Abstract
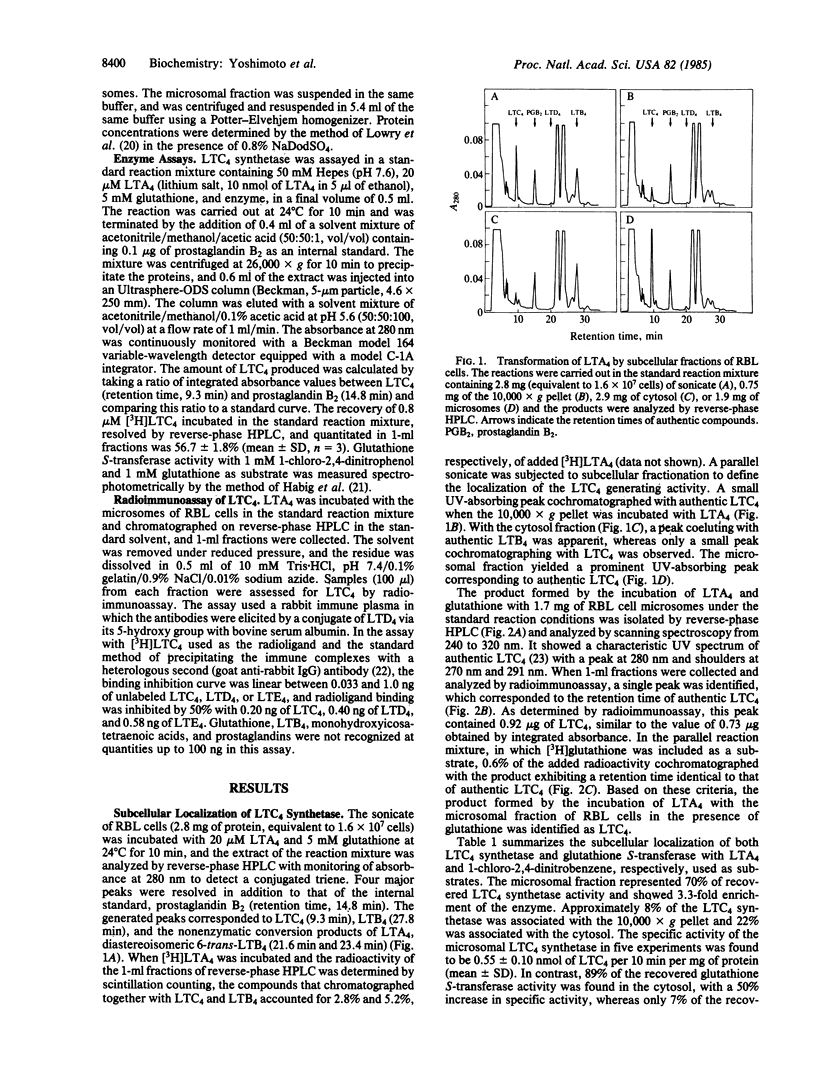
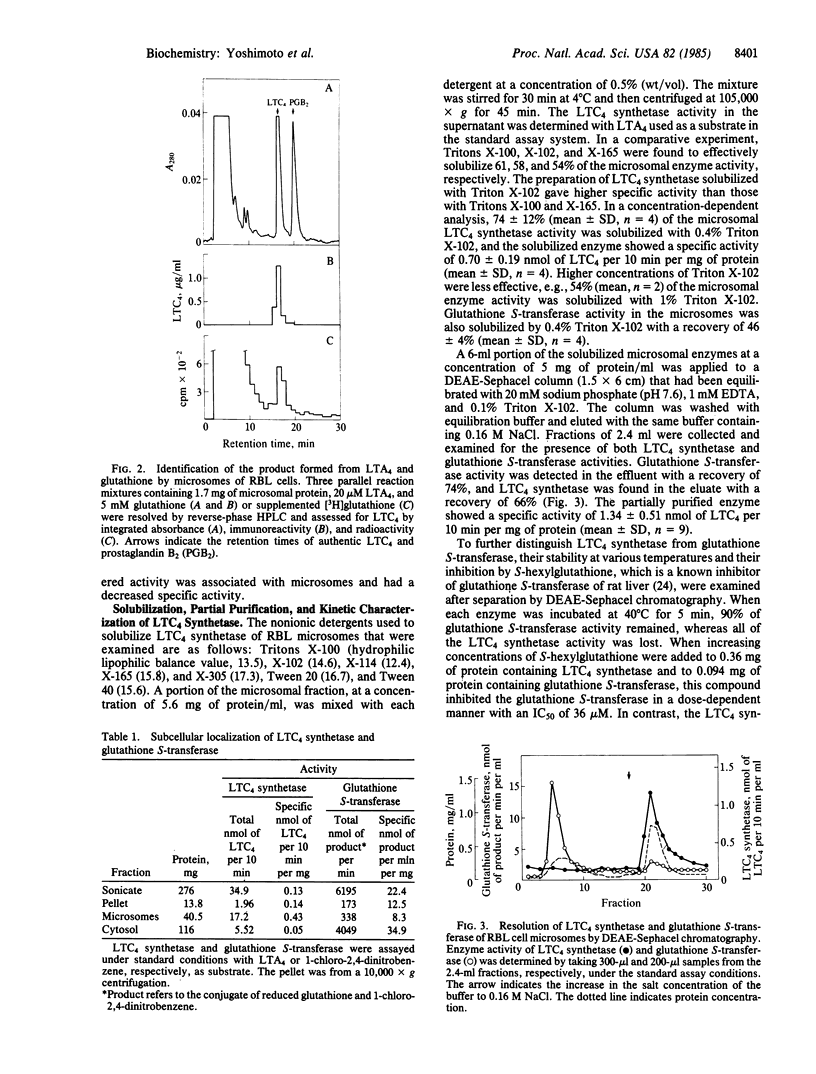
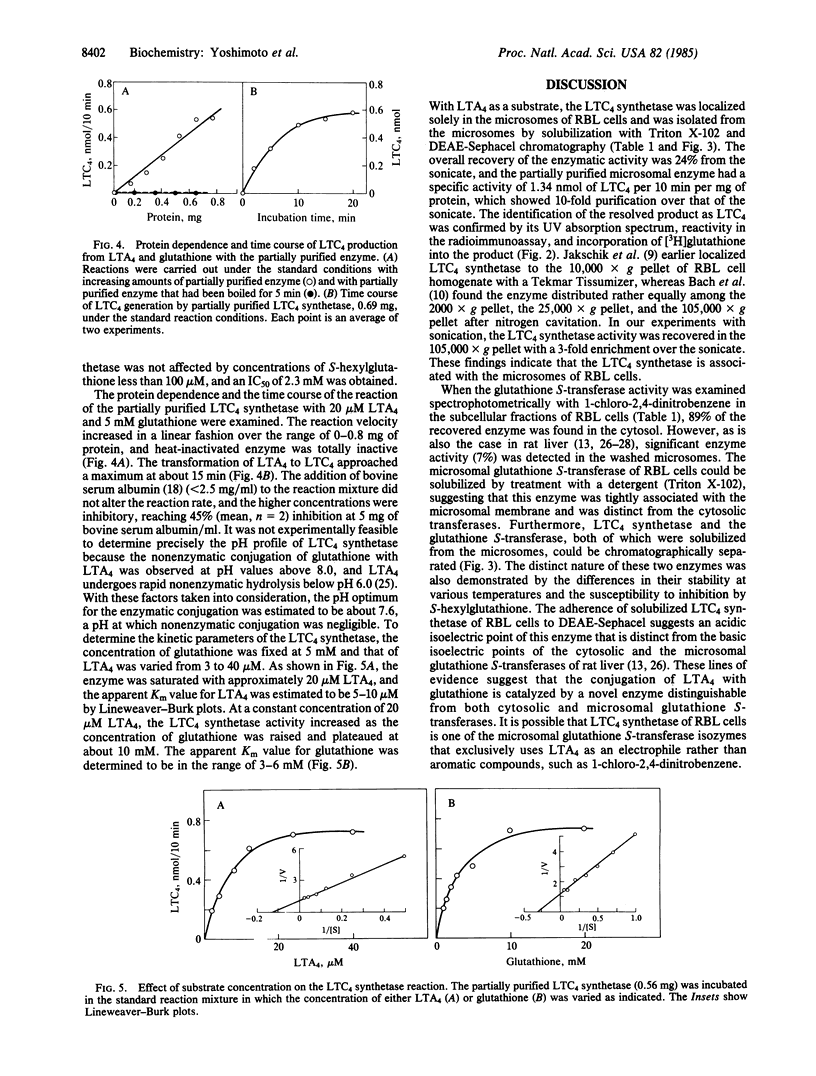
When leukotriene (LT) A4 was incubated with subcellular fractions of sonicated rat basophilic leukemia (RBL) cells in the presence of glutathione, the enzyme producing LTC4, designated LTC4 synthetase, was found in the 105,000 X g pellet (microsomes) with a 3-fold enrichment in specific activity over that of the sonicate. The identification of the reaction product as LTC4 was confirmed by its identical retention time on reverse-phase HPLC to that of synthetic LTC4, the incorporation of [3H]glutathione into the product, its reactivity in a radioimmunoassay, and its UV absorption spectrum. In contrast, glutathione S-transferase activity, measured spectrophotometrically with 1-chloro-2,4-dinitrobenzene, was detected predominantly in the 105,000 X g supernatant (89%) and also in the microsomes (7%). The microsomal glutathione S-transferase and LTC4 synthetase were solubilized with 0.4% Triton X-102 and separated by DEAE-Sephacel chromatography; the former appeared in the effluent and the latter in the eluate after the addition of 0.16 M NaCl to the equilibration buffer. Solubilized, microsomal glutathione S-transferase was inhibited by S-hexylglutathione with an IC50 of 36 microM and was stable at 40 degrees C for 5 min, whereas LTC4 synthetase was only slightly inhibited (IC50, 2.3 mM) by S-hexylglutathione and retained no activity after incubation at 40 degrees C for 5 min. The partially purified LTC4 synthetase showed a specific activity of 1.34 +/- 0.51 nmol of LTC4 per 10 min per mg of protein (mean +/- SD, n = 9), representing a 10-fold purification from the sonicate and catalyzed the dose- and time-dependent production of LTC4 from LTA4 and glutathione. The apparent Km values for LTA4 and glutathione were estimated by Lineweaver-Burk plots to be 5-10 microM and 3-6 mM, respectively. These results indicate that the conjugation of LTA4 with glutathione to form LTC4 is catalyzed by a unique microsomal enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askelöf P., Guthenberg C., Jakobson I., Mannervik B. Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J. 1975 Jun;147(3):513–522. doi: 10.1042/bj1470513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Morton D. R., Jr Solubilization and characterization of the leukotriene C4 synthetase of rat basophil leukemia cells: a novel, particulate glutathione S-transferase. Arch Biochem Biophys. 1984 May 1;230(2):455–465. doi: 10.1016/0003-9861(84)90426-0. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Morton D. R., Wynalda M. A. Albumin stabilizes leukotriene A4. J Biol Chem. 1982 May 10;257(9):4680–4683. [PubMed] [Google Scholar]
- Fitzpatrick F., Haeggström J., Granström E., Samuelsson B. Metabolism of leukotriene A4 by an enzyme in blood plasma: a possible leukotactic mechanism. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5425–5429. doi: 10.1073/pnas.80.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F., Liggett W., McGee J., Bunting S., Morton D., Samuelsson B. Metabolism of leukotriene A4 by human erythrocytes. A novel cellular source of leukotriene B4. J Biol Chem. 1984 Sep 25;259(18):11403–11407. [PubMed] [Google Scholar]
- Friedberg T., Bentley P., Stasiecki P., Glatt H. R., Raphael D., Oesch F. The identification, solubilization, and characterization of microsome-associated glutathione S-transferases. J Biol Chem. 1979 Dec 10;254(23):12028–12033. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Harper T., Murphy R. C. Leukotriene C4 and D4 formation by particulate enzymes. J Biol Chem. 1982 May 25;257(10):5346–5349. [PubMed] [Google Scholar]
- Jakschik B. A., Kuo C. G. Characterization of leukotriene A4 and B4 biosynthesis. Prostaglandins. 1983 Jun;25(6):767–782. doi: 10.1016/0090-6980(83)90002-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine L., Morgan R. A., Lewis R. A., Austen K. F., Clark D. A., Marfat A., Corey E. J. Radioimmunoassay of the leukotrienes of slow reacting substance of anaphylaxis. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7692–7696. doi: 10.1073/pnas.78.12.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Jensson H., Alin P., Orning L., Hammarström S. Transformation of leukotriene A4 methyl ester to leukotriene C4 monomethyl ester by cytosolic rat glutathione transferases. FEBS Lett. 1984 Oct 1;175(2):289–293. doi: 10.1016/0014-5793(84)80753-x. [DOI] [PubMed] [Google Scholar]
- Maycock A. L., Anderson M. S., DeSousa D. M., Kuehl F. A., Jr Leukotriene A4: preparation and enzymatic conversion in a cell-free system to leukotriene B4. J Biol Chem. 1982 Dec 10;257(23):13911–13914. [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Meijer J., Depierre J. W., Ernster L. Characterization of rat-liver microsomal glutathione S-transferase activity. Eur J Biochem. 1980 Feb;104(1):167–174. doi: 10.1111/j.1432-1033.1980.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Griffith O. W., Hamill A. L., Cohn Z. A. Glutathione metabolism in resting and phagocytizing peritoneal macrophages. J Biol Chem. 1982 Feb 25;257(4):2002–2008. [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Clark D. A., Goto G., Marfat A., Corey E. J. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem Biophys Res Commun. 1980 Feb 12;92(3):954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B. Leukotriene A4: enzymatic conversion to leukotriene C4. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1679–1687. doi: 10.1016/0006-291x(80)91367-4. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Shimizu T., Jörnvall H., Samuelsson B. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J Biol Chem. 1984 Oct 25;259(20):12339–12345. [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]



