Abstract
Pseudomonas syringae pv. actinidiae (Psa) is an emerging phytopathogen causing bacterial canker disease in kiwifruit plants worldwide. Quorum sensing (QS) gene regulation plays important roles in many different bacterial plant pathogens. In this study we analyzed the presence and possible role of N-acyl homoserine lactone (AHL) quorum sensing in Psa. It was established that Psa does not produce AHLs and that a typical complete LuxI/R QS system is absent in Psa strains. Psa however possesses three putative luxR solos designated here as PsaR1, PsaR2 and PsaR3. PsaR2 belongs to the sub-family of LuxR solos present in many plant associated bacteria (PAB) that binds and responds to yet unknown plant signal molecules. PsaR1 and PsaR3 are highly similar to LuxRs which bind AHLs and are part of the canonical LuxI/R AHL QS systems. Mutation in all the three luxR solos of Psa showed reduction of in planta survival and also showed additive effect if more than one solo was inactivated in double mutants. Gene promoter analysis revealed that the three solos are not auto-regulated and investigated their possible role in several bacterial phenotypes.
Introduction
Quorum sensing (QS) is an intercellular communication system in bacteria that links bacterial cell density to gene expression via the production and detection of signal molecules [1], [2]. In Gram-negative bacteria, N-acyl homoserine lactones (AHL) signal molecules are most commonly used; they are produced by an AHL synthase, which belongs to the LuxI-protein family and a transcriptional regulator belonging to the LuxR family. The LuxR-family protein forms a complex with the cognate AHL at threshold (‘quorum’) concentration and affects the transcription of target genes [3]. QS-dependent regulation in bacteria is most often involved in the coordinated community action of bacteria like antibiotic production, biofilm formation, conjugation, bioluminescence, production of extracellular enzymes, virulence factors and pigment formation [2], [4]–[6]. Well-characterized examples of QS-dependent regulation of phenotypic functions in Pseudomonas include the LasI/LasR and RhlI/RhlR of the opportunistic human pathogen P. aeruginosa [7], [8], the AhlI/AhlR system of the plant pathogen P. syringae pv. syringae [9], the PfsI/PfsR and PfvI/PfvR of the emerging plant pathogen P. fuscovaginae [10], the PcoI/R system of P. corrugata [11], the two QS systems PhzI/R and CsaI/R of plant beneficial P. aureofaciens [12]–[14], the PupI/PupR of plant growth-promoting P. putida [15], [16], and the MupI/MupR QS system of plant growth promoting P. fluorescencs NCIMB 10586 [17].
The AHLs molecules produced by different LuxI-family synthases vary in length of the acyl chain (from 4 to 18 carbon atoms) and in their substitution (eg an hydroxyl or oxo substitution) in the third carbon position of the acyl chain [3]. LuxR proteins are approximately 250 amino acids long and consist of two domains; an AHL-binding domain at the N-terminal region [18], [19] and a DNA-binding helix-turn-helix (HTH) domain at the C-terminal region [20]–[22]. The AHL-binding domain recognizes AHLs most often resulting in its ability to bind target DNA in gene promoter regions at a conserved sites called a lux box [23], [24]. QS LuxRs display surprisingly low homologies (18–25%); 95% however share 9 highly conserved amino acid residues [6], [25]. Six of these are hydrophobic or aromatic and form the cavity of the AHL-binding domain and the remaining three are in the HTH domain [26].
In a typical AHL QS system, luxI/R genes are almost always located genetically adjacent to each other. In many proteobacteria, additional QS luxR-type genes also have been found that are unpaired to a cognate luxI synthase. An analysis of 265 proteobacterial genomes by Case et al. in 2008 showed that 68 had a canonical paired luxI/R system and out of these 68, 45 contained more luxRs than luxIs; another set of 45 genomes contained only QS luxR genes. These QS LuxR proteins lacking a genetically linked LuxI have been termed “orphans” [27] and more recently “solos” [28]. LuxR solos have the same modular structure; an AHL binding domain in the N-terminus and a DNA binding HTH domain at the C-terminus as like other LuxRs from canonical LuxI/R systems. LuxR solos can result in the increase of the regulatory targets of the canonical complete AHL QS systems by responding to endogenous AHLs or they are responsible for eavesdropping by detecting exogenous AHLs molecules. For example, QscR from P. aeruginosa responds to endogenously produced AHLs [29], [30] while SdiA of Salmonella enterica and E. coli which do not produces AHLs and eavesdrops on AHLs produced by neighboring bacteria [31]–[34].
A sub-group of LuxR solos has been recently discovered which are only found in plant-associated bacteria (PAB) that do not bind AHLs but to plant produced compounds [35], [36]. These LuxRs are very closely associated to QS LuxRs differing in the conservation of one or two of the six highly conserved amino acids in the AHL-binding domain. Five members of this subfamily have been studied and these are XccR of Xanthomonas campestris pv. campestris (Xcc), OryR of Xanthomonas oryzae pv. oryzae (Xoo), PsoR of Pseduomonas fluorescens, XagR of Xanthomonas axonopodis pv. glycines (Xag) and NesR in Sinorhizobium meliloti [37]–[42]. OryR of the rice vascular pathogen Xoo is involved in virulence, it responds to plant signals and activates the expression of the neighboring proline iminopeptidase (pip) and of motility genes [38], [39], [43]. XccR of the crucifer pathogen Xcc also responds to a yet unidentified plant compound and regulates the neighboring pip gene [42]. XagR of the soybean pathogen Xag which causes bacterial leaf pustule on soybean (Glycine max) is also involved in virulence [37]. Like XccR in Xcc and OryR in Xoo, also XagR in Xag activates pip transcription in planta thought to be due to a plant compound(s) which are over-produced by the plant in response to pathogen attack by Xag. Two of these PAB LuxR-type proteins have been studied in plant-beneficial bacteria, namely PsoR of P. fluorescens and NesR of S. meliloti [40], [41]. PsoR responds to plant compounds of several plant species and plays a role in biocontrol in rhizospheric P. fluorescens [41], NesR of S. meliloti has been associated with survival under stress and utilization of various carbon sources [40].
Pseudomonas syringae pv. actinidiae (Psa) is one of the emerging pathogens of Pseudomonas group which causes trunk canker, twig wilting and leaf spot on kiwifruit species (Actinidia deliciosa and chinensis) [44]. Psa was first described in Japan in 1984 [45] and later was isolated in South Korea [46] and Italy [47]. In 2008, a re-emergence of Psa was found on A. chinensis (kiwigold) plants in central Italy and caused a huge economic loss [48]. This outbreak was caused by a different population of Psa from the original one that caused fewer problems in 1992 [49]. More recently, Psa has been isolated in several countries including China [50], Portugal [51], France [52], Australia [53], Chile [54], New Zealand [55], Spain [56], Switzerland [57] and Turky [58].
The virulence mechanisms of Psa are largely unknown since no significant genetic and molecular studies have been thus far performed. Comparative genomic studies comparing genome sequences of several Psa strains suggests that a canonical AHL QS system is absent in Psa [49], [59]–[61]. Here we report that Psa does not produce AHLs and does not contain a complete canonical AHL QS system but possesses three LuxR solos. One of these belongs to the sub-family of PAB solos which do not respond to AHLs but to plant signals [41]. The other two LuxR solos might be involved in the signaling with neighboring bacteria by AHLs eavesdropping. Here we also report genetic studies of these three solos and their potential roles in virulence.
Results and Discussion
A Canonical AHL QS System is Absent in P. syringae pv. actinidiae (Psa)
It was of interest to determine if Psa produces AHLs and thus possesses a canonical AHL QS system. Purification of AHLs was performed on spent supernatants of 11 Psa isolates of Italy both from kiwigreen (A. deliciosa) and kiwigold (A. chinensis) (see Table 1); it was established that all the Psa strains did not produce detectable AHL molecules in TLC plates using three different AHL bacterial biosensors which can detect a wide range of structurally different AHLs (see Materials and Methods and data not shown). These results suggest that AHL mediated LuxI/R type QS is either absent in Psa strains or AHLs could not be detected by the analysis performed in this study as these might be produced in very low quantities or have structures which are not detected by the sensors. Soon after this analysis, the draft genome sequences of several Psa strains from Italy [49] and later from China, New Zealand, Japan, Korea and Chile were published [59]–[61]; none of these genomes contained canonical luxI/R pairs. It was concluded that Psa does not produce AHLs and consequently does not possess a complete AHL QS system. Plant pathogenic P. syringae strains display diverse and host-specific interactions with different plant species. Specific strains are classified to one of the over 50 known pathovars based on their ability to infect various plant species (www.pseudomonas-syringae.org). A few pathovars have been reported to be able to produce AHLs, these include P. syringae pv. syringae, P. syringae pv. tabaci, P. syringae pv. maculicola [9], [62], [63]; however many are believed not to produce AHL signal molecules.
Table 1. Bacterial strains used in this study.
| Strains | Relevant characteristicsa | Reference/Source |
| E. coli | ||
| DH5a | Cloning strain, Nalr | [72] |
| PRK2013 | Helper strain for tri-parental conjugation, Kmr | [77] |
| E. coli (pSB401) | Biosensor strain; Tcr | [70], [73] |
| Biosensors | ||
| A. tumefaciens NT1 | Harbouring pZLR4 plasmid, β-galactosidase reporter system, Gmr | [95] |
| C. violaceum CV026 | Violacin pigment reporter system, Kmr | [79] |
| Pseudomonas syringae pv. actinidiae ( Psa ) | ||
| Psa 10,22 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 10,24 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 10,25 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 10,29 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 10,30 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 11,41 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 11,47 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 11,50 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 11,51 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 12,56 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa 12,64 | Wild type; Italian isolate; Nfr | Lab collection |
| Psa-mR1 | psaR1::pKNOCK; Nfr, Kmr; derivative of wild type | This work |
| Psa-mR2 | psaR2::pKNOCK; Nfr, Kmr; derivative of wild type | This work |
| Psa-mR3 | psaR3:: in-frame deletion mutant generated by pEX19Gm plasmid; Nfr;derivative of wild type | This work |
| Psa-mR1+pBBR-psaR1 | Psa-mR1 carrying full length psaR1 in pBBR; Nfr, Kmr, Gmr; derivative of Psa-mR1 | This work |
| Psa-mR2+pBBR-psaR2 | Psa-mR2 carrying full length psaR2 in pBBR; Nfr, Kmr, Gmr; derivative of Psa-mR2 | This work |
| Psa-mR3+pBBR-psaR3 | Psa-mR3 carrying full length psaR3 in pBBR; Nfr, Gmr, derivative of Psa-mR3 | This work |
| Psa-mR3+pcos-psaR3 | Psa-mR3 carrying cosmid clone for psaR3; Nfr, Tcr, derivative of Psa-mR3 | This work |
| Psa-mR3+ Psa-mR1 | Psa-mR3 and Psa-mR1 double mutant; Nfr, Kmr; derivative of Psa-mR3 | This work |
| Psa-mR3+ Psa-mR2 | Psa-mR3 and Psa-mR2 double mutant; Nfr, Kmr; derivative of Psa-mR3 | This work |
| Psa-mR3+ Psa-mR1+ pcos-psaR3+pBBR-psaR1 | Psa-mR3 and Psa-mR1 double mutant carrying cosmid clone for psaR3 and fulllength psaR1 in pBBR; Nfr, Kmr, Tcr, Gmr; derivative of Psa-mR3+ Psa-mR1 | This work |
| Psa-mR3+ Psa-mR2+ pcos-psaR3+pBBR-psaR2 | Psa-mR3 and Psa-mR2 double mutant carrying cosmid clone for psaR3 and fulllength psaR2 in pBBR; Nfr, Kmr, Tcr, Gmr; derivative of Psa-mR3+ Psa-mR2 | This work |
Nalr, Kmr, Tcr, Gmr, and Nfr indicate resistance to nalidixic acid, kanamycin, tetracycline, gentamycin and nitrofurantoin respectively.
Psa has Two QS LuxR Solos and One PAB LuxR Solo
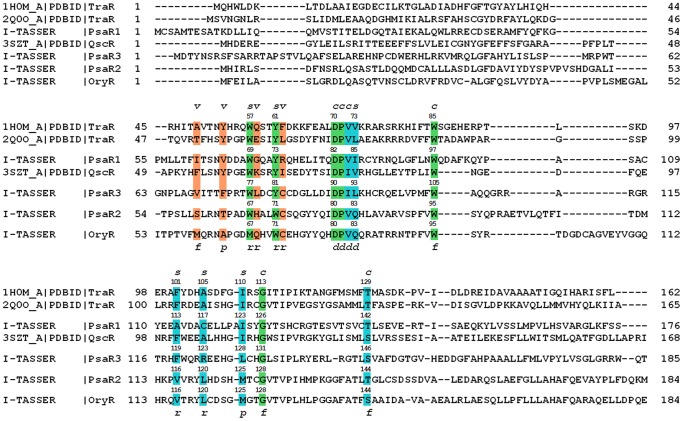
Numerous sequenced proteobacterial genomes have QS-related LuxR AHL sensors/regulators which lack a cognate LuxI AHL synthase [64]. These unpaired QS LuxR-family proteins have been recently called solos [28] and possess the typical modular structure having an acyl-homoserine lactone binding domain at their N-terminus and a helix-turn-helix DNA binding domain at their C-terminus. Interestingly Psa possesses three such LuxR solos (Table S3), designated here as PsaR1, PsaR2 and PsaR3, and these could be playing roles in detecting and responding to exogenous signals. One of these solos, PsaR2, most likely belongs to a sub-family of LuxR solos only found in plant-associated bacteria (PAB) which binds and responds to yet unknown plant signals [35]. This family has an imperfect AHL-binding domain with substitutions either in one or two of the highly conserved amino acids in the AHL binding domain, more precisely, W57 and Y61 (numbers in respect to TraR) were found substituted by methionine (M) and tryptophan (W) respectively (see below; Table 2). The other two LuxR solos appear to be related to AHL-LuxRs as they shared the nine conserved amino acid residues that were shown to be important for AHL binding (Table 2) [6], [25]. Generation of a phylogenetic tree for the subset of LuxR proteins that we used for alignment indicated that PsaR2 was grouped with PAB LuxR solos from other plant associated Pseudomonas species. PsaR1 and PsaR3 were grouped with QS associated LuxR proteins further suggesting that these two LuxR solos might be binding to AHL signal molecules (data not shown). The neighboring genes of the three luxR solos were also mapped (as depicted in Table S3). Importantly, we found that psaR2 has adjacently located the pip gene encoding for an proline iminopeptidase; notably all the sub-family of PAB luxR solos genes possess this locus next to it [35].
Table 2. Nine key amino acids in the alignment of LuxR proteins from subset of plant associated bacteria.
| Category of LuxRs | Subset of LuxR proteins from Plant associated bacteria | Key amino acids in auto-inducer binding domain | Key amino acids in HTH domain | |||||||
| AHL LuxRs | AAZ50597.1-TraR-At | W57 | Y61 | D70 | P71 | W85 | G113 | E178 | L182 | G188 |
| AAA25874.1-LasR-Pa | W | Y | D | P | W | G | E | L | G | |
| AAC44036.1-RhlR-Pa | W | Y | D | P | W | G | E | L | G | |
| ACM50924.1-ExpR-Pcc | W | Y | D | P | W | G | E | L | G | |
| AAC38403.1-CarR-Pcc | W | Y | D | P | W | G | E | L | G | |
| AAA82097.1-EsaR-Pantoea | W | Y | D | P | W | G | E | L | G | |
| PAB LuxR solos | YP_199907.1-OryR-Xoo | M | W | D | P | W | G | E | L | G |
| ZP_08179515.1-LuxR-Xcv | M | W | D | P | W | G | E | L | G | |
| YP_242384.1-AhyR-Xcc | M | W | D | P | W | G | E | L | G | |
| NP_643297.1-AhyR-Xac | M | W | D | P | W | G | E | L | G | |
| XagR-Xag | M | W | D | P | W | G | E | L | G | |
| ZP_03522316.1-LuxR-Re | M | W | D | P | W | G | E | L | G | |
| YP_765467.1-LuxR-Rl | M | W | D | P | W | G | E | L | G | |
| AGG75406.1-NesR-Sm | M | W | D | P | W | G | E | L | G | |
| AAY94512.1-PsoR-Pf-5 | W | W | D | P | W | G | E | L | G | |
| YP_004351870.1-NarL-Pbb | W | W | D | P | W | G | E | L | G | |
| YP_276356.1-LuxR-Psp | W | W | D | P | W | G | E | L | G | |
| BAD15091-LuxR-Paf | W | W | D | P | W | G | E | L | G | |
| ZP_07006441.1-AhyR-Psn | W | W | D | P | W | G | E | L | G | |
| AHL LuxR solo | PsaR1-Psa | W | Y | D | P | W | G | E | L | G |
| PAB LuxR solo | PsaR2-Psa | W | W | D | P | W | G | E | L | G |
| AHL LuxR solo | PsaR3-Psa | W | Y | D | P | W | G | E | L | G |
Position of nine key amino acids in LuxR proteins are indicated using AAZ50597.1-TraR-At as a reference sequence. Amino acid substitution in respective position has been indicated by bold letters. At; Agrobacterium tumefaciens, Pa; Pseudomonas aeruginosa PAO1, Pcc; Pectobacterium carotovorum subsp. carotovorum, Pantoea; Pantoea stewartii subsp. stewartii DC283, Xoo; Xanthomonas oryzae pv. oryzae KACC 10331, Xcv; Xanthomonas vesicatoria ATCC 35937, Xcc; Xanthomonas campestris pv. campestris str. 8004, Xac; Xanthomonas axonopodis pv. citri str. 306, Xag; Xanthomonas axonopodis pv. glycines, Re; Rhizobium etli GR56, Rl; Rhizobium leguminosarum bv. viciae 3841, Sm; Sinorhizobium meliloti 2011, Pf-5; Pseudomonas protegens Pf-5, Pbb; Pseudomonas brassicacearum subsp. brassicacearum NFM421, Psp; Pseudomonas syringae pv. phaseolicola 1448A, Paf; Pseudomonas azotoformans, Psn; Pseudomonas savastanoi pv. savastanoi NCPPB 3335, Psa; Pseudomonas syringae pv. actinidiae.
In-silico 3D Architecture and Cartography of the Ligand Binding Sites in PsaR1, PsaR2 and PsaR3
In order to gain insights on PsaRs substrate specificity the 3D architecture and cartography of the ligand-binding sites have been dissected exploiting a structure-based homology model of the three LuxR solos obtained using I-TASSER [65]. The protein sequences of the PsaR1, PsaR2 and PsaR3 regulatory domains were structurally aligned to four different LuxR family proteins (Figure 1): three of them related to canonical LuxR family (TraR from Sinorhizobium fredii [PDB_ID 2Q0O] [66], QscR from Pseudomonas aeruginosa [PDB_ID 3SZT] [67] and TraR from Agrobacterium tumefaciens [PDB_ID 1H0M] [68] and one from PAB LuxR family (OryR from Xanthomonas oryzae). A comparative structural analysis of the cartography of the regulatory domains of PsaR1, PsaR2 and PsaR3 according to the 3D molecular descriptors [69] suggested that in addition to the six conserved hydrophobic/aromatic residues previously reported (Table 2) [6], [26] to delineate the binding site (named Cluster 1 and colored in green in Figures 1, 2 and 3), two additional clusters of residues directly involved in ligand binding have been identified: Cluster 2 (colored in cyan in Figures 1, 2 and 3) that is reasonably conserved and Cluster 3 (colored in orange in Figures 1, 2 and 3) that is quite variable. The contribution of the three clusters to the binding site topology pinpoints to a tripartite architecture (TraR sequence numbering has been taken as reference): i) a conserved core, encompassing residues of Clusters 1 and 2 delimiting the binding site floor and the distal wall (residues 70, 71, 72, 85, 110, 113, 129) shared by QS LuxRs and PAB LuxR solos; ii) a specificity patch, encompassing residues of Clusters 1 and 2 that mainly delimit the binding site roof and the nearby regions of the proximal and distal walls (residues 57, 61, 73, 101, 105) conserved only within the members of the QS LuxRs or the PAB LuxR solos, respectively; iii) a variable part (variability patch), encompassing residues of Cluster 3 delimiting the binding site proximal wall and the nearby regions of the roof and of the floor (residues 49, 53, 58, 62), less conserved even within the members of QS LuxR or PAB LuxR solos respectively.
Figure 1. Structure-based multiple sequence alignment of the regulatory domains of the three Psa solos with QS LuxRs and with the prototype of the PAB LuxR solos subfamily.
The residues belonging to Cluster 1, to Cluster 2 and Cluster 3 are highlighted in green, cyan and in orange, respectively. The 3D architecture of the boundaries of the ligand-binding site is schematized by r (roof), f (floor), p (proximal wall) and d (distal wall) and its tripartite topology by c (conserved core), s (specificity patch) and v (variable patch).
Figure 2. Comparison of the ligand-binding sites of the three Psa solos with the prototypes of QS LuxRs and PAB LuxR solos subfamily.
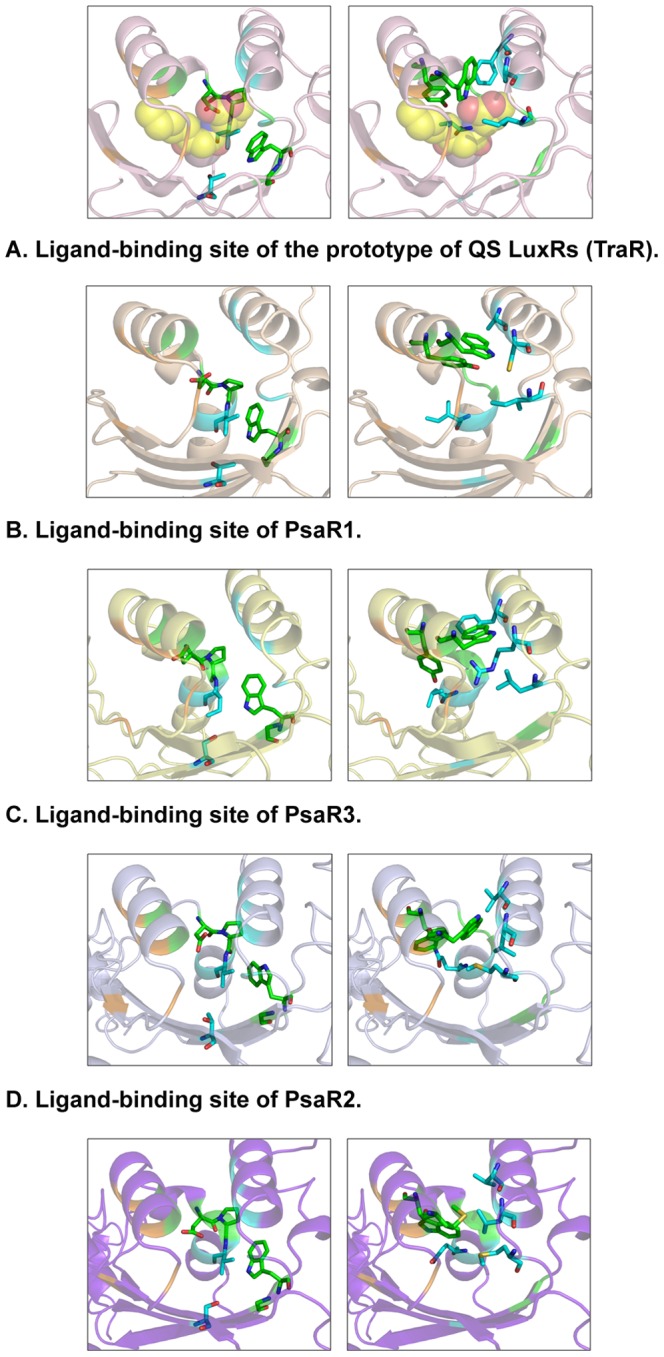
Mapping the protein residues defining the three Clusters (Cluster 1, Cluster 2 and Cluster 3 colored in green, cyan and in orange, respectively) showing the amino acid side chains that delineate the conserved core (left column) and the specificity patch (right column), respectively on the X-ray crystal structure of TraR in complex with OC8-HSL (PDB_ID 1H0M) [68] (A) and on the 3D structure-based homology models of PsaR1 (B), PsaR3 (C), PsaR2 (D) and OryR (E). The carbon, nitrogen and oxygen atoms of the OC8-HSL ligand shown in (A) are represented by spheres and are colored in yellow, blue and red respectively. Figures produced by Pymol [94].
Figure 3. Multiple sequence alignment of the regulatory domain of PsaR2 with the PAB LuxR solos subfamily.
The residues belonging to Cluster 1, Cluster 2 and Cluster 3 are highlighted in green, cyan and in orange, respectively.
The detailed molecular cartography of the regulatory domains was extended to the structure-based homology models of the three Psa solos, mainly focusing on Clusters 1 and Cluster 2. To this end we have not further discussed Cluster 3 due to its high variability likely to be responsible for the different selectivity towards molecules that belong to the same family of ligands or for the modulation of the degree of “promiscuity” towards members of the same family of compounds. Indeed, the 3D molecular mapping unveils that all of them share the conserved binding site core (marked by c in Figure 1 and represented in the left column of Figures 2B, 2C and 2D). The prototypes of QS LuxRs (TraR) and of PAB LuxR solos (OryR) are, for comparison, shown in the left column of Figures 2A and 2E respectively.
The identified binding-site molecular determinants of the PsaR1 and PsaR3 regulatory domains suggest a likely shared specificity towards AHL compounds. All the residues defining the specificity patch (marked by s in Figure 1 and represented in the right column of Figures 2B and 2C for PsaR1 and PsaR3 respectively) indeed differ from those of the PAB LuxR solos subfamily whose prototype OryR is shown in Figure 2E. Interestingly, most of the residues are distinctive of the canonical QS LuxRs whose prototype TraR is shown in Figure 2E. The residues belonging to Cluster 1 and delimiting the roof of the binding site, i.e. PasR1 W69 and PsaR3 W77 (TraR W57) and PsaR1 Y73 and PsaR3 Y81 (TraR Y61) are shared with QS LuxRs, whereas between the two residues, i.e. PsaR3 F119 (TraR F101) and PsaR1 A113, belonging to Cluster 2, only the former is conserved. Moreover, PsaR1 A113 also differs from the highly conserved V116 in PAB LuxR solos.
Similarly, Cluster 2 residues PsaR1 C117 (TraR A105) and PsaR3 R123 are conserved nor in the canonical QS LuxRs nor in the PAB LuxR solos families, the latter being characterized by the occurrence of the highly conserved residue L120. In the distal wall Cluster 2 conserved hydrophobic/aliphatic residues PsaR1 I85 (TraR V73), PsaR3 L93, are substituted by a highly conserved residue Q83 in PAB LuxR solos.
Interestingly, the molecular determinants of the PsaR2 binding site (Figure 2D) closely resemble those of the PAB LuxR solos (Figure 2E), highlighting a different binding specificity, most likely towards plant compounds unrelated to AHLs. Indeed all the residues belonging to the specificity patch are conserved with respect to the PAB LuxR solos subfamily and differ with respect to the canonical QS LuxRs family (Figure 3). The residue PsaR2 and OryR W71, belonging to Cluster 1, among the residues that delimit the roof of the binding site, is highly conserved in all members of the PAB LuxR solos subfamily. The residue TraR Y61 is the corresponding residue that is conversely highly conserved in all members of the QS LuxRs family. Alike, the two PsaR2 and OryR residues V116 and L120 are replaced by the quite conserved TraR F101 and A105 residues respectively. The distal wall residue PsaR2 and OryR Q83, belonging to Cluster 2, is highly conserved in the PAB LuxR solos subfamily, whereas it is substituted by a conserved hydrophobic/aliphatic residue (V/L/M), TraR V73, in QS LuxRs.
PsaR1, R2 and R3 Solos are Required for in planta Survival
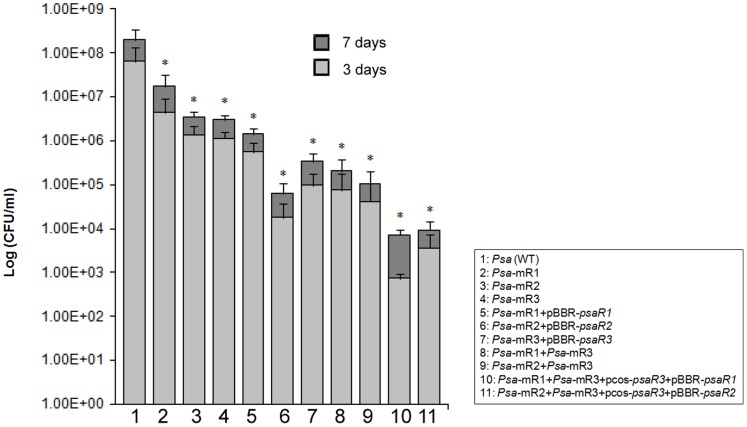
In order to assess the possible roles of the three luxR solos in plant virulence towards kiwifruit, all the three luxR solos were mutated creating three independent knock-out mutants. In addition, we have also generated two double mutants having two of the solos inactivated; namely the psa-mR1+psa-mR3 and psa-mR2+psa-mR3 double mutants. All these mutants were inoculated on A. deliciosa and A. chinosa kiwifruit leaves and bacterial multiplication and survival was determined after the 3rd and 7th day after inoculation by bacterial count (cfu/ml). All the three luxR solos mutants were found significantly impaired in in planta survival and multiplication compared to wild type Psa (Figure 4). Cfu/ml for psaR1 mutant was approximately 10 fold less compared to wild type level on the 3rd and 7th day after infection. The cfu/ml count for psaR2 and psaR3 mutants were found at least 100 fold less than the wild type level. The solo double mutants showed a further reduction compared to respective luxR solo single mutants. Compared to wild type the double mutants showed at least 1000 fold less cfu/ml count in the kiwifruit leaf after 3rd and 7th day of observation. These results implicate the three solos as being important for in planta growth and multiplication. We did not observe the recovery of these in planta survival phenotype upon complementation by providing the wild-type gene in trans in a plasmid on the single and double mutated luxR solos (Figure 4). We do not know the reason for this, however it has been previously observed that over-expression of this sub-family of luxR solos can have unexpected phenotypes and do not result in recovery of phenotypes [37], [43].
Figure 4. In planta survival of Pseudomonas syringae pv. actinidiae strains in Actinidia deliciosa cv Hayward leaf.
Histogram reporting in planta survival of Psa strains: The average bacterial count (log cfu/ml) of three independent experiments is reported with standard deviations for 3rd and 7th day after bacterial inoculation (1–2×106 cfu/ml) in Actinidia deliciosa cv. Hayward leaf. Statistical significance with respect to Psa wild type is indicated with one asterisk (P<0.01).
psaR1, psaR2 and psaR3 are not Auto-regulated and psaR2 does not Regulate pip in vitro
In order to study the expression and possible auto-regulation of the three luxR solos, we cloned the promoter of psaR1, psaR2 and psaR3 genes in a promoter probe vector harboring a promoterless lacZ gene. Gene promoter studies revealed that psaR2 was highly expressed compared to the other solos and that the three genes are not autoregulated under the conditions we tested (Table 3). As mentioned above, PsaR2 belongs to the sub-family of solos found in many plant associated bacteria (PAB) and which respond to yet unknown plant signal molecules [35]. All members of this LuxR solo sub-family contain an adjacent proline iminopeptidase (pip) gene which is regulated by the solo. We cloned the pip promoter in promoter probe vector and introduced it into the wild type and psaR2 mutant. We performed β-galactosidase assay in the presence and absence of plant kiwifruit leaf macerate extract; no significant increase in pip gene expression was observed in the presence of the plant extract (Table 4). Induction of pip gene expression is not always possible via the solos using plant extracts as the signal molecule might not be present in large amounts in the particular tissue and/or growth stage of the plant.
Table 3. Expression and auto-regulation of psaR1, psaR2 and psaR3.
| Strains | Average Miller unit | Standard deviation |
| Psa (WT)+pMP-psaR1 | 54.99a | 1.42 |
| Psa-mR1+pMP-psaR1 | 47.15a | 2.29 |
| Psa (WT)+pMP-psaR2 | 759.06b | 13.23 |
| Psa-mR2+pMP-psaR2 | 769.00b | 19.38 |
| Psa (WT)+pMP-psaR3 | 60.85a | 4.41 |
| Psa-mR3+pMP-psaR3 | 64.44a | 0.29 |
Statistical analyses (Student’s t test) were performed to compare the significance difference in promoter activity between wild type Psa strain and respective mutants. a, Not significant difference to a at P<0.05; b, significant difference to a at P<0.001 but not significant difference to b at P<0.05.
Table 4. Expression and regulation of psa-pip.
| Media | Strains | Average Miller unit | Standard deviation |
| KB | Psa (WT)+pMP-psa-pip | 91.75a | 2.21 |
| KB+Kiwi | Psa (WT)+pMP-psa-pip | 126.82c | 7.68 |
| KB | Psa-mR2+pMP-psa-pip | 103.60b | 3.47 |
| KB+Kiwi | Psa-mR2+pMP-psa-pip | 122.62c | 7.56 |
Expression of psa-pip was assessed in presence and absence of kiwi leaf extract for wild type (WT) and psaR2 mutant (Psa-mR2). Statistical analyses (Student’s t test) were performed to compare the significant difference in promoter analysis between wild type Psa strain and Psa-mR2 mutant in the presence and absence of kiwi extract. a, significant difference to b at P<0.01 and significant difference to c at P<0.05. c, not significant difference to c at P<0.05 but significant difference to a and b at P<0.05.
Response of PsaR1 and PsaR3 to AHLs
In order to test if PsaR1 and PsaR3 respond to any AHL signals, we performed an assay in E. coli harboring the pMULTIAHLPROM plasmid carrying a synthetic tandem promoter of seven different luxI gene promoters transcriptionally fused to a promoterless lacZ which respond to several different LuxR family proteins [70]. We introduced the pBBR empty vector as well as pBBR constructs containing either psaR1 or psaR3 in E. coli (pMULTIAHLPROM) and determined lacZ activities providing many structurally different exogenous AHLs. If PsaR1 and PsaR3 bind an AHL and recognize at least one of the promoters in pMULTIAHLPROM, this will result in an increase in lacZ activity. We analyzed all the structurally different AHLs having C4-12 acyl chains, unsubstituted at position C3 or having a ketone or a hydroxy. Results show that promoter activity was statistically significantly (P<0.05) increased only in the presence of OH-C6-AHL (28% increase) and OH-C8-AHL (16% increase) for PsaR1 and in the presence of OH-C6-AHL (38% increase), OH-C8-AHL (34% increase) OH-C10-AHL (28% increase) and OH-C12-AHL (23% increase) for PsaR3 compared to the same growth conditions in the absence of added exogenous AHLs and to the empty vector control (Figure 5). The background promoter activity that we observed could be to the SdiA solo present in E. coli which is known to respond to several AHLs [31]. In summary, we have detected a response if PsaR1 and PsaR3 respond to AHLs, future studies needs to involve biochemical analysis and identification of potential target gene(s).
Figure 5. Response of PsaR1 and PsaR3 to AHLs.
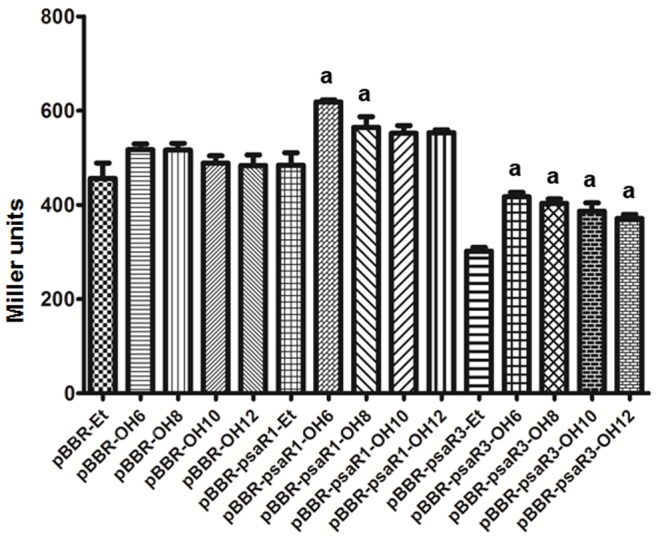
Histogram reporting gene promoter activity of E. coli harboring pMULTIAHLPROM in the presence of either pBBR, pBBR-psaR1 and pBBR-psaR3 plasmids and different hydroxy AHLs (OH-C6-AHL, OH-C8-AHL, OH-C10-AHL and OH-C12-AHL). All measures were performed in biological triplicates, and the mean Miller units with standard deviations are shown. Promoter activity was statistically significantly (P<0.05) increased (a) in the presence of OH-C6-AHL and OH-C8-AHL for PsaR1 compared to respective pBBR empty vector control. Similarly, for PsaR3 statistically significantly (P<0.05) increase (a) in lacZ expression was observed in the presence of OH-C6-AHL, OH-C8-AHL, OH-C10-AHL and OH-C12-AHL compared to respective pBBR empty vector controls.
Phenotypic Studies on the Three LuxR Solos
It was of interest to determine if any of the three LuxR solos were involved in regulating phenotypes which are known to be relevant to bacterial communities and potentially important for virulence. Bacterial movement via swarming and swimming was tested as described in the Materials and Methods section. Mutants of psaR1 and psaR2 swarmed just like the wild type whereas the psaR3 swarmed less (Table 5). The psaR1-psaR3 and psaR2-psaR3 double mutants also displayed a reduced swarming phenotype (Table 5). These results indicate that psaR3 is involved in regulating swarming in Psa. Complementation experiments of psaR3 and the two double mutants using the wild type psaR3 gene harboured in a plasmid resulted in a more pronounced defect in swarming. The reason for this is not known, it is possible that extra copies of the regulator might result in causing this defect in swarming. A very similar trend of the role of psaR3 was observed when testing its role in swimming (Table 5). In order to test resistance to oxidative stress we tested all Psa luxR solo mutants for H2O2 sensitivity on a plate assay as described in the Materials and Methods section. The three single solo mutants displayed similar resistance to H2O2 as the wild type whereas the two double mutants were found to be more sensitive to H2O2 as they both formed a visually larger clearing zone compared to wild type. Complemented strains were also found more sensitive to H2O2, particularly the over expression of psaR1 in double mutant background was found more drastically affected for H2O2 sensitivity (data not shown). We also tested Psa for several secreted enzyme activities and have detected lipase activity; results from plate assay indicated that psaR1 and psaR2 do not affect lipase activity whereas the psaR3 mutant resulted in a decrease of lipase secretion compared to wild type Psa. The double mutant in psaR1 or psaR2 in the psaR3 mutant background were also decreased for the lipase activity. Complementation of psaR3 did not restore the phenotype whereas psaR1 and psaR2 expression in trans increased the lipase secretion in plate assay (Table 6, Figure S1). The AHL independent role of these solos in controlling these phenotypes can be due to background activity or possible a negative effect of AHLs. In the future performing the same experiments in the presence of AHLs will help to determine role of AHLs.
Table 5. Swarming and swimming movement score of Pseudomonas syringae pv. actinidiae strains.
| Psa Strains | Swarming (0.8% KBA)Average ± S.D. in mm | Swarming (0.6% KBA)Average ± S.D. in mm | Swimming 0.3% KBA)Average ± S.D. in mm |
| Psa (WT) | 65.33±1.5 | 80.00±0.5 | 66.50+/−5.8 |
| Psa-mR1 | 64.00±1.48 | 75.00±3.13 | 65.00±5.22 |
| Psa-mR2 | 63.00±1.04a | 77.50±2.71 | 65.50±3.73 |
| Psa-mR3 | 55.00±1.04a | 72.00±2.09a | 37.50±7.83a |
| Psa-mR1+pBBR-psaR1 | 54.33±0.49a | 79.00±1.04 | 62.00±2.95 |
| Psa-mR2+pBBR-psaR2 | 50.00±1.20a | 77.00±2.33 | 59.00±1.04 |
| Psa-mR3+pBBR-psaR3 | 37.00±0.60a | 72.50±2.71a | 37.50±7.96a |
| Psa-mR3+Psa-mR1 | 30.25±0.62a | 69.50±3.92a | 33.00±3.13a |
| Psa-mR3+Psa-mR2 | 30.58±0.80a | 65.50±1.50a | 27.00±3.14a |
| Psa-mR3+Psa-mR1+ pcos-psaR3+ pBBR-psaR1 | 7.50±0.522a | 10.50±0.90a | 29.00±1.04a |
| Psa-mR3+Psa-mR2+ pcos-psaR3+ pBBR-psaR2 | 21.00±1.04a | 57.50±2.61a | 40.00±4.35a |
Mean values and standard deviations were calculated for swarming and swimming bacterial movement obtained from three replications each on 0.8%, 0.6% and 0.3% KBA. Statistical analyses (Student’s t test) were performed to compare the significant difference in bacterial movement between wild type Psa strain and mutated and complemented strains. a, significant difference to WT at P<0.0001.
Table 6. Lipase secretion score of Pseudomonas syringae pv. actinidiae strains.
| Psa Strains | Lipase secretion score in LB Agar-tributyrinplate Average ± S.D. in mm |
| Psa (WT) | 4.00±0.00 |
| Psa-mR1 | 3.83±0.29 |
| Psa-mR2 | 3.83±0.29 |
| Psa-mR3 | 2.83±0.29a |
| Psa-mR1+pBBR-psaR1 | 4.25±0.25 |
| Psa-mR2+pBBR-psaR2 | 4.17±0.29 |
| Psa-mR3+pBBR-psaR3 | 2.00±0.00a |
| Psa-mR3+Psa-mR1 | 2.50±0.00a |
| Psa-mR3+Psa-mR2 | 2.50±0.00a |
| Psa-mR3+Psa-mR1+ pcos-psaR3+ pBBR-psaR1 | 4.83±0.29ab |
| Psa-mR3+Psa-mR2+ pcos-psaR3+ pBBR-psaR2 | 3.17±0.29a |
Mean values and standard deviations were calculated for halo obtained from three replications of lipase secretion in LB Agar-tributyrin plates. Statistical analyses (Student’s t test) were performed to compare the significant difference in lipase secretion between wild type Psa strain and mutated and complemented strains. a, significant difference to WT at P<0.05. b, significant difference to ‘a’ at P<0.01.
Concluding Remarks
The emerging pathogen Pseudomonas syringae pv. actinidiae (Psa) causing bacterial canker of kiwifruit crops has been isolated in several countries and genome based comparison studies of many isolates had been suggested the presence of variations among different strains [49], [59]–[61]. Analysis of the genome and experiments presented here lead to the conclusion that a canonical AHL LuxI/R QS system is absent in Psa. We found, however, that Psa possesses three LuxR solos; two of these could possibly be binding to AHLs, whereas one was found to belong to a sub-family of plant associated bacteria (PAB) solos which responds to yet unidentified plant signals [35], [41]. The genetic inactivation of these three putative luxR solos (psaR1, psaR2 psaR3) either alone or in combination of double mutation affected in planta survival implicating them in in planta fitness. The PsaR3 solo was found to be involved in motility and lipase production.
The PAB PsaR2 solo is most likely involved in responding to a plant signal and ortholog proteins in Xanthomonas, Pseudomonas and Sinorhizobium have been shown to be regulating plant-associated traits. It is therefore likely that this interkingdom system is also involved in regulating genes in Psa implicated in virulence, growth or persistence in the kiwifruit plant.
In summary, this study shows that Psa possesses three LuxR solos which is rather unusual as most commonly proteobacteria possess only one. The PsaR2 solo is most likely involved in interkingdom signaling whereas PsaR1 and PsaR3 could be responding to exogenous AHLs produced by neighboring bacteria. It must be kept in mind however that very few LuxR solos have been studied and they could be involved in responding to other types of signals. For example a recent study has reported that a LuxR solo from Photorhabdus luminescens responds to an endogenous signal which is not an AHL [71] thus it cannot be excluded that PsaR1 and PsaR3 be part of a novel QS system involving new types of signals.
Materials and Methods
Bacterial Strains, Media and Culture Conditions
The bacterial strains used in this study are listed in Table 1. Escherichia coli DH5α [72] was grown in LB medium at 37°C. Agrobacterium tumefaciens, C. violaceum CV026 and E. coli (pSB401) biosensors [70], [73] were grown as recommended. Pseudomonas syringae pv. actinidiae (Psa) was grown either in LB medium, KB medium or NSA medium at 25°C (room temperature). The following antibiotic concentrations were used: Nitrofurantoin (Nf) 150 µg/ml; ampicillin (Amp) 100 µg/ml; kanamycin (Km) 100 µg/ml; tetracycline (Tc) 10 µg/ml (E. coli), 40 µg/ml (Psa); gentamycin (Gm) 10 µg/ml (E. coli), 40 µg/ml (Psa and A. tumefaciens).
Recombinant DNA Techniques
Plasmids used or generated in this study and details on their construction are listed in Table S1. Routine DNA manipulation steps such as digestion with restriction enzymes, agarose gel electrophoresis, purification of DNA fragments, ligations with T4 ligase, radioactive labeling by random priming and transformation of E. coli etc. standard procedures were performed as described previously [74]. Colony hybridizations were performed using N+Hybond membrane (Amersham Biosciences); plasmids were purified using the EuroGold plasmid columns (Euro Clone) or with the alkaline lysis method [75]; total DNA from Pseudomonas strains were isolated by Sarkosyl/Pronase lysis as described previously [76]. PCR amplifications were performed using Go-Taq DNA polymerase or pfu DNA polymerase (Promega). The oligonucleotide primers used in this study are listed in Table S2. Automated sequencing was performed by Macrogen sequence service (Europe). Triparental matings between E. coli and Psa were carried out with the helper strain E. coli DH5α (pRK2013) [77].
AHLs Extraction and Detection
Culture supernatant extracts of Psa strains were analyzed on C18 reverse-phase TLC plates as described previously [78]. In brief, different isolates of Psa strains were grown in KB medium at room temperature. 30 hrs grown 50 ml cultures were pelleted down and supernatants were further extracted with similar volume of ethyl acetate containing 0.1% acetic acid by vortexing. The lower organic phase was discarded and upper watery phase was transferred to a glass beaker and dried over-night in a laminar hood. Dried AHLs in glass beaker were then dissolved in the 10 ml of same extraction solvent using magnetic stirrer. The dissolved AHLs were further concentrated to a final volume of 100–200 µl using vacuum dryer. Extraction debris were removed by pelleting at 13000 rpm for two minutes and clear extracts were loaded onto a pre warmed TLC sheet and run by 70% methanol. After completion of the TLC run, it was dried in laminar hood and was then overlaid with a thin layer of AB top agar seeded either with A. tumefaciens NTL4/pZLR4 in the presence of X-Gal (100 µg/ml), as described previously [78], or Luria-Bertani top agar seeded with C. violaceum CVO26 [79] or E. coli pSB401 [80].
Bioinformatic Search for luxR Solos and their Analysis
We looked for the genes annotated as LuxR in the draft genome of Psa. All the LuxR sequences obtained in genome search were further analysed for autoinducer binding domain at conserved domain database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Three LuxR sequences with autoinducer binding domain that were selected and used in this study have been designated as PsaR1, PsaR2 and PsaR3. We retrieved the protein sequences of known LuxR solos from several plant associated bacteria using PUBMED and aligned them using Clustal Omega service available at http://www.ebi.ac.uk/Tools/msa/clustalo/. Multiple aligned LuxR solos were further exported as tiff file and edited for domains and highlighting the key amino acid residues. A phylogeny was also generated for these aligned sequences at Clustal Omega service.
Homology Modeling and Structural Alignments
Three dimensional structure-based homology models were built using I-TASSER [65]. The top-scored models (with C-scores of 0.60, of 0.78 and −0.45 respectively) were based on TraR from Sinorhizobium fredii [PDB_ID 2Q0O] [66] for PsaR1 and on QscR from Pseudomonas aeruginosa [PDB_ID 3SZT] [67] for both PsaR2 and PsaR3 and were validated by two complementary protein model quality predictors. The correctness of the selected models was assessed by ProQ [81] and exploiting PSIPRED [82] for secondary structure prediction, resulting in the predicted LGscores and MaxSub values of 3.542, 3.897 and 3.339, and 0.451, 0.362 and 0.368, respectively. The overall quality of the models obtained was validated by a neural network approach using AIDE [83], the statistical indicators TM-score and RMSD being 0.63, 0.67 and 0.72, and 6.63 Å, 5.36 Å and 4.88 Å, respectively.
Sequence alignment was performed by Expresso [84] that exploits structural aligners algorithms like SAP [85] or TMalign [86] to generate structure-based alignments of the templates, used to obtain the structure-based homology models, and TraR from Agrobacterium tumefaciens (PDB_ID 1H0M [68], the prototype of canonical QS LuxR family. The achieved score (the total consistency value) of 97 is highly reliable, being 100 the full agreement between the considered alignment and its associated primary library that has been computed as a first step of the consistency-based protocol exploited by Expresso. Then, the structure-based homology model of OryR from Xanthomonas oryzae [69], a prototype of PAB LuxR solos, and the three structure-based homology models of LuxR solos from Psa were structurally aligned based on the secondary structure prediction according to I-TASSER [65].
Construction of Psa luxR Solos Mutants
The psaR3 in frame deletion mutant was generated using the pEX19Gm plasmid as described previously [87]. Briefly, deleting the internal region (249 bp) of psaR3 gene, two external fragments; Frag1 (527 bp) and Frag2 (539 bp) were PCR amplified using primers listed in Table S2 and sequentially cloned in pEX19Gm as mentioned in Table S1. The resulting pEX19Gm-derivative plasmid, listed in Table S1, was introduced in Psa 10,22 by conjugation. Clones with a chromosomal insertion of the pEX19Gm plasmids were selected on LB agar plates supplemented with 50 µg/ml Gm and 150 µg/ml Nf. Plasmid excision from the chromosome was subsequently selected on LB agar plates supplemented with 10% (w/v) sucrose. The psaR1 and psaR2 mutants were generated using plasmid integration by pKNOCK-Km suicide delivery system. Briefly, an internal fragment of psaR1 (372 bp) and psaR2 (390 bp) were PCR amplified by using primers listed in Table S2 and sequentially cloned in pKNOCK-Km yielding pKNOCK-psaR1 and pKNOCK-psaR2 as mentioned in Table S1. pKNOCK-psaR1 and pKNOCK-psaR2 plasmids were further used as a suicide delivery system and psaR1 and psaR2 mutants were created as previously described [88]. Psa mutant strains were verified by PCR analysis and sequencing.
Complementation of Psa luxR Solo Mutants
We PCR amplified the full length psaR1, psaR2 and psaR3 genes using primers listed in Table S2 and cloned in the pBBR-Gm vector [89] as mentioned in Table S1. pBBR plasmids containing full length luxR solo genes, pBBR-psaR1, pBBR-psaR2 and pBBR-psaR3 (Table S1), were introduced in mutants Psa-mR1, Psa-mR2 and Psa-mR3 respectively by conjugation. Positive clones were selected on LBA plates supplemented with 50 µg/ml Gm, 50 µg/ml Km and 150 µg/ml Nf.
In order to complement the double mutants, a cosmid library was constructed of Psa 10,22 strain by using the cosmid pLAFR3 [90] as vector. Insert DNA was prepared by partial EcoRI digestion of the genomic DNA and then ligated into the corresponding site in pLAFR3. The ligated DNA was then packaged into λ phage heads using Gigapack III Gold packaging extract (Stratagene) and the phage particles were transduced to E. coli HB101 as recommended by the supplier. In order to identify the cosmid containing the luxR genes, the cosmid library was screened using full length psaR3 gene as a radiolabelled probe in colony hybridization. We obtained a cosmid clone containing psaR3 (pcos-psaR3) and was harbored together with a pBBR clone containing one of the other luxR solos (i.e. pBBR-psaR1 and pBBR-psaR2; Table S1). In this way Psa-mR3+Psa-mR1 and Psa-mR3+Psa-mR2 double mutants were complemented.
β-galactosidase Activity, Lipase, Motility and H2O2 Sensitivity Assay
The psaR1, psaR2, psaR3 and pip gene promoter regions were PCR amplified using primers listed in Table S2 and cloned into promoter probe vector pMP220 which harbours a promoterless lacZ gene as described in Table S1. pMP-psaR1, pMP-psaR2 and pMP-psaR3 were then introduced independently into the WT and derivative Psa-mR1, Psa-mR2 and Psa-mR3 mutants by conjugation. pMP-pip was introduced only in the WT and in the Psa-mR2 mutant. β-galactosidase assays were performed as previously described [91]. Average Miller unit values and standard deviations were calculated from three independent experiments.
Lipase secretion phenotype for Psa strains were performed as mentioned previously with some modifications [92]. Briefly, for plate assays 1 ml of tributyrin solution was added to a 10 ml of LB broth and sonicated using a sonicator (four pulse of 60–80 Hrtz) until the solution to become homogenous white. This homogenous tributyrin mix was added to pre-warmed 400 ml of LB Agar media, mixed well and poured onto petriplates. All the Psa strains grown for 36 hrs were harvested washed with LB media and adjusted to OD 1.0 at OD600. 1 µl of equalized Psa strains were spotted onto dried LB Agar-tributyrin plates and incubated at room temperature for further periodical observation. Plates were scanned at 7th day and lipase halo data were scored. Mean values and standard deviations were calculated and statistical analysis were performed for three replicates.
For bacterial motility assays, all the Psa strains were grown in KB broth for 24 hrs at room temperature and adjusted to OD = 1.0. 2 µl of adjusted cultures were spotted onto 0.5 mm filter disc placed in the centre of 0.6% and 0.8% KB agar plates for swarming motility. Similarly for swimming motility 2 µl of adjusted cultures were spotted directly in the centre of 0.3% KB agar plates. The plates were incubated at room temperature and the diameter of swarming and swimming were measured in three dimensions after 24 hrs and 48 hrs and the mean values were calculated. All the experiments were performed in triplicate and the mean values and standard deviations are presented.
In order to measure the H2O2 sensitivity, Psa strains grown in KB broth at room temperature were adjusted to OD = 1.0. 100 µl of adjusted bacterial culture were added to 25 ml of pre warmed 0.6% KBA, mixed well and poured on petri plates. Four microliter of 33.3% H2O2 was pipetted onto 3 MM Whatman paper disks (0.5 cm diameter) and these disks were placed in the centre and on top of the bacterial plates and incubated at room temperature overnight. The zone of bacterial inhibition, in mm, was taken as a measure of H2O2 sensitivity. Plates were scanned and zone of inhibition was measured in three dimensions and the mean values, standard deviations and statistics were calculated from three independent replications.
AHL Response to QS LuxR Solos
In order to assess if QS LuxR solos PsaR1 and PsaR3 respond to AHLs, we performed promoter activity of E. coli harboring pMULTIAHLPROM [70] in the presence of either PsaR1 or PsaR3 and different AHLs. Briefly, pBBR, pBBR-psaR1 and pBBR-psaR3 plasmids (Table S1) were introduced into E. coli (pMULTIAHLPROM). β-galactosidase activity was performed for these strains in the presence of different AHL molecules and ethyl acetate as a baseline control. Average Miller unit values and standard deviations were calculated from three independent experiments.
In planta Survival Assay
Psa in planta survival assay was performed as described previously [49]. For the survival assay, one-year-old, potted plants of A. deliciosa cv. Hayward were used. The plants were maintained in a climatic room and watered regularly. For inoculation, Psa strains were grown for 48 hrs on NSA medium supplemeted with antibiotics, at 23–25°C. Bacterial culture were pelleted down washed with sterile saline (0,85% NaCl in distilled water) and adjusted to 1–2×106 cfu/ml in sterile saline. Leaf areas of approximately 1 cm in diameter were inoculated using a needleless sterile syringe with the bacterial suspension. For each strain, 10 leaves were inoculated in four sites and control plants were treated in the similar manner using sterile saline. In order to determine in planta bacterial growth, leaf disks of about 0.5 cm of diameter were sampled from inoculation site at 3rd and 7th days post inoculation, ground in 1 ml of sterile saline, and serial ten-fold dilutions were plated onto NSA supplemented with antibiotics. Colonies were counted two days after incubation at 23–25°C. Cfu/ml determined for each strain were plotted as log values on excel graph. Confirmation of colony identity was achieved by following well established procedures [48], [49], [93].
Supporting Information
Lipase secretion in LB Agar-tributyrin plate assay. Figure showing lipase secretion phenotype on LB Agar-tributyrin plate for Psa strains. (1) Wild type, (2) psa-mR1, (3) psa-mR2, (4) psa-mR3, (5) psa-mR1+pBBR-psaR1, (6) psa-mR2+pBBR-psaR2, (7) psa-mR3+pBBR-psaR3, (8) psa-mR1+psa-mR3, (9) psa-mR2+psa-mR3, (10) psa-mR1+psa-mR3+pcos-psaR3+pBBR-psaR1, (11) psa-mR2+psa-mR3+pcos-psaR3+pBBR-psaR2.
(TIF)
Plasmids used in this study.
(DOC)
List of oligonucleotide primers used in this study.
(DOC)
Flanking genes to psaR1, psaR2 and psaR3 in Pseudomonas syringae pv. Actinidiae.
(DOC)
Acknowledgments
We acknowledge Iris Bertani, Giulia Devescovi, Giuliano Degrassi, and Kumari Sonal Choudhary for their interest and technical assistance. We are very grateful to Rob Jackson (University of Reading, UK) for his constant input and interest.
Funding Statement
HKP was funded by an ICGEB Pre-doctoral fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311: 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuqua C, Greenberg EP (2002) Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol 3: 685–695. [DOI] [PubMed] [Google Scholar]
- 3. Fuqua C, Parsek MR, Greenberg EP (2001) Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35: 439–468. [DOI] [PubMed] [Google Scholar]
- 4. Bassler BL (2002) Small talk. Cell-to-cell communication in bacteria. Cell 109: 421–424. [DOI] [PubMed] [Google Scholar]
- 5. Von Bodman SB, Bauer WD, Coplin DL (2003) Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol 41: 455–482. [DOI] [PubMed] [Google Scholar]
- 6. Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25: 365–404. [DOI] [PubMed] [Google Scholar]
- 7. Smith RS, Iglewski BH (2003) Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 112: 1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steindler L, Bertani I, De Sordi L, Schwager S, Eberl L, et al. (2009) LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas aeruginosa beneficial to plants. Appl Environ Microbiol 75: 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinones B, Dulla G, Lindow SE (2005) Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant Microbe Interact 18: 682–693. [DOI] [PubMed] [Google Scholar]
- 10. Mattiuzzo M, Bertani I, Ferluga S, Cabrio L, Bigirimana J, et al. (2011) The plant pathogen Pseudomonas fuscovaginae contains two conserved quorum sensing systems involved in virulence and negatively regulated by RsaL and the novel regulator RsaM. Environ Microbiol 13: 145–162. [DOI] [PubMed] [Google Scholar]
- 11. Licciardello G, Bertani I, Steindler L, Bella P, Venturi V, et al. (2007) Pseudomonas corrugata contains a conserved N-acyl homoserine lactone quorum sensing system; its role in tomato pathogenicity and tobacco hypersensitivity response. FEMS Microbiol Ecol 61: 222–234. [DOI] [PubMed] [Google Scholar]
- 12. Wood DW, Gong F, Daykin MM, Williams P, Pierson LS 3rd (1997) N-acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30–84 in the wheat rhizosphere. J Bacteriol 179: 7663–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood DW, Pierson LS 3rd (1996) The phzI gene of Pseudomonas aureofaciens 30–84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168: 49–53. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Pierson LS 3rd (2001) A second quorum-sensing system regulates cell surface properties but not phenazine antibiotic production in Pseudomonas aureofaciens. Appl Environ Microbiol 67: 4305–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertani I, Venturi V (2004) Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationary-phase RpoS sigma factor and the global regulator GacA. Appl Environ Microbiol 70: 5493–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steidle A, Allesen-Holm M, Riedel K, Berg G, Givskov M, et al. (2002) Identification and characterization of an N-acylhomoserine lactone-dependent quorum-sensing system in Pseudomonas putida strain IsoF. Appl Environ Microbiol 68: 6371–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Sayed AK, Hothersall J, Thomas CM (2001) Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology 147: 2127–2139. [DOI] [PubMed] [Google Scholar]
- 18. Shadel GS, Devine JH, Baldwin TO (1990) Control of the lux regulon of Vibrio fischeri. J Biolumin Chemilumin 5: 99–106. [DOI] [PubMed] [Google Scholar]
- 19. Slock J, VanRiet D, Kolibachuk D, Greenberg EP (1990) Critical regions of the Vibrio fischeri luxR protein defined by mutational analysis. J Bacteriol 172: 3974–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi SH, Greenberg EP (1991) The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc Natl Acad Sci U S A 88: 11115–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi SH, Greenberg EP (1992) Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J Bacteriol 174: 4064–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devine JH, Shadel GS, Baldwin TO (1989) Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc Natl Acad Sci U S A 86: 5688–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens AM, Greenberg EP (1997) Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J Bacteriol 179: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang RG, Pappas KM, Brace JL, Miller PC, Oulmassov T, et al. (2002) Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417: 971–974. [DOI] [PubMed] [Google Scholar]
- 26. Fuqua C, Winans SC, Greenberg EP (1996) Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol 50: 727–751. [DOI] [PubMed] [Google Scholar]
- 27. Fuqua C (2006) The QscR quorum-sensing regulon of Pseudomonas aeruginosa: an orphan claims its identity. J Bacteriol 188: 3169–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramoni S, Venturi V (2009) LuxR-family ‘solos’: bachelor sensors/regulators of signalling molecules. Microbiology 155: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 29. Chugani SA, Whiteley M, Lee KM, D'Argenio D, Manoil C, et al. (2001) QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 98: 2752–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP (2006) A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J Bacteriol 188: 3365–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmer BM (2004) Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol Microbiol 52: 933–945. [DOI] [PubMed] [Google Scholar]
- 32. Ahmer BM, van Reeuwijk J, Timmers CD, Valentine PJ, Heffron F (1998) Salmonella typhimurium encodes an SdiA homolog, a putative quorum sensor of the LuxR family, that regulates genes on the virulence plasmid. J Bacteriol 180: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michael B, Smith JN, Swift S, Heffron F, Ahmer BM (2001) SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J Bacteriol 183: 5733–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao Y, Martinez-Yamout MA, Dickerson TJ, Brogan AP, Wright PE, et al. (2006) Structure of the Escherichia coli quorum sensing protein SdiA: activation of the folding switch by acyl homoserine lactones. J Mol Biol 355: 262–273. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez JF, Venturi V (2013) A novel widespread interkingdom signaling circuit. Trends Plant Sci 18: 167–174. [DOI] [PubMed] [Google Scholar]
- 36. Venturi V, Fuqua C (2013) Chemical signaling between plants and plant-pathogenic bacteria. Annu Rev Phytopathol 51: 17–37. [DOI] [PubMed] [Google Scholar]
- 37. Chatnaparat T, Prathuangwong S, Ionescu M, Lindow SE (2012) XagR, a LuxR homolog, contributes to the virulence of Xanthomonas axonopodis pv. glycines to soybean. Mol Plant Microbe Interact 25: 1104–1117. [DOI] [PubMed] [Google Scholar]
- 38. Ferluga S, Bigirimana J, Hofte M, Venturi V (2007) A LuxR homologue of Xanthomonas oryzae pv. oryzae is required for optimal rice virulence. Mol Plant Pathol 8: 529–538. [DOI] [PubMed] [Google Scholar]
- 39. Ferluga S, Venturi V (2009) OryR is a LuxR-family protein involved in interkingdom signaling between pathogenic Xanthomonas oryzae pv. oryzae and rice. J Bacteriol 191: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patankar AV, Gonzalez JE (2009) An orphan LuxR homolog of Sinorhizobium meliloti affects stress adaptation and competition for nodulation. Appl Environ Microbiol 75: 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramoni S, Gonzalez JF, Johnson A, Pechy-Tarr M, Rochat L, et al. (2011) Bacterial subfamily of LuxR regulators that respond to plant compounds. Appl Environ Microbiol 77: 4579–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Jia Y, Wang L, Fang R (2007) A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol Microbiol 65: 121–136. [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez JF, Myers MP, Venturi V (2013) The inter-kingdom solo OryR regulator of Xanthomonas oryzae is important for motility. Mol Plant Pathol 14: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scortichini M, Marcelletti S, Ferrante P, Petriccione M, Firrao G (2012) Pseudomonas syringae pv. actinidiae: a re-emerging, multi-faceted, pandemic pathogen. Mol Plant Pathol 13: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takikawa Y, Serizawa S, Ichikawa T, Tsuyumu S, Goto M (1989) Pseudomonas syringae pv. actinidiae pv. nov.: the causal bacterium of canker of kiwifruit in Japan. Ann Phytopathol Soc Jpn 55: 437–444. [Google Scholar]
- 46. Koh YJ, Cha BJ, Chung HJ, Lee DH (1994) Outbreak and spread of bacterial canker of kiwifruit. Korean Journal of Plant Pathology 10: 68–72. [Google Scholar]
- 47. Scortichini M (1994) Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathology 43: 1035–1038. [Google Scholar]
- 48. Ferrante P, Scortichini M (2010) Molecular and phenotypic features of Pseudomonas syringae pv. actinidiae isolated during recent epidemics of bacterial canker on yellow kiwifruit (Actinidia chinensis) in central Italy. Plant Pathology 59: 954–962. [Google Scholar]
- 49. Marcelletti S, Ferrante P, Petriccione M, Firrao G, Scortichini M (2011) Pseudomonas syringae pv. actinidiae draft genomes comparison reveal strain-specific features involved in adaptation and virulence to Actinidia species. PLoS One 6: e27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.CABI (2008) Pseudomonas syringae pv. actinidiae. [Distri -bution map]. Octo-ber (edition 1) map 1043. CABI, Wallingford, UK.
- 51.Balestra GM, Renzi M, Mazzaglia A (2010) First report on bacterial canker of Actinidia deliciosa caused by Pseudomonas syringae pv. actinidiae in Portugal. New Disease Reports 22.
- 52. Vanneste JL, Poliakoff F, Audusseau C, Cornish DA, Paillard S, et al. (2011) First report of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker of kiwifruit in France. Plant Disease 95: 1311–1311. [DOI] [PubMed] [Google Scholar]
- 53.Australia B (2011) Pest risk analysis report for Pseudomonas syringae pv. actinidiae associated with Actinidia (kiwifruit) propagative material. Department of Agriculture, Fisheries and Forestry, Canberra.
- 54.Anonymous (2011) Bacterial canker, kiwifruit–Chile: First report (O'Higgins, Maule). ProMED mail: International Society For Infectious Disease.
- 55. Everett K, Taylor R, Romberg M, Rees-George J, Fullerton R, et al. (2011) First report of Pseudomonas syringae pv. actinidiae causing kiwifruit bacterial canker in New Zealand. Australasian Plant Disease Notes 6: 67–71. [Google Scholar]
- 56. Abelleira A, López MM, Peñalver J, Aguín O, Mansilla JP, et al. (2011) First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Spain. Plant Disease 95: 1583–1583. [DOI] [PubMed] [Google Scholar]
- 57.Service E (2011) First report of Pseudomonas syringae pv. actinidiae in Switzerland.
- 58. Bastas KK, Karakaya A (2011) First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Turkey. Plant Disease 96: 452–452. [DOI] [PubMed] [Google Scholar]
- 59. Butler MI, Stockwell PA, Black MA, Day RC, Lamont IL, et al. (2013) Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PLoS One 8: e57464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mazzaglia A, Studholme DJ, Taratufolo MC, Cai R, Almeida NF, et al. (2012) Pseudomonas syringae pv. actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage. PLoS One 7: e36518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McCann HC, Rikkerink EH, Bertels F, Fiers M, Lu A, et al. (2013) Genomic analysis of the Kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathog 9: e1003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dumenyo CK, Mukherjee A, Chun W, Chatterjee A (1998) Genetic and physiological evidence for the production of N-acyl homoserine lactones by Pseudomonas syringae pv. syringae and other fluorescent plant pathogenic Pseudomonas species. European Journal of Plant Pathology 104: 569–582. [Google Scholar]
- 63. Quinones B, Pujol CJ, Lindow SE (2004) Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol Plant Microbe Interact 17: 521–531. [DOI] [PubMed] [Google Scholar]
- 64. Case RJ, Labbate M, Kjelleberg S (2008) AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J 2: 345–349. [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen G, Jeffrey PD, Fuqua C, Shi Y, Chen L (2007) Structural basis for antiactivation in bacterial quorum sensing. Proc Natl Acad Sci U S A 104: 16474–16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lintz MJ, Oinuma K, Wysoczynski CL, Greenberg EP, Churchill ME (2011) Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc Natl Acad Sci U S A 108: 15763–15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, et al. (2002) The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J 21: 4393–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Covaceuszach S, Degrassi G, Venturi V, Lamba D (2013) Structural insights into a novel interkingdom signaling circuit by cartography of the ligand-binding sites of the homologous quorum sensing LuxR-family. Int J Mol Sci 14: 20578–20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steindler L, Devescovi G, Subramoni S, Venturi V (2008) A versatile plasmid biosensor useful to identify quorum sensing LuxR-family orphans in bacterial strains. J Microbiol Methods 73: 273–275. [DOI] [PubMed] [Google Scholar]
- 71. Brachmann AO, Brameyer S, Kresovic D, Hitkova I, Kopp Y, et al. (2013) Pyrones as bacterial signaling molecules. Nat Chem Biol. 9: 573–578. [DOI] [PubMed] [Google Scholar]
- 72. Grant SG, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87: 4645–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Steindler L, Venturi V (2007) Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol Lett 266: 1–9. [DOI] [PubMed] [Google Scholar]
- 74.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Edited by Press CSHL. Cold Spring Harbor, N.Y.
- 75. Birnboim HC (1983) A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol 100: 243–255. [DOI] [PubMed] [Google Scholar]
- 76. Better M, Lewis B, Corbin D, Ditta G, Helinski DR (1983) Structural relationships among Rhizobium meliloti symbiotic promoters. Cell 35: 479–485. [DOI] [PubMed] [Google Scholar]
- 77. Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76: 1648–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shaw PD, Ping G, Daly SL, Cha C, Cronan JE Jr, et al. (1997) Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci U S A 94: 6036–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, et al. (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143 (Pt 12): 3703–3711. [DOI] [PubMed] [Google Scholar]
- 80. Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, et al. (1998) Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163: 185–192. [DOI] [PubMed] [Google Scholar]
- 81. Wallner B, Fang H, Elofsson A (2003) Automatic consensus-based fold recognition using Pcons, ProQ, and Pmodeller. Proteins 53 Suppl 6534–541. [DOI] [PubMed] [Google Scholar]
- 82. Buchan DW, Ward SM, Lobley AE, Nugent TC, Bryson K, et al. (2010) Protein annotation and modelling servers at University College London. Nucleic Acids Res 38: W563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mereghetti P, Ganadu ML, Papaleo E, Fantucci P, De Gioia L (2008) Validation of protein models by a neural network approach. BMC Bioinformatics 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Armougom F, Moretti S, Poirot O, Audic S, Dumas P, et al. (2006) Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acids Res 34: W604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Taylor WR (2000) Protein structure comparison using SAP. Methods Mol Biol 143: 19–32. [DOI] [PubMed] [Google Scholar]
- 86. Zhang Y, Skolnick J (2005) TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res 33: 2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP (1998) A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212: 77–86. [DOI] [PubMed] [Google Scholar]
- 88.Alexeyev MF (1999) The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26: 824–826, 828. [DOI] [PubMed]
- 89. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, et al. (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166: 175–176. [DOI] [PubMed] [Google Scholar]
- 90. Staskawicz B, Dahlbeck D, Keen N, Napoli C (1987) Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol 169: 5789–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stachel SE, An G, Flores C, Nester EW (1985) A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J 4: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Smeltzer MS, Hart ME, Iandolo JJ (1992) Quantitative spectrophotometric assay for staphylococcal lipase. Appl Environ Microbiol 58: 2815–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferrante P, Scortichini M (2009) Identification of Pseudomonas syringae pv. actinidiae as causal agent of bacterial canker of yellow kiwifruit (Actinidia chinensis Planchon) in central Italy. Journal of Phytopathology 157: 768–770. [Google Scholar]
- 94.DeLano WL (2002) The PyMOL molecular graphics system. Palo Alto, CA, USA, DeLano Scientific LLC.
- 95. Farrand SK, Qin Y, Oger P (2002) Quorum-sensing system of Agrobacterium plasmids: analysis and utility. Methods Enzymol 358: 452–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lipase secretion in LB Agar-tributyrin plate assay. Figure showing lipase secretion phenotype on LB Agar-tributyrin plate for Psa strains. (1) Wild type, (2) psa-mR1, (3) psa-mR2, (4) psa-mR3, (5) psa-mR1+pBBR-psaR1, (6) psa-mR2+pBBR-psaR2, (7) psa-mR3+pBBR-psaR3, (8) psa-mR1+psa-mR3, (9) psa-mR2+psa-mR3, (10) psa-mR1+psa-mR3+pcos-psaR3+pBBR-psaR1, (11) psa-mR2+psa-mR3+pcos-psaR3+pBBR-psaR2.
(TIF)
Plasmids used in this study.
(DOC)
List of oligonucleotide primers used in this study.
(DOC)
Flanking genes to psaR1, psaR2 and psaR3 in Pseudomonas syringae pv. Actinidiae.
(DOC)