahid SM, Nawab SN, Mansoor Q, Javaid A, et al. (2012) Distribution of ACE I/D polymorphism in the patients of diabetes and nephropathy in Pakistan. Int J Hum Genet 12: 133–138. [Google Scholar]
- 28. Rahimi Z, Vaisi-Raygani A, Rahimi Z, Parsian A (2012) Concomitant presence of endothelial nitric oxide 894T and angiotensin II-converting enzyme D alleles are associated with diabetic nephropathy in a Kurdish population from Western Iran. Nephrology 17: 175–181. [DOI] [PubMed] [Google Scholar]
- 29. El-Baz R, Settin A, Ismaeel A, Khaleel AA, Abbas T, et al. (2012) MTHFR C677T, A1298C and ACE I/D polymorphisms as risk factors for diabetic nephropathy among type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst 13: 472–477. [DOI] [PubMed] [Google Scholar]
- 30. Zsom M, Fulop T, Zsom L, Barath A, Maroti Z, et al. (2011) Genetic polymorphisms and the risk of progressive renal failure in elderly Hungarian patients. Hemodial Int 15: 501–508. [DOI] [PubMed] [Google Scholar]
- 31. Al-Harbi EM, Farid EM, Gumaa KA, Masuadi EM, Singh J (2011) Angiotensin-converting enzyme gene polymorphisms and T2DM in a case-control association study of the Bahraini population. Mol Cell Biochem 350: 119–125. [DOI] [PubMed] [Google Scholar]
- 32. Jung ES, Kim SM, Cha RH, Oh YK, Kim YS, et al. (2011) Impact of polymorphisms of the genes encoding angiotensin II-forming enzymes on the progression of IgA nephropathy. Nephron Clin Pract 118: c122–129. [DOI] [PubMed] [Google Scholar]
- 33.Ali A, Vasudevan R, Ismail P, Thiam Seong CL, Chakravarthi S (2011) Analysis of insertion/deletion polymorphisms of the angiotensin converting enzyme gene in Malaysian end-stage renal disease patients. J Renin Angiotensin Aldosterone Syst [Epub ahead of print]. [DOI] [PubMed]
- 34. Huang HD, Lin FJ, Li XJ, Wang LR, Jiang GR (2010) Genetic polymorphisms of the renin-angiotensin-aldosterone system in Chinese patients with end-stage renal disease secondary to IgA nephropathy. Chin Med J (Engl) 123: 3238–3242. [PubMed] [Google Scholar]
- 35. Jayapalan JJ, Muniandy S, Chan SP (2010) Null association between ACE gene I/D polymorphism and diabetic nephropathy among multiethnic Malaysian subjects. Indian J Hum Genet 16: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naresh VV, Reddy AL, Sivaramakrishna G, Sharma PV, Vardhan RV, et al. (2009) Angiotensin converting enzyme gene polymorphism in type II diabetics with nephropathy. Indian J Nephrol 19: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ezzidi I, Mtiraoui N, Kacem M, Chaieb M, Mahjoub T, et al. (2009) Identification of specific angiotensin-converting enzyme variants and haplotypes that confer risk and protection against type 2 diabetic nephropathy. Diabetes Metab Res Rev 25: 717–724. [DOI] [PubMed] [Google Scholar]
- 38. Nikzamir A, Esteghamati A, Feghhi M, Nakhjavani M, Rashidi A, et al. (2009) The insertion/deletion polymorphism of the angiotensin-converting enzyme gene is associated with progression, but not development, of albuminuria in Iranian patients with type 2 diabetes. J Renin Angiotensin Aldosterone Syst 10: 109–114. [DOI] [PubMed] [Google Scholar]
- 39. Anbazhagan K, Sampathkumar K, Ramakrishnan M, Gomathi P, Gomathi S, et al. (2009) Analysis of polymorphism in renin angiotensin system and other related genes in South Indian chronic kidney disease patients. Clin Chim Acta 406: 108–112. [DOI] [PubMed] [Google Scholar]
- 40. Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, et al. (2009) ACE variants interact with the RAS pathway to confer risk and protection against type 2 diabetic nephropathy. DNA Cell Biol 28: 141–150. [DOI] [PubMed] [Google Scholar]
- 41. Mollsten A, Kockum I, Svensson M, Rudberg S, Ugarph-Morawski A, et al. (2008) The effect of polymorphisms in the renin-angiotensin-aldosterone system on diabetic nephropathy risk. J Diabetes Complications 22: 377–383. [DOI] [PubMed] [Google Scholar]
- 42. Eroglu Z, Cetinkalp S, Erdogan M, Kosova B, Karadeniz M, et al. (2008) Association of the angiotensinogen M235T and angiotensin-converting enzyme insertion/deletion gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. J Diabetes Complications 22: 186–190. [DOI] [PubMed] [Google Scholar]
- 43. Arfa I, Abid A, Nouira S, Elloumi-Zghal H, Malouche D, et al. (2008) Lack of association between the angiotensin-converting enzyme gene (I/D) polymorphism and diabetic nephropathy in Tunisian type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst 9: 32–36. [DOI] [PubMed] [Google Scholar]
- 44. Tripathi G, Sharma RK, Baburaj VP, Sankhwar SN, Jafar T, et al. (2008) Genetic risk factors for renal failure among north Indian ESRD patients. Clin Biochem 41: 525–531. [DOI] [PubMed] [Google Scholar]
- 45. Movva S, Alluri RV, Komandur S, Vattam K, Eppa K, et al. (2007) Relationship of angiotensin-converting enzyme gene polymorphism with nephropathy associated with Type 2 diabetes mellitus in Asian Indians. J Diabetes Complications 21: 237–241. [DOI] [PubMed] [Google Scholar]
- 46. Uddin M, Azam M, Chowdhury N, Akhteruzzaman S (2007) Angiotensin I-converting enzyme gene polymorphism in type 2 diabetic patients with nephropathy. J Med Sci 7: 682–685. [Google Scholar]
- 47. Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, et al. (2006) Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant 21: 979–983. [DOI] [PubMed] [Google Scholar]
- 48. So WY, Ma RC, Ozaki R, Tong PC, Ng MC, et al. (2006) Angiotensin-converting enzyme (ACE) inhibition in type 2, diabetic patients– interaction with ACE insertion/deletion polymorphism. Kidney Int 69: 1438–1443. [DOI] [PubMed] [Google Scholar]
- 49. Shestakova MV, Vikulova OK, Gorashko NM, Voronko OE, Babunova NB, et al. (2006) The relationship between genetic and haemodynamic factors in diabetic nephropathy (DN): Case-control study in type 1 diabetes mellitus (T1DM). Diabetes Res Clin Pract 74: S41–S50. [Google Scholar]
- 50. Ng DP, Placha G, Choo S, Chia KS, Warram JH, et al. (2006) A disease haplotype for advanced nephropathy in type 2 diabetes at the ACE locus. Diabetes 55: 2660–2663. [DOI] [PubMed] [Google Scholar]
- 51. Prasad P, Tiwari AK, Kumar KM, Ammini AC, Gupta A, et al. (2006) Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Med Genet 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Degirmenci I, Kebapci N, Basaran A, Efe B, Gunes HV, et al. (2005) Frequency of angiotensin-converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Int J Clin Pract 59: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 53. van der Sman-de Beer F, Verhagen C, Rombach SM, Boorsma P, van Manen JG, et al. (2005) ACE I/D polymorphism is associated with mortality in a cohort study of patients starting with dialysis. Kidney Int 68: 2237–2243. [DOI] [PubMed] [Google Scholar]
- 54. Canani LH, Costa LA, Crispim D, Goncalves Dos Santos K, Roisenberg I, et al. (2005) The presence of allele D of angiotensin-converting enzyme polymorphism is associated with diabetic nephropathy in patients with less than 10 years duration of Type 2 diabetes. Diabet Med 22: 1167–1172. [DOI] [PubMed] [Google Scholar]
- 55. Fabris B, Bortoletto M, Candido R, Barbone F, Cattin MR, et al. (2005) Genetic polymorphisms of the renin-angiotensin-aldosterone system and renal insufficiency in essential hypertension. J Hypertens 23: 309–316. [DOI] [PubMed] [Google Scholar]
- 56. Lau YK, Woo KT, Choong HL, Zhao Y, Tan HB, et al. (2004) Renin-angiotensin system gene polymorphisms: its impact on IgAN and its progression to end-stage renal failure among Chinese in Singapore. Nephron Physiol 97: p1–8. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki H, Sakuma Y, Kanesaki Y, Eiro M, Asahi K, et al. (2004) Close relationship of plasminogen activator inhibitor-1 4G/5G polymorphism and progression of IgA nephropathy. Clin Nephrol 62: 173–179. [DOI] [PubMed] [Google Scholar]
- 58. Stratta P, Bermond F, Guarrera S, Canavese C, Carturan S, et al. (2004) Interaction between gene polymorphisms of nitric oxide synthase and renin-angiotensin system in the progression of membranous glomerulonephritis. Nephrol Dial Transplant 19: 587–595. [DOI] [PubMed] [Google Scholar]
- 59. Lochynska K, Ciechanowicz A, Pawlaczyk K, Oko A, Czekalski S (2003) Angiotensin-converting enzyme gene insertion/deletion polymorphism in newly diagnosed primary chronic glomerulonephritis. Adv Clin Exp Med 12: 161–165. [Google Scholar]
- 60. Papp F, Friedman AL, Bereczki C, Haszon I, Kiss E, et al. (2003) Renin-angiotensin gene polymorphism in children with uremia and essential hypertension. Pediatr Nephrol 18: 150–154. [DOI] [PubMed] [Google Scholar]
- 61. Aucella F, Margaglione M, Vigilante M, Gatta G, Grandone E, et al. (2003) PAI-1 4G/5G and ACE I/D gene polymorphisms and the occurrence of myocardial infarction in patients on intermittent dialysis. Nephrol Dial Transplant 18: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 62. Okuno S, Utsugi T, Ohno T, Ohyama Y, Uchiyama T, et al. (2003) Angiotensin-converting enzyme gene polymorphism as a potent risk factor for developing microalbuminuria in Japanese patients with type 2 diabetes mellitus: a 9-year follow-up study. J Int Med Res 31: 290–298. [DOI] [PubMed] [Google Scholar]
- 63. Ortiz MA, De Prado A, Donate T, Gallart L, Claramunt H, et al. (2003) Angiotensin-converting enzyme polymorphism gene and evolution of nephropathy to end-stage renal disease. Nephrology (Carlton) 8: 171–176. [DOI] [PubMed] [Google Scholar]
- 64. Wang AY, Chan JC, Wang M, Poon E, Lui SF, et al. (2003) Cardiac hypertrophy and remodeling in relation to ACE and angiotensinogen genes genotypes in Chinese dialysis patients. Kidney Int 63: 1899–1907. [DOI] [PubMed] [Google Scholar]
- 65. Dixit M, Mansur A, Dixit N, Gilman J, Santarina L, et al. (2002) The role of ACE gene polymorphism in rapidity of progression of focal segmental glomerulosclerosis. J Postgrad Med 48: 266–269 discussion 269. [PubMed] [Google Scholar]
- 66. Losito A, Kalidas K, Santoni S, Ceccarelli L, Jeffery S (2002) Polymorphism of renin-angiotensin system genes in dialysis patients–association with cerebrovascular disease. Nephrol Dial Transplant 17: 2184–2188. [DOI] [PubMed] [Google Scholar]
- 67. Yoon HJ, Kim H, Kim HL, Lee SG, Zheng SH, et al. (2002) Interdependent effect of angiotensin-converting enzyme and platelet-activating factor acetylhydrolase gene polymorphisms on the progression of immunoglobulin A nephropathy. Clin Genet 62: 128–134. [DOI] [PubMed] [Google Scholar]
- 68. Fradin S, Goulet-Salmon B, Chantepie M, Grandhomme F, Morello R, et al. (2002) Relationship between polymorphisms in the renin-angiotensin system and nephropathy in type 2 diabetic patients. Diabetes Metab 28: 27–32. [PubMed] [Google Scholar]
- 69. Drouet M, Aupetit C, Denizot Y, Bois M, Bridoux F, et al. (2002) Analysis of three genetic markers in IgA nephropathy patients from a single region. Clin Nephrol 57: 253–260. [DOI] [PubMed] [Google Scholar]
- 70. Nicod J, Frey BM, Frey FJ, Ferrari P (2002) Role of the alpha-adducin genotype on renal disease progression. Kidney Int 61: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 71. Araz M, Yilmaz N, Gungor K, Okan V, Kepekci Y, et al. (2001) Angiotensin-converting enzyme gene polymorphism and microvascular complications in Turkish type 2 diabetic patients. Diabetes Res Clin Pract 54: 95–104. [DOI] [PubMed] [Google Scholar]
- 72. Azar ST, Zalloua PA, Medlej R, Halabi G (2001) The DD genotype of the ACE gene polymorphism is associated with diabetic nephropathy in the type-1 diabetics. Endocr Res 27: 99–108. [DOI] [PubMed] [Google Scholar]
- 73. Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P (2001) Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int 60: 46–54. [DOI] [PubMed] [Google Scholar]
- 74. Wang JG, Staessen JA, Tizzoni L, Brand E, Birkenhager WH, et al. (2001) Renal function in relation to three candidate genes. Am J Kidney Dis 38: 1158–1168. [DOI] [PubMed] [Google Scholar]
- 75. Taniwaki H, Ishimura E, Matsumoto N, Emoto M, Inaba M, et al. (2001) Relations between ACE gene and ecNOS gene polymorphisms and resistive index in type 2 diabetic patients with nephropathy. Diabetes Care 24: 1653–1660. [DOI] [PubMed] [Google Scholar]
- 76. Viswanathan V, Zhu Y, Bala K, Dunn S, Snehalatha C, et al. (2001) Association between ACE gene polymorphism and diabetic nephropathy in South Indian patients. JOP 2: 83–87. [PubMed] [Google Scholar]
- 77. Hadjadj S, Belloum R, Bouhanick B, Gallois Y, Guilloteau G, et al. (2001) Prognostic value of angiotensin-I converting enzyme I/D polymorphism for nephropathy in type 1 diabetes mellitus: a prospective study. J Am Soc Nephrol 12: 541–549. [DOI] [PubMed] [Google Scholar]
- 78. Wu S, Xiang K, Zheng T, Sun D, Weng Q, et al. (2000) Relationship between the renin-angiotensin system genes and diabetic nephropathy in the Chinese. Chin Med J (Engl) 113: 437–441. [PubMed] [Google Scholar]
- 79. Hsieh MC, Lin SR, Hsieh TJ, Hsu CH, Chen HC, et al. (2000) Increased frequency of angiotensin-converting enzyme DD genotype in patients with type 2 diabetes in Taiwan. Nephrol Dial Transplant 15: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 80. van Ittersum FJ, de Man AM, Thijssen S, de Knijff P, Slagboom E, et al. (2000) Genetic polymorphisms of the renin-angiotensin system and complications of insulin-dependent diabetes mellitus. Nephrol Dial Transplant 15: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 81. Solini A, Giacchetti G, Sfriso A, Fioretto P, Sardu C, et al. (1999) Polymorphisms of angiotensin-converting enzyme and angiotensinogen genes in type 2 diabetic sibships in relation to albumin excretion rate. Am J Kidney Dis 34: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 82. De Cosmo S, Margaglione M, Tassi V, Garrubba M, Thomas S, et al. (1999) ACE, PAI-1, decorin and Werner helicase genes are not associated with the development of renal disease in European patients with type 1 diabetes. Diabetes Metab Res Rev 15: 247–253. [DOI] [PubMed] [Google Scholar]
- 83. Miura J, Uchigata Y, Yokoyama H, Omori Y, Iwamoto Y (1999) Genetic polymorphism of renin-angiotensin system is not associated with diabetic vascular complications in Japanese subjects with long-term insulin dependent diabetes mellitus. Diabetes Res Clin Pract 45: 41–49. [DOI] [PubMed] [Google Scholar]
- 84. Kuramoto N, Iizuka T, Ito H, Yagui K, Omura M, et al. (1999) Effect of ACE gene on diabetic nephropathy in NIDDM patients with insulin resistance. Am J Kidney Dis 33: 276–281. [DOI] [PubMed] [Google Scholar]
- 85. Huang XH, Rantalaiho V, Wirta O, Pasternack A, Hiltunen TP, et al. (1998) Angiotensin-converting enzyme insertion/deletion polymorphism and diabetic albuminuria in patients with NIDDM followed Up for 9 years. Nephron 80: 17–24. [DOI] [PubMed] [Google Scholar]
- 86. Walder B, Spanaus KS, Weinreich T, Sawicki PT, Widmer U (1998) Genetic heterogeneity in the renin-angiotensin system and the risk of diabetic nephropathy: Association with the angiotensinogen gene, but not with the ACE gene. J Clin Basic Cardiol 1: 55–58. [Google Scholar]
- 87. Freire MB, van Dijk DJ, Erman A, Boner G, Warram JH, et al. (1998) DNA polymorphisms in the ACE gene, serum ACE activity and the risk of nephropathy in insulin-dependent diabetes mellitus. Nephrol Dial Transplant 13: 2553–2558. [DOI] [PubMed] [Google Scholar]
- 88. Grzeszczak W, Zychma MJ, Lacka B, Zukowska-Szczechowska E (1998) Angiotensin I-converting enzyme gene polymorphisms: relationship to nephropathy in patients with non-insulin dependent diabetes mellitus. J Am Soc Nephrol 9: 1664–1669. [DOI] [PubMed] [Google Scholar]
- 89. Young RP, Chan JC, Critchley JA, Poon E, Nicholls G, et al. (1998) Angiotensinogen T235 and ACE insertion/deletion polymorphisms associated with albuminuria in Chinese type 2 diabetic patients. Diabetes Care 21: 431–437. [DOI] [PubMed] [Google Scholar]
- 90. Penno G, Chaturvedi N, Talmud PJ, Cotroneo P, Manto A, et al. (1998) Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 47: 1507–1511. [DOI] [PubMed] [Google Scholar]
- 91. Fernandez-Llama P, Poch E, Oriola J, Botey A, Coll E, et al. (1998) Angiotensin converting enzyme gene I/D polymorphism in essential hypertension and nephroangiosclerosis. Kidney Int 53: 1743–1747. [DOI] [PubMed] [Google Scholar]
- 92. Frost D, Pfohl M, Clemens P, Haring HU, Beischer W (1998) Evaluation of the insertion/deletion ACE gene polymorphism as a risk factor for carotid artery intima-media thickening and hypertension in young type 1 diabetic patients. Diabetes Care 21: 836–840. [DOI] [PubMed] [Google Scholar]
- 93. Pei Y, Scholey J, Thai K, Suzuki M, Cattran D (1997) Association of angiotensinogen gene T235 variant with progression of immunoglobin A nephropathy in Caucasian patients. J Clin Invest 100: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marre M, Jeunemaitre X, Gallois Y, Rodier M, Chatellier G, et al. (1997) Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: Genetique de la Nephropathie Diabetique (GENEDIAB) study group. J Clin Invest 99: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barnas U, Schmidt A, Illievich A, Kiener HP, Rabensteiner D, et al. (1997) Evaluation of risk factors for the development of nephropathy in patients with IDDM: insertion/deletion angiotensin converting enzyme gene polymorphism, hypertension and metabolic control. Diabetologia 40: 327–331. [DOI] [PubMed] [Google Scholar]
- 96. Ringel J, Beige J, Kunz R, Distler A, Sharma AM (1997) Genetic variants of the renin-angiotensin system, diabetic nephropathy and hypertension. Diabetologia 40: 193–199. [DOI] [PubMed] [Google Scholar]
- 97. Schmidt S, Strojek K, Grzeszczak W, Bergis K, Ritz E (1997) Excess of DD homozygotes in haemodialysed patients with type II diabetes. The Diabetic Nephropathy Study Group. Nephrol Dial Transplant 12: 427–429. [DOI] [PubMed] [Google Scholar]
- 98. Kawada N, Moriyama T, Yokoyama K, Yamauchi A, Ando A, et al. (1997) Renin-angiotensin system component gene polymorphisms in Japanese maintenance haemodialysis patients. Nephrology 3: 521–526. [Google Scholar]
- 99. Kario K, Kanai N, Nishiuma S, Fujii T, Saito K, et al. (1997) Hypertensive nephropathy and the gene for angiotensin-converting enzyme. Arterioscler Thromb Vasc Biol 17: 252–256. [DOI] [PubMed] [Google Scholar]
- 100. Chowdhury TA, Dronsfield MJ, Kumar S, Gough SL, Gibson SP, et al. (1996) Examination of two genetic polymorphisms within the renin-angiotensin system: no evidence for an association with nephropathy in IDDM. Diabetologia 39: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 101. McLaughlin KJ, Harden PN, Ueda S, Boulton-Jones JM, Connell JM, et al. (1996) The role of genetic polymorphisms of angiotensin-converting enzyme in the progression of renal diseases. Hypertension 28: 912–915. [DOI] [PubMed] [Google Scholar]
- 102. Oh TG, Shin CS, Park KS, Kim SY, Cho BY, et al. (1996) Relationships between angiotensin I converting enzyme gene polymorphism and renal complications in Korean IDDM patients. Korean J Intern Med 11: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schmidt A, Kiener HP, Barnas U, Arias I, Illievich A, et al. (1996) Angiotensin-converting enzyme polymorphism in patients with terminal renal failure. J Am Soc Nephrol 7: 314–317. [DOI] [PubMed] [Google Scholar]
- 104. Doi Y, Yoshizumi H, Yoshinari M, Iino K, Yamamoto M, et al. (1996) Association between a polymorphism in the angiotensin-converting enzyme gene and microvascular complications in Japanese patients with NIDDM. Diabetologia 39: 97–102. [DOI] [PubMed] [Google Scholar]
- 105. Ohno T, Kawazu S, Tomono S (1996) Association analyses of the polymorphisms of angiotensin-converting enzyme and angiotensinogen genes with diabetic nephropathy in Japanese non-insulin-dependent diabetics. Metabolism 45: 218–222. [DOI] [PubMed] [Google Scholar]
- 106. Mizuiri S, Hemmi H, Inoue A, Yoshikawa H, Tanegashima M, et al. (1995) Angiotensin-converting enzyme polymorphism and development of diabetic nephropathy in non-insulin-dependent diabetes mellitus. Nephron 70: 455–459. [DOI] [PubMed] [Google Scholar]
- 107. Yorioka T, Suehiro T, Yasuoka N, Hashimoto K, Kawada M (1995) Polymorphism of the angiotensin converting enzyme gene and clinical aspects of IgA nephropathy. Clin Nephrol 44: 80–85. [PubMed] [Google Scholar]
- 108. Fujisawa T, Ikegami H, Shen GQ, Yamato E, Takekawa K, et al. (1995) Angiotensin I-converting enzyme gene polymorphism is associated with myocardial infarction, but not with retinopathy or nephropathy, in NIDDM. Diabetes Care 18: 983–985. [DOI] [PubMed] [Google Scholar]
- 109. Panagiotopoulos S, Smith TJ, Aldred GP, Baker EJ, Jacklin CJ, et al. (1995) Angiotensin-converting enzyme (ACE) gene polymorphism in type II diabetic patients with increased albumin excretion rate. J Diabetes Complications 9: 272–276. [DOI] [PubMed] [Google Scholar]
- 110. Tarnow L, Cambien F, Rossing P, Nielsen FS, Hansen BV, et al. (1995) Lack of relationship between an insertion/deletion polymorphism in the angiotensin I-converting enzyme gene and diabetic nephropathy and proliferative retinopathy in IDDM patients. Diabetes 44: 489–494. [DOI] [PubMed] [Google Scholar]
- 111. Schmidt S, Stier E, Hartung R, Stein G, Bahnisch J, et al. (1995) No association of converting enzyme insertion/deletion polymorphism with immunoglobulin A glomerulonephritis. Am J Kidney Dis 26: 727–731. [DOI] [PubMed] [Google Scholar]
- 112. Yoshida H, Mitarai T, Kawamura T, Kitajima T, Miyazaki Y, et al. (1995) Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest 96: 2162–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dudley CR, Keavney B, Stratton IM, Turner RC, Ratcliffe PJ (1995) U.K. Prospective Diabetes Study. XV: Relationship of renin-angiotensin system gene polymorphisms with microalbuminuria in NIDDM. Kidney Int 48: 1907–1911. [DOI] [PubMed] [Google Scholar]
- 114. Harden PN, Geddes C, Rowe PA, McIlroy JH, Boulton-Jones M, et al. (1995) Polymorphisms in angiotensin-converting-enzyme gene and progression of IgA nephropathy. Lancet 345: 1540–1542. [DOI] [PubMed] [Google Scholar]
- 115. Doria A, Warram JH, Krolewski AS (1994) Genetic predisposition to diabetic nephropathy. Evidence for a role of the angiotensin I–converting enzyme gene. Diabetes 43: 690–695. [DOI] [PubMed] [Google Scholar]
- 116. Marre M, Bernadet P, Gallois Y, Savagner F, Guyene TT, et al. (1994) Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes 43: 384–388. [DOI] [PubMed] [Google Scholar]
- 117. Powrie JK, Watts GF, Ingham JN, Taub NA, Talmud PJ, et al. (1994) Role of glycaemic control in development of microalbuminuria in patients with insulin dependent diabetes. BMJ 309: 1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, et al. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86: 1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wolf G, Neilson EG (1993) Angiotensin II as a renal growth factor. J Am Soc Nephrol 3: 1531–1540. [DOI] [PubMed] [Google Scholar]
- 120. Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J (2001) Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens 10: 321–329. [DOI] [PubMed] [Google Scholar]
- 121. Kobori H, Nangaku M, Navar LG, Nishiyama A (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287. [DOI] [PubMed] [Google Scholar]
- 122. Ahmed N (2005) Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pract 67: 3–21. [DOI] [PubMed] [Google Scholar]
- 123. Biller H, Zissel G, Ruprecht B, Nauck M, Busse Grawitz A, et al. (2006) Genotype-corrected reference values for serum angiotensin-converting enzyme. Eur Respir J 28: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 124. Reckelhoff JF, Zhang H, Granger JP (1998) Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439. [DOI] [PubMed] [Google Scholar]
- 125. Lim YK, Retnam L, Bhagavath B, Sethi SK, bin Ali A, et al. (2002) Gonadal effects on plasma ACE activity in mice. Atherosclerosis 160: 311–316. [DOI] [PubMed] [Google Scholar]
- 126. Ewing JA, Rouse BA (1978) Hirsutism, race and testosterone levels: comparison of East Asians and Euroamericans. Hum Biol 50: 209–215. [PubMed] [Google Scholar]
- 127. Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, Henderson BE (2006) Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 15: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 128. Ellis L, Nyborg H (1992) Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids 57: 72–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)
Search strategies. Web sites and uniform resource locator: MEDLINE: http://www.ncbi.nlm.nih.gov/pubmed Cochrane Library: http://www.thecochranelibrary.com Embase: https://www.embase.com
(DOC)