Abstract
The composition of a bone can be described in terms of the mineral phase, hydroxyapatite, the organic phase, which consists of collagen type I, noncollagenous proteins, other components and water. The relative proportions of these various components vary with age, site, gender, disease and treatment. Any drug therapy could change the composition of a bone. This review, however, will only address those pharmaceuticals used to treat or prevent diseases of bone: fragility fractures in particular, and the way they can alter the composition. As bone is a heterogeneous tissue, its composition must be discussed in terms of the chemical makeup, properties of its chemical constituents and their distributions in the ever-changing bone matrix. Emphasis, in this review, is placed on changes in composition as a function of age and various diseases of bone, particularly osteoporosis. It is suggested that while some of the antiosteoporotic drugs can and do modify composition, their positive effects on bone strength may be balanced by negative ones.
Introduction
Bone is a heterogeneous composite material consisting, in decreasing order, of a mineral phase, hydroxyapatite (Ca10(PO4)6(OH)2) (analogous to geologic ‘hydroxyapatite'),1 an organic phase (∼90% type I collagen, ∼5% noncollagenous proteins (NCPs), ∼2% lipids by weight)2 and water. Proteins in the extracellular matrix of bone can also be divided as follows: (a) structural proteins (collagen and fibronectin) and (b) proteins with specialized functions, such as those that (i) regulate collagen fibril diameter, (ii) serve as signaling molecules, (iii) serve as growth factors, (iv) serve as enzymes and (v) have other functions. The relative amount of each of these constituents present in a given bone varies with age,3 site,4 gender,5 ethnicity6 and health status.7 The amount, proper arrangement and characteristics of each of these components (quantity and quality) define the properties of bone. The tendency of bones to fracture depends on the quantity of mineralized tissue present (size and density) often measured by clinicians as bone mineral density or BMD8 and several other factors, grouped together as ‘bone quality'.8,9 ‘Bone quality' factors include composition (weight percent of each component), mineralization (organization of the mineral and its crystallite size and perfection), collagen content and collagen crosslinks, morphology,10 microarchitecture11 and the presence of microcracks.12 Each of these factors varies with health, disease and drug therapies. Their distribution in the heterogeneous tissue also varies with these perturbations. The focus of this review will be on the composition of bone and its site-specific variation. Materials present, their characteristics and their distribution will be discussed here. Readers are referred to the references above for more information on morphology, microarchitecture and the presence of microcracks, which will not be discussed.
Bone mineral
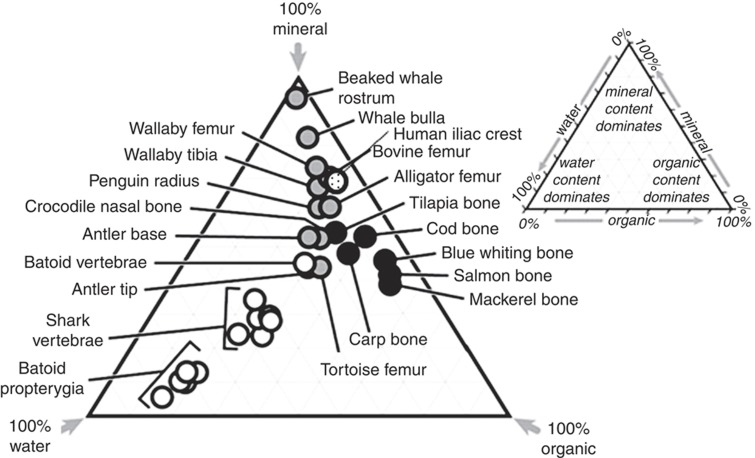
Hydroxyapatite is the principal component of the mineral phase of bone. This was demonstrated more than 60 years ago using X-ray diffraction, now viewed as the ‘gold standard' for such determinations.1 The quantity of mineral present in bone can be determined by a variety of techniques13 including gravimetric analyses (ash weight determination), analysis of calcium and phosphate contents, spectroscopic and densitometric analyses including bone mineral density distribution (BMDD), bone mineral density (BMD) and micro-computed tomography (micro-CT). Such methods show that the mineral content of bone ranges from ∼30%/dry weight (in the skate or ray appendicular skeletal element, the propterygium) to 98%/dry weight in the stapes of the human ear. Most bones have ∼60–70% mineral/dry weight, depending upon site, species and stage of development (Figure 1).13,14
Figure 1.
Bone composition. Ternary diagram illustrating the composition of mature bones in different species (adapted from Currey J.D. ‘Bones'. Princeton University Press: Princeton, NJ, 2002, p 436.) and reproduced with permission from Journal of Experimental Biologists, Figure 7B in ‘Comparison of structural, architectural and mechanical aspects of cellular and acellular bone in two teleost fish'.14 The speckled circle is the average of data from five iliac crest biopsies of females aged 69–75 years.
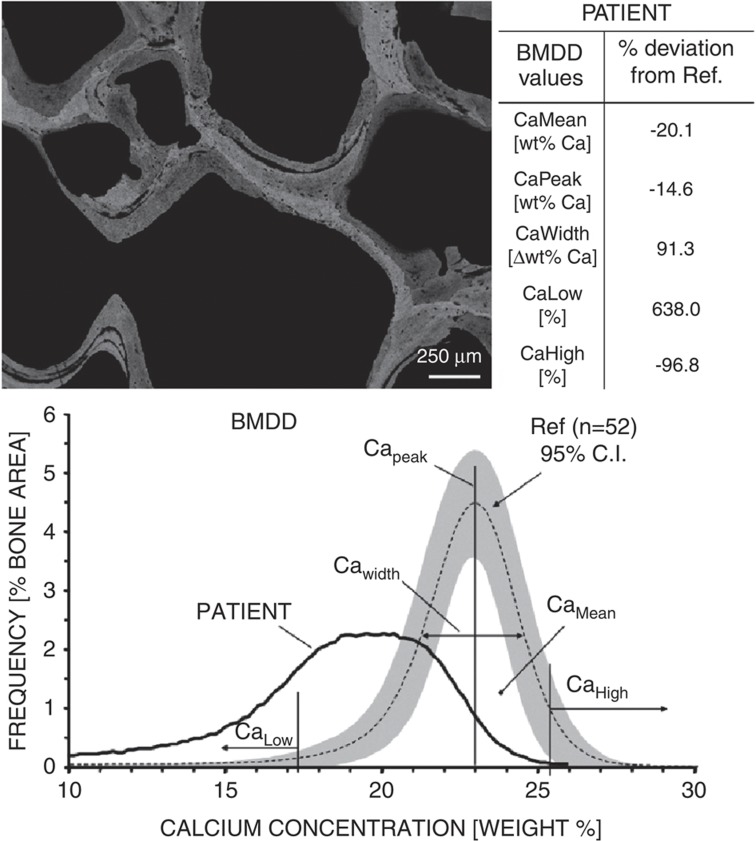
Variation in the distribution of mineral and its properties in bone can be illustrated by a variety of imaging techniques, discussed here, including BMDD, Raman and infrared spectroscopic imaging. It can also be determined by microprobe or synchrotron radiation-induced micro-X-ray fluorescence elemental analysis and mapping15 including trace elements such as strontium, aluminum, zinc or lead. In contrast, backscattered electron imaging in the scanning electron microscope is highly sensitive to the average atomic number of the bone material that is dominated by calcium. This technique is not a tool to identify specific elements in bone. Quantitative backscattered electron imaging is used for mapping the calcium concentrations and for the determination of bone mineralization density distribution (frequency distribution of Ca concentrations within the bone sample, BMDD; Figure 2).16 Parameters obtained from BMDD include the average and mode Ca content and the full-width at half-maximum of the BMDD peak, which is a measure of the heterogeneity of mineralization. Deviations from normal calcium distributions have been reported to date in: osteomalacia,17 osteoporosis18 and idiopathic osteoporosis19 (peak shifted to the left of normal), classical and new forms of osteogenesis imperfecta16,20 (peak shifted to the right of normal) and treatment with some but not all bisphosphonates examined by this technique.18,21,22
Figure 2.
BMDD distribution of bone. Measurement of bone mineralization density distribution (BMDD) using quantitative backscattered electron imaging (qBEI) in a transiliac bone biopsy sample (left insert) from a 39-year-old women with coeliac disease (PATIENT). The qBEI image shows a detail of the cancellous bone compartment. Bright gray levels mean high and dark gray levels mean low mineral content. The trabecular features display severe under mineralization and mineralization defects. Thus, the BMDD curve is extremely shifted to the left toward lower matrix mineralization (decreased CaMean, CaPeak, CaHigh and increased CaLow) compared with the normal reference BMDD (Ref.). In addition, the peak width of the BMDD (CaWidth) is distinctly enlarged, indicating an enormous heterogeneity in mineralization of the bone matrix. Examinations by histological staining techniques revealed a severe osteomalacia combined with signs of secondary hyperparathyroidism. Description of the individual BMDD indices derived from the BMDD curve: *CaMean, the weighted mean calcium concentration of the bone area obtained by the integrated area under the BMDD curve; *CaPeak, the peak position of the histogram, which indicates the most frequently occurring calcium concentration (mode calcium concentration); *CaWidth, the full-width at half-maximum of the distribution, describing the variation in mineralization density (heterogeneity); *CaLow, the percentage of bone area that is mineralized below the 5th percentile of the reference BMDD of normal adults, that is, below 17.68 weight percent calcium. This parameter corresponds normally to the amount of bone area undergoing primary mineralization. *CaHigh, the percentage of bone area that is mineralized above the 95th percentile of the reference BMDD of normal adults that is above 25.30 weight percent calcium. This parameter corresponds to bone matrix having achieved plateau level of normal mineralization. Figures courtesy of P Roschger.
Variation in phosphate distribution is visualized by both Fourier transform infrared microscopic imaging (FTIRI)23 and Raman microscopy and imaging (Raman).24,25 These types of vibrational spectroscopic imaging describe the distribution of any elemental pair or larger moiety that vibrates when excited by incident light. Relevant vibrations for bone are those in phosphate, protein and lipid groups. The precise location of the vibrations, often given in wave numbers or reciprocal wavelength, reflects the molecular environment in which the vibrating ions are found. In addition, as with BMDD, the line width at half-maximum of any of the broadened peaks indicates the heterogeneity (number of pixels with different values in the section analyzed) for that particular vibration. The spatial resolution of the FTIRI experiment, unless synchrotron radiation is used, is ∼7 μm. Raman spectroscopy, in contrast, has a spatial resolution of ∼1 μm. Raman spectral data is not affected by the presence of water, making the analysis of non-dehydrated samples, not possible for FTIRI, yet possible using Raman. Vibrations that are strong in the infrared spectra are weak in Raman spectra, and vice versa. These two are complimentary techniques, providing information on both the mineral phosphate and the organic matrix distribution in tissues (Figures 3, 4, 5). The data from these images can be presented as ‘chemical photographs' (as in Figures 3 and 5) or as numerical averages (Figure 6). Data in Figure 6 compares the composition of cancellous bone in patients treated with bisphosphonate followed by 1 year of teriparatide (PTH) treatment, as determined by Raman spectroscopy. The lower half of Figure 6 shows baseline values as determined by FTIRI in an on-going study of female patients with and without fragility fractures. The mean heterogeneity for each of these parameters is also shown. An important observation is that the fracture cases had reduced heterogeneities relative to unfractured controls. We believe (discussed later) that this loss of compositional heterogeneity may allow microcrack propagation resulting in a weakened, more brittle tissue as the microcracks spread. Whether this change in heterogeneity is due to oversuppression or other mechanisms is yet to be determined.
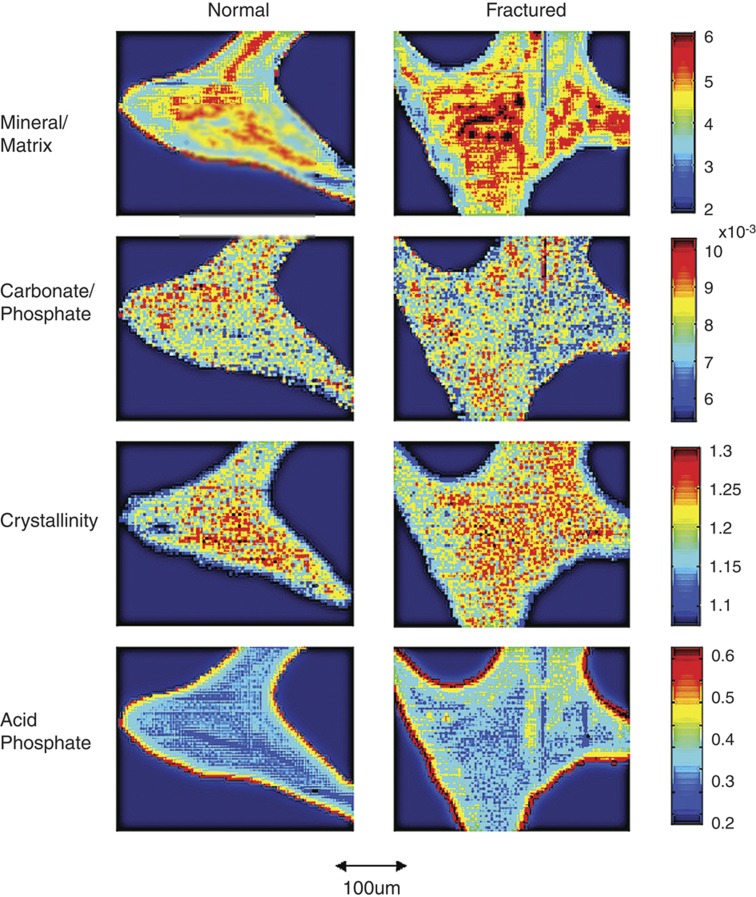
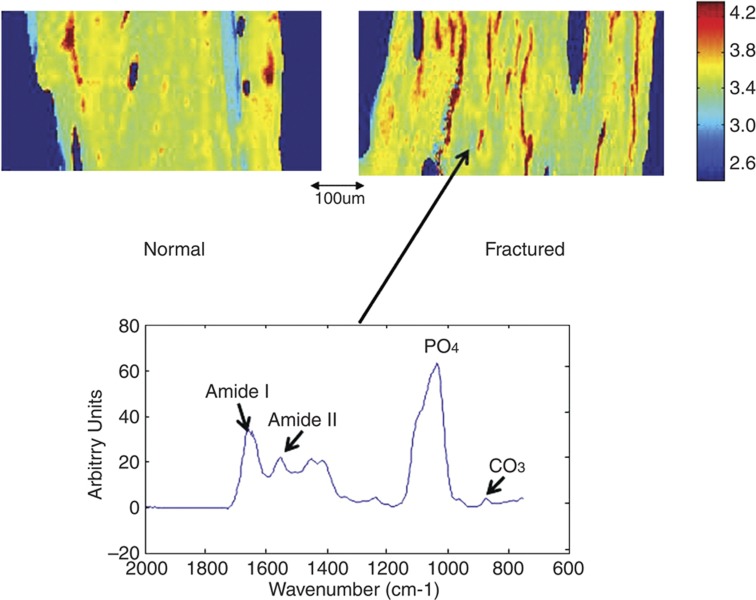
Figure 3.
FTIRI images. Typical FTIR images of mineral compositional parameters in normal and osteoporotic bone trabeculae from iliac crest biopsies of a woman with fractures and her age-matched control. Left side, normal; right side, osteoporotic (fractured). Images are shown for: mineral/matrix ratio, carbonate/phosphate ratio, crystallinity (1030/1020, cm−1) and acid phosphate substitution. The arrow indicates the size scale for all images. The color scales for each parameter for both normal and fractured images are shown.
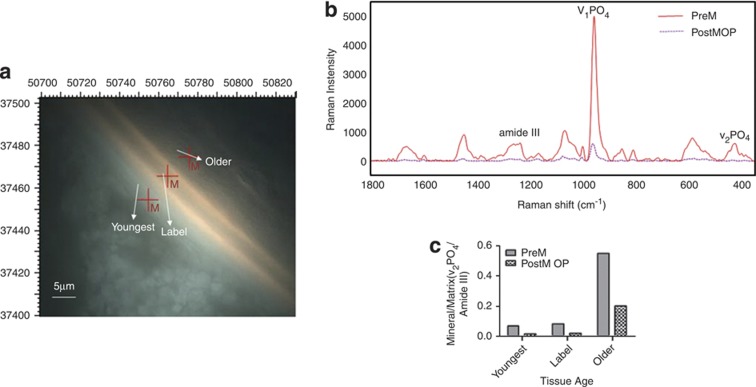
Figure 4.
Raman analysis. (a) Image as seen under the Raman microscope showing the double tetracycline labels used to define newly formed trabecular bone, the three areas examined are shown. (b) Typical Raman spectra of bone from a premenopausal woman and a woman with osteoporosis. (c) Mean values for the indicated parameters calculated from the Raman spectra of these two patients. Figure courtesy of Dr Lefteris Paschalis.
Figure 5.
FTIR image of the organic matrix in osteoporosis. Typical cortical bone image of collagen maturity (ratio of intensity of 1660/1690, cm−1) in iliac crest biopsies from age-matched women without and with a fracture. The magnification is indicated by the arrow. The infrared spectra corresponding to the pixel at the tip of the arrow is shown. The major peaks used in the analysis of mineral and matrix are indicated in this figure.
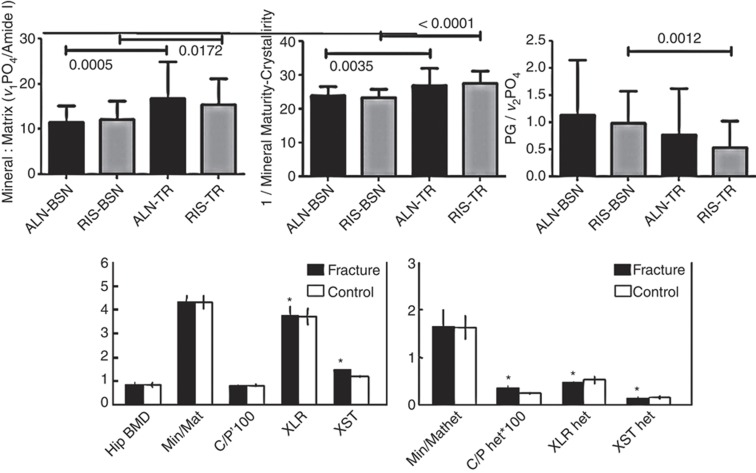
Figure 6.
Compositional changes in bone as detected by Raman and FTIRI. Top row of figures show Raman compositional data indicating changes from baseline in newly formed trabecular bone caused by 1 year treatment with teriparatide (TP) following longer treatment with alendronate (ALN) or risedronate (RIS). Differences between baseline and TP treatment are indicated. Mineral-to-matrix, inverse of crystallinity and proteoglycan-to-mineral ratios are shown (adapted with permission from Figures 2a, 3 and 4 in Gamsjaeger et al.51. Lower row shows FTIRI lack of compositional changes in iliac crest biopsies from a group of 48 women, half with fractures and half without who had comparable hip BMD values. The first figure shows mean and standard deviation in cancellous bone. The second figure illustrates the mean and standard deviation of the heterogeneity of these parameters. *P<0.05.
Using FTIRI spectroscopy, biopsies from patients with low-energy (fragility) fractures were found to exhibit differences in their mineral composition, relative to the same tissue in fracture-free controls of similar age and sex. Using these FTIRI analyses of 54 iliac crest biopsies from patients ranging in age from 30 to 84 years, with and without fragility fractures,26 models were constructed to determine which FTIRI parameters were associated with fracture. The mineral parameters significantly associated with fracture in the constructed model were high cortical mineral/matrix ratio (increased fracture risk) and high cancellous crystallinity (increased fracture risk). Carbonate-to-phosphate ratio was increased in both areas, however, not significantly. Raman analysis performed on femoral cancellous bone near the fracture site of women with fractures similarly demonstrated a higher carbonate/amide I area ratio than in those without fractures. Iliac crest biopsies in those fractured patients also revealed a higher carbonate/phosphate ratio in cortical bone samples of women with fractures.27 These compositional changes and the loss of heterogeneity reflect the persistence of older more mature bone (increased mineral/matrix ratio and carbonate/phosphate ratio and a lower acid phosphate substitution) along with the absence of new bone formation.
Changes in mineral composition also occur in other bone diseases associated with increased fracture risk in addition to osteoporosis. In most types of OI (or brittle bone disease), as in osteoporosis, the mineral content (mineral/matrix ratio) is increased.28 In osteoporosis, this increase is attributable to the lack of osteoid associated with increased remodeling and decreased bone formation, whereas in OI there is a lesser amount of collagen because OI patients make an improper collagen matrix.28 In osteomalacia, the mineral/matrix ratio is unchanged when mineralized tissue alone is examined.29 Crystallinity (measured based on different vibrations in FTIRI and Raman30) generally shows parallel trends. These trends, reflecting the changes in mineral properties in different diseases, are summarized in Table 1 along with other compositional parameters measured by FTIRI and Raman. Carbonate/phosphate ratio, based on the same vibrations in both techniques varies similarly. The extent of acid phosphate substitution31 varies inversely with crystallinity. For example, in chronic kidney disease, abnormalities in phosphate transport and clearance lead to osteoporosis and increased crystallinity when bone turnover is high, with no significant changes occurring when bone turnover is low.32
Table 1. Mineral property distribution in diseased human bone revealed by FTIRI and Raman analysis.
| Parameter/condition | Compared with healthy age-matched similar site | |||||||
|---|---|---|---|---|---|---|---|---|
|
Cortical |
Cancellous |
|||||||
| |
Min/matrix |
CO3/P |
XST |
HPO4 |
Min/matrix |
CO3/P |
XST |
HPO4 |
| Aging52 primates | Inc | Inc | Inc | Dec | Inc | Inc | Inc | Dec |
| Osteogenesis imperfecta53,54,a | ||||||||
| Type I | NC | Dec | Dec | NA | NC | Dec | Dec | NA |
| Type III | ||||||||
| Type IV | ||||||||
| Type VII | Inc | NC | Dec | NA | Inc | NC | Dec | NA |
| Type VIII | Inc | NC | Dec | NA | Inc | NC | Dec | NA |
| Osteomalaciab | NC | NA | NC | NA | NC | NA | NC | NA |
| Osteoporosisc | Inc | NC | Inc | NA | NC | NC | Inc | NA |
| Renal osteodystrophy32,55 | NC | NC | NC | NA | Dec | NC | NC/INC | NA |
Abbreviations: CO3/P, carbonate-to-phosphate ratio; Dec, decreased relative to appropriate control; HPO4, acid phosphate substitution; Inc, increased relative to appropriate control; Min/mat, mineral-to-matrix ratio; NA, not measured; NC, unchanged relative to appropriate control. XST=crystallinity.
aA Boskey, E Carter, CL Raggio, unpublished data,
bSee Faibish et al.29
Bone matrix
Protein composition of bone was classically determined following demineralization of the tissue and isolation and characterization of the component proteins. Collagen, predominantly type I, accounted for the majority of the matrix, but other proteins, the so-called NCPs, accounted for ∼5% of the total bone weight. The major components of the NCPs were identified as belonging to the SIBLING (small integrin-binding N-glycosylated), SLRP (small leucine-rich proteoglycans), GLA protein (γ-carboxyglutamic acid protein) and CCN protein (small secreted cysteine-rich protein) families. Today, using proteomics and gene expression profiling, it is known that there are thousands of proteins in the bone matrix, some of which (based on whole-genome analysis) have been associated with changes in BMD, but most are yet to be identified and their functions determined. Major structural proteins and NCPs will be reviewed here.
Structural proteins
Collagen
The most abundant protein in the bone matrix is type I collagen, a unique triple helical molecule consisting of two identical amino-acid chains and one that is different. The collagen molecules that consist of repeating glycine–X–Y residues are often hydroxylated and glycosylated. This gives rise to some of collagen's unique crosslinking ability, in turn, making the collagen lattice ideal for its functions. These include: providing elasticity to the tissues, stabilizing the extracellular matrix, supporting or templating initial mineral deposition and binding other macromolecules. In different types of OI, mutations in the collagen genes are reflected in the inability of the OI bones to mineralize properly. Reviewed elsewhere,28 the origin of the mineralization defect is unknown. This defect may be in the altered structure of the collagen itself, or in the inability of extracellular NCPs, which regulate the mineralization process, to bind to the defective collagen, and hence regulate mineralization.
Chemical analyses of the crosslinks in bone collagen have demonstrated two types of crosslinks, those formed enzymatically and those that occur by glycation.33 Both types of crosslinks (enzymatic and non-enzymatic) increase with age, are altered in disease (Table 2) and affect the mechanical strength of the collagenous matrix. Enzymatic crosslinks are believed to enhance mechanical strength, whereas the advanced glycosylation end-products, which are elevated in uncontrolled diabetics and in oxidative stress, make for a more brittle bone. Distribution of total crosslinks in the bone matrix can be visualized in FTIRI data (Figure 5). Such analyses agree with the high-performance liquid chromatography chemical analysis of crosslinks.34 The number of these crosslinks, which reflect collagen maturity, is increased in osteoporotic individuals, especially in the center of the remaining cancellous bone. The heterogeneity of this distribution is decreased in consequence of age, osteoporosis and treatment with bisphosphonates.35
Table 2. Variation in collagen crosslinking in human bones with age, disease and therapy (relative to control values).
| FTIRI collagen maturity | Enzymatic crosslinks | AGEs | |
|---|---|---|---|
| Agea | Increasesb | Increases56 | Increases in cortical bone56 |
| Osteogenesis imperfectac | Increasedb | No change57 | No change57 |
| Osteomalaciac | No changed | ND | ND |
| Renal osteodystrophyc | No change55 | Increased58 (rats) | Increased58 (rats) |
| Osteoporosisc | Increased59 | Increasede | Increasede |
| +Alendronatef | No change60,61 | No change (dogs)62 | Decreased (dogs)62 |
| +Risedronatef | No change (dogs)61 | No change (dogs)62 | Decreased (dogs)62 |
| +Zoledronatef | No changeb | ND | ND |
| +PTHf | No changeb | Increased (monkeys)63 | Decreased (monkeys)63 |
| +Estrogenf | Increased64 | ND | ND |
| +Raloxifene (SERM)f | No change | ND | Decreased |
Abbreviations: AGE, advanced glycosylation end-products; FTIRI, Fourier transform infrared microscopic imaging; ND, not determined; PTH, parathyroid hormone; SERM, selective estrogen receptor modulator.
avs Younger individuals,
bA Boskey, E Carter, CL Raggio, unpublished data,
cvs Age-matched control,
dSee Faibish et al.29,
eSee Vashishth.33,
fvs Untreated osteoporotic.
Fibronectin
Fibronectin, a minor constituent of bone matrix, is one of the first proteins produced by osteoblasts, and directs the initial deposition of collagen fibrils.36 Continued presence of fibronectin is also required to maintain the integrity of the collagenous matrix.37 Studies with a variety of different conditional knockout animals demonstrated that while osteoblasts produce fibronectin, they are not responsible for the presence of the same in the bone extracellular matrix; rather, the bone matrix fibronectin is derived from circulating liver fibronectin.38 In primary biliary cirrhosis, the incidence of osteoporosis is markedly elevated. This higher incidence is due to an increased production of a fibronectin isoform that lessens osteoblastic bone formation.39
Noncollagenous proteins
There are several families of proteins that account for a small proportion of the extracellular matrix, which, as reviewed elsewhere,40 serve important functions in matrix organization, cell signaling, metabolism and mineralization. Other than early studies showing a reduced NCP content of osteoporotic bone,41 little has been written on how these proteins change in expression or distribution in osteoporosis or other bone diseases, with or without anabolic or antiresorptive therapies. Known changes in the expression of NCPs in health and disease are summarized in Table 3. As shown in Table 3, recent gene-wide association studies have identified several NCPs that may be related to fracture risk. In terms of actual measurement of protein content, findings to date are that osteocalcin and osteopontin are important for fracture resistance,42 their concentrations are reduced in older osteonal bone43 and osteopontin may retard crack propagation.42 As a number of NCPs can and do interact with collagen fibrils,40 they may function as ‘glue', enhancing bone's resistance to fracture.42 Recent studies have shown compositional differences between lamellae and interlamellar areas of cortical bone. The interlamellar areas have lower collagen content and increased concentration of NCPs.44 The significance of such differences is as yet unknown. No published studies to date have examined changes in the expression of enzymes and signaling factors in the matrix of patients with metabolic bone disease.
Table 3. Variation of noncollagenous bone protein concentrations in healthy and diseased human and animal bonesa.
| Protein | Bone conc. in osteoporosis | Bone conc. in OI (types I–IV) | Bone conc. after drug treatment |
|---|---|---|---|
| Albumin | Reduced65 | Increased | ? |
| Alpha 2-HS Glycoprotein (fetuin) | Unchanged65 | Increased66 | ? |
| Bone Gla protein (osteocalcin) | Reduced67 | Increased66 | ? |
| Fibronectin | ? | Increased68 | ? |
| Matrix Gla protein | Reduced69 | ? | +ALN not affected (mice)70 |
| Large proteoglycans | ? | Decreased67 | +ZOL reduced71,72 |
| SLRPS | |||
| Biglycan | Depleted72,73 | Decreased68 | ? |
| Decorin | Depleted72 | No change68 | ? |
| Osteoadherin | No change74 | ? | ? |
| SIBLINGS | |||
| BSP | Associated with BMD75 | No change76 | +ALN (rats) reduced77+PTH (mice) reduced78+Sr ranelate no effect79 |
| DMP1 | Increased (mouse)80 | No change76 | +Ca supplement increased81 |
| DPP | ? | Decreased80 | ? |
| MEPE | Reduced82,83Associated with BMD82,83,84 | No change76 | +Ca supplement decreased81 |
| Osteopontin | Reduced85 | No change76 | +PTH serum levels lowered86 |
| Osteonectin | Reduced (mouse)87Associated with BMD in males88 | Reduced68 | +ALN (rats) reduced77 |
| Thrombospondins | Associated with BMD89,90 | TSP1 increased76 | ? |
| Matrix metalloproteases | |||
| MMP13 | Reduced;81,84 associated with BMD82 | ? | +ALN (rats) reduced91 |
| ADAMTS18 | Associated with BMD in Japanese women82 | ? | ? |
| Phosphatases | |||
| FAM210A | Reduced;82 associated with BMD84 | ? | ? |
| Alkaline phosphatase | Associated with BMD90No change in staining (rats)91 | Decreased staining91 | +ZOL-enhanced staining92 |
| Tartrate-resistant acid phosphatase | Increased93,94 | Increased staining (mice)95 | +Estrogen reduced92 |
Abbreviations: ADAMTS18, A Disintegrin And Metalloprotease with ThromboSpondin repeats; ‘Associations', refer to gene expression studies; ALN, alendronate; BMD, bone mineral density; BSP, bone sialoprotein; Conc., relative concentration; DMP1, dentin matrix protein 1; DPP, dentin phosphoprotein; ?, no data available; MEPE, matrix extracellular phosphorylated glycoprotein; MMP13, matrix metalloproteinase 13; OI, osteogenesis imperfecta; PTH, parathyroid hormone; SIBLING, small integrin-binding N-glycosylated; SLRPS, small leucine-rich proteoglycans; TSP1, thrombospondin-1; ZOL, zoledronate.
aSee review by Boskey and Robey40 for the function of these proteins and animal models in which the diseases mentioned are noted. Items without reference numbers are discussed in that text.
Lipids
Less than 3% of the total bone matrix is fat soluble. Lipids are important for cell function, surrounding the cell body, regulating the flux of ions and signaling molecules into and out of the cell. The distribution of lipids in the matrix can be observed from histology, based on sudanophilia, from FTIR and Raman analysis or by nuclear magnetic resonance (NMR).45 There are no recent published studies on lipid composition associated with fragility fractures or other bone diseases in humans. Thirty years ago, we did analyze the lipid composition of femoral heads from patients with avascular necrosis, reporting increased cholesterol content.46
Water
The water content of bone may be demonstrated by proton NMR and can be assessed quantitatively by Raman spectroscopy47 and gravimetric methods.48 Water serves many functions, including filling the pores, interacting with collagen fibrils and binding to mineral crystals.49 Unfortunately, the precise role of water in determining the mechanical competence of bone has not been determined. Analysis of water content shows a direct relationship between water and cortical porosity, which occurs with aging and osteoporosis (Figure 7) and is a key feature of renal osteodystrophy50 and its associated osteoporosis. It is assumed, but not yet demonstrated, that decreases in porosity caused by bisphosphonate treatment will result in a lesser water content in both osteoporosis and renal osteodystrophy.
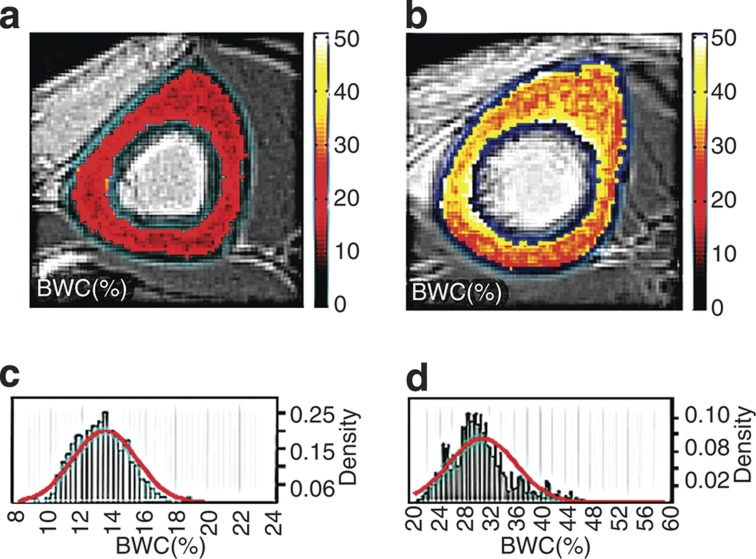
Figure 7.
Water distribution in midshaft tibia cortical bone is demonstrated by three-dimensional ultrashort echo-time magnetic resonance imaging. (a) A young 33-year-old woman without osteoporosis, and (b) is a 64-year-old woman. Note the marked increase in bone water content (BWC) shown by both the concentration images (on the top) and their histograms (c and d, respectively, on the bottom) (reproduced from Yoder et al.49 (Figure 5) with permission from John Wiley & Sons Ltd).
Composition changes in aging and disease
Bone is a dynamic as well as a heterogeneous tissue; therefore, it is not surprising to see changes in composition as a function of age.3 The types of compositional changes that have been reported are summarized in Table 4. These are distinct from the changes discussed above that are associated with bone disease, fragility fractures or treatments to prevent such fractures. Important is the observation that some age-dependent changes in composition are due to alterations in cell activity and protein expression as well as changes in the concentration and post-translational modification40 of those NCPs that regulate matrix composition and mineralization. Therapies to limit the extent of bone disease are directed against these very same cells.
Table 4. Age-related changes in healthy bone composition (cortical and cancellous considered together).
| Bone mineral densitya and tissue mineral densityb | Increase with age |
|---|---|
| Mineral to organic matrix ratioc | Increase with age |
| Calcium-to-phosphate ratiod | Increase with age |
| Carbonate-to-phosphate ratioc | Increase with age |
| Crystal size and perfection (crystallinity)c,e | Increase with age |
| Acid phosphate substitutionc | Decrease with age |
| Matrix heterogeneityb,c | Decrease with age |
| Total collagen crosslinks (collagen maturity)c | Increase with age |
| Collagen enzymatic crosslinksd | Increase with age |
| Collagen AGEsd | Increase with age |
Abbreviations: AGE, advanced glycation end-products; BMDDD, bone mineral density distribution; DXA, dual photon absorptiometry; FTIRI, Fourier transform infrared microscopic imaging; XRD, X-ray diffraction.
aDetermined by DXA,
bDetermined by microcomputed tomography,
cDetermined by FTIRI, BMDD and Raman spectroscopy,
dDetermined by chemical analyses,
eDetermined by XRD.
Compositional changes induced by therapies for osteoporosis
The interesting and important observation is that treatments for osteoporosis while decreasing fracture incidence do not consistently correct the above compositional abnormalities. Therapies currently used (Table 5), other than supplements of calcium, vitamin D and phosphate, fall into two classes: anabolic agents and antiresorptive agents. These therapies all increase or maintain BMD. They each, however, have distinct effects on compositional properties and heterogeneity of their distribution. The most informative of the studies in Table 5 are those based on biopsies before and after treatment. Not every study examined the same tissue site, dose of drug or duration of treatment, and many did not compare the resulting data to the same tissue site in an untreated control. The few instances where this comparison was carried out, and the therapy returned the parameter in question to healthy control values are emphasized in Table 5. Where no human data exists, animal data is included, with the caveat that this does not mean the same alterations will occur in man. Bisphosphonates, alendronate in particular, decreased the heterogeneity of the tissue, rather than increasing it, as would be appropriate for a mechanically strong tissue. Parathyroid hormone, an anabolic agent, and strontium ranelate both increase heterogeneity, determined by FTIRI, Raman and micro-CT measurements. Calcitriol's effect on bone composition has not been determined, neither were the effects of sclerostin antibodies. Odanatacib, the cathepsin K inhibitor, has been shown to affect composition based on BMDD measurements.
Table 5. Effects of current therapies on cancellous bone compositional properties.
| Ca content (BMDD) | Min/mat | BV/TV | TbN | TbS | XLR | XST | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| Antiresorptives | ||||||||
| Estrogen | N96 | I64 | N96 | N96 | N96 | I64 | D64 | NA |
| Calcitonin | NA | D97 | I97 | I97 | D97 | NA | NA | NA |
| Ibandronate | NA | I98 | N99 | N99 | N99 | NA | I98 | N,99 D98 |
| Alendronate | I18,22,100 | N60 | I101 | N101 | N101 | N60 | N60 | D,18,60,98,100N,22,99 |
| Odanaticib | I102 | I102 | NA | NA | NA | NA | NA | D102 |
| Risedronate | I102 | D*103 | NA | NA | NA | D*103 | N*103 | D104 |
| Zoledronate | I103 | I105,106 | I21 | I21 | D21 | NA | D*105,106 | D71 |
| Anabolics | ||||||||
| PTH | D16 | Da | N107 | N107 | N107 | D | Da | I,16,a |
| Antisclerostin | I15 | NA | I15 | NA | NA | NA | NA | NA |
| Other | ||||||||
| SrRAN | I108,109 | N109,110 | N111 | N111 | N111 | N110 | N111 | I108 |
Abbreviations: BMDD, bone mineral density distribution; BV/TV, bone volume fraction; D, decreased; I, increased; Min/mat, mineral/matrix ratio; N, no change; NA, not measured; TbN, trabecular number; TbS, trabecular separation; XLR, collagen maturity; XST, crystallinity.
I, D, N and NA show changes relative to untreated osteoporotic patients. Differences in reported values may be because of site, duration of treatment or method of analysis. Where no human data was available, other species are shown in italics. Bold indicates treatments that were reported to normalize indicated property to that in healthy controls.
aA Boskey, E Carter, CL Raggio, unpublished data.
Most of the antiresorptive therapies for osteoporosis (estrogen, bisphosphonates, calcitonin, cathepsin K inhibitors) increase mineral/matrix ratio, decrease crystallinity and return other FTIR and Raman parameters to less osteoporotic values without returning these values to normalcy. The anabolic agent PTH and strontium-ranelate, which may have both catabolic and anabolic properties, correct many of these FTIRI properties and increase tissue heterogeneity. Many of the therapies lead to retention of existing ‘older' bone. Older bone has increased collagen maturity and increased crystal size. Anabolic agents, in contrast, stimulate new bone formation and the tissue acquired has characteristics of younger bone.
Loss of material heterogeneity, in fracture mechanics, is associated with an increase in brittleness, hence a greater risk of fracture. The importance of heterogeneity is seen in lumber structure and in development of stronger cements and plastics. Bisphosphonate treatment usually results in an increase of bone mineral density and bone volume, or in its maintenance. Bisphosphonate treatment is often accompanied by a decrease in heterogeneity. The reason for this event is as yet uncertain. It may be that there is a balance in these two opposing effects. The use of bisphosphonates results in an increase in BMD and bone volume, hence an increase in bone stiffness and strength. The use of bisphosphonates also causes a decrease in bone heterogeneity, which in turn increases bone brittleness. If this balance is disturbed, as in ‘oversuppression' of bone turnover, failure may occur. Thus, the composition of bone in the healthy individual must be maintained and adjusted, similar to the structure described by Wolff's law, so as to optimize the function of bone.
Conclusions
This review of mineral and matrix properties in healthy and diseased bones demonstrates that these properties show both age- and disease-dependent changes. Bone disease and therapies for these diseases also affect the composition of bone. Bisphosphonates increase bone quantity but their effects on bone quality are variable. The effects of many other agents used in the treatment of osteoporosis are still under investigation. Some bisphosphonates decrease tissue heterogeneity, which may in turn increase brittleness. The origins of this effect and the significance of the alteration in bone quality remain to be determined.
Acknowledgments
Dr Boskey's work on osteoporosis, as detailed in this review, has been supported by NIAMS Grant No. AR041325. I am grateful to Dr Judah Gerstein for his assistance in editing this manuscript.
Footnotes
Dr Boskey's work on FTIRI and osteoporosis has been funded by the NIH. The author declares no conflict of interest.
References
- Boskey A. Mineralization of bones and teeth. Elements Mag 2007;3:385–392. [Google Scholar]
- Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int 2003;14:S35–S42. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Coleman R. Aging and bone. J Dent Res 2010;89:1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E, Meredith DS, Nguyen JT, Boskey AL. Bone tissue composition varies across anatomic sites in the proximal femur and the iliac crest. J Orthop Res 2012;30:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson CL, Paggiosi MA, Crabtree N, Steel SA, McCloskey E, Duncan EL et al. Analysis of body composition in individuals with high bone mass reveals a marked increase in fat mass in women but not men. J Clin Endocrinol Metab 2013;98:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie WD. Clinical review: ethnic differences in bone mass—clinical implications. J Clin Endocrinol Metab 2012;97:4329–4340. [DOI] [PubMed] [Google Scholar]
- Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt 2005;10:031102–031108. [DOI] [PubMed] [Google Scholar]
- Ruppel ME, Miller LM, Burr DB. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int 2008;19:1251–1265. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol 2009;23:741–753. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ. Functional interactions among morphologic and tissue quality traits define bone quality. Clin Orthop Relat Res 2011;469:2150–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JM, Burghardt AJ, Kazakia G, Majumdar S. Noninvasive imaging of bone microarchitecture. Ann NY Acad Sci 2011;1240:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapurlat RD, Delmas PD. Bone microdamage: a clinical perspective. Osteoporos Int 2009;20:1299–1308. [DOI] [PubMed] [Google Scholar]
- Currey JD. Bones Structure and Mechanics. Princeton: Princeton University Press, 2002. [Google Scholar]
- Cohen L, Dean M, Shipov A, Atkins A, Monsonego-Ornan E, Shahar R. Comparison of structural, architectural and mechanical aspects of cellular and acellular bone in two teleost fish. Exp Biol 2012;215:1983–1993. [DOI] [PubMed] [Google Scholar]
- Doublier A, Farlay D, Jaurand X, Vera R, Boivin G. Effects of strontium on the quality of bone apatite crystals: a paired biopsy study in postmenopausal osteoporotic women. Osteoporos Int 2013;24:1079–1087. [DOI] [PubMed] [Google Scholar]
- Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone 2008;42:456–466. [DOI] [PubMed] [Google Scholar]
- Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P et al. Constant mineralization density distribution in cancellous human bone. Bone 2003;32:316–323. [DOI] [PubMed] [Google Scholar]
- Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 2001;29:185–191. [DOI] [PubMed] [Google Scholar]
- Misof BM, Gamsjaeger S, Cohen A, Hofstetter B, Roschger P, Stein E et al. Bone material properties in premenopausal women with idiopathic osteoporosis. J Bone Miner Res 2012;27:2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl-Zelman N, Morello R, Lee B, Rauch F, Glorieux FH, Misof BM et al. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone 2010;46:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misof BM, Roschger P, Gabriel D, Paschalis EP, Eriksen EF, Recker RR et al. Annual intravenous zoledronic acid for three years increased cancellous bone matrix mineralization beyond normal values in the HORIZON biopsy cohort. J Bone Miner Res 2013;28:442–448. [DOI] [PubMed] [Google Scholar]
- Roschger P, Lombardi A, Misof BM, Maier G, Fratzl-Zelman N, Fratzl P et al. Mineralization density distribution of postmenopausal osteoporotic bone is restored to normal after long-term alendronate treatment: qBEI and sSAXS data from the fracture intervention trial long-term extension (FLEX). J Bone Miner Res 2010;25:48–55. [DOI] [PubMed] [Google Scholar]
- Boskey AL. Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging. Curr Osteoporos Rep 2006;4:71–75. [DOI] [PubMed] [Google Scholar]
- Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res 2011;469:2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanci M, Roschger P, Paschalis EP, Klaushofer K, Fratzl P. Bone osteonal tissues by Raman spectral mapping: orientation–composition. J Struct Biol 2006;156:489–496. [DOI] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A et al. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res 2009;24:1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreadie BR, Morris MD, Chen TC, Sudhaker Rao D, Finney WF, Widjaja E et al. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006;39:1190–1195. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Doty S. Mineralized tissue: histology, biology and biochemistry. In Shapiro J, Glorieux F, Byers P, Sponsellor P (eds)Osteogenesis Imperfecta: A Translational Approach to Brittle Bone Disease. New York: Elsevier, 2013. [Google Scholar]
- Faibish D, Gomes A, Boivin G, Binderman I, Boskey A. Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone 2005;36:6–12. [DOI] [PubMed] [Google Scholar]
- Turunen MJ, Saarakkala S, Rieppo L, Helminen HJ, Jurvelin JS, Isaksson H. Comparison between infrared and Raman spectroscopic analysis of maturing rabbit cortical bone. Appl Spectrosc 2011;65:595–603. [DOI] [PubMed] [Google Scholar]
- Spevak L, Flach CR, Hunter T, Mendelsohn R, Boskey A. Fourier transform infrared spectroscopic imaging parameters describing acid phosphate substitution in biologic hydroxyapatite. Calcif Tissue Int 2013;92:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol 2012;23:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 2007;5:62–66. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R et al. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 2011;49:1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Lukashova L, Power J, Loveridge N, Reeve J, Boskey AL. Fourier transform infrared imaging of femoral neck bone: reduced heterogeneity of mineral-to-matrix and carbonate-to-phosphate and more variable crystallinity in treatment-naive fracture cases compared with fracture-free controls. J Bone Miner Res 2013;28:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins a11b1 and a2b1. J Biol Chem 2002;277:37377–37381. [DOI] [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell–matrix adhesions. Mol Biol Cell 2002;13:3546–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentmann A, Kawelke N, Moss D, Zentgraf H, Bala Y, Berger I et al. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J Bone Miner Res 2010;25:706–715. [DOI] [PubMed] [Google Scholar]
- Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A et al. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res 2008;23:1278–1286. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Robey PG. The regulatory role of matrix proteins in mineralization of bone. In Robert Marcus MD, Feldman D, Dempster DW, Luckey M (eds) Osteoporosis. New York: Elsevier, 2013; pp 235–258. [Google Scholar]
- Grynpas MD, Tupy JH, Sodek J. The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone 1994;15:505–513. [DOI] [PubMed] [Google Scholar]
- Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 2012;10:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga GE, Karim L, Colón W, Vashishth D. Biochemical characterization of major bone–matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol Cell Proteomics 2011;10:M110006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsamenis OL, Chong HM, Andriotis OG, Thurner PJ. Load-bearing in cortical bone microstructure: selective stiffening and heterogeneous strain distribution at the lamellar level. J Mech Behav Biomed Mater 2013;17:152–165. [DOI] [PubMed] [Google Scholar]
- Reid DG, Shanahan CM, Duer MJ, Arroyo LG, Schoppet M, Brooks RA et al. Lipids in biocalcification: contrasts and similarities between intimal and medial vascular calcification and bone by NMR. J Lipid Res 2012;53:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Raggio CL, Bullough PG, Kinnett JG. Changes in the bone tissue lipids in persons with steroid- and alcohol-induced osteonecrosis. Clin OrthopRelat Res 1983;172:289–295. [PubMed] [Google Scholar]
- Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues. J Biomed Opt 2000;5:259–268. [DOI] [PubMed] [Google Scholar]
- Jabłoński M, Gun'ko VM, Golovan AP, Leboda R, Skubiszewska-Zięba J, Pluta R et al. Textural characteristics of model and natural bone tissues and interfacial behavior of bound water. J Colloid Interface Sci 2013;392:446–462. [DOI] [PubMed] [Google Scholar]
- Yoder CH, Pasteris JD, Worcester KN, Schermerhorn DV. Structural water in carbonated hydroxylapatite and fluorapatite: confirmation by solid state (2)HNMR. Calcif Tissue Int 2012;90:60–67. [DOI] [PubMed] [Google Scholar]
- Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology 2008;248:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsjaeger S, Buchinger B, Zoehrer R, Phipps R, Klaushofer K, Paschalis EP. Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 2011;49:1160–1165. [DOI] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Burket JC, Havill LM, DiCarlo E, Doty SB, Mendelsohn R et al. Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res 2009;24:1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K, Barnes AM, Fratzl-Zelman N, Whyte MP, Hefferan TE, Makareeva E et al. COL1 C-propeptide cleavage site mutations cause high bone mass osteogenesis imperfecta. Hum Mutat 2011;32:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanleene M, Saldanha Z, Cloyd KL, Jell G, Bou-Gharios G, Bassett JH et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 2011;117:1053–1060. [DOI] [PubMed] [Google Scholar]
- Isaksson H, Turunen MJ, Rieppo L, Saarakkala S, Tamminen IS, Rieppo J et al. Infrared spectroscopy indicates altered bone turnover and remodeling activity in renal osteodystrophy. J Bone Miner Res 2010;25:1360–1366. [DOI] [PubMed] [Google Scholar]
- Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M et al. Advanced glycation end products and bone loss during aging. Ann NY Acad Sci 2005;1043:710–717. [DOI] [PubMed] [Google Scholar]
- Vetter U, Weis MA, Mörike M, Eanes ED, Eyre DR. Collagen crosslinks and mineral crystallinity in bone of patients with osteogenesis imperfecta. J Bone Miner Res 1993;8:133–137. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone 2011;48:1260–1267. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res 2004;19:2000–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD et al. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res 2012;27:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone 2010;46:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int 2008;19:329–337. [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R et al. Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1–34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int 2012;22:2373–2383. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Boskey AL, Kassem M, Eriksen EF. Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res 2003;18:955–959. [DOI] [PubMed] [Google Scholar]
- Quelch KJ, Cole WG, Melick RA. Noncollagenous proteins in normal and pathological human bone. Calcif Tissue Int 1984;36:545–549. [DOI] [PubMed] [Google Scholar]
- Vetter U, Fisher LW, Mintz KP, Kopp JB, Tuross N, Termine JD et al. Osteogenesis imperfecta: changes in noncollagenous proteins in bone. J Bone Miner Res 1991;6:501–505. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Narusawa K, Onishi H, Miura M, Hijioka A, Kanazawa Y et al. Lower osteocalcin and osteopontin contents of the femoral head in hip fracture patients than osteoarthritis patients. Osteoporos Int 2011;22:587–597. [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Robey PG, Vetter UK. Extracellular matrix stoichiometry in osteoblasts from patients with osteogenesis imperfecta. J Bone Miner Res 1995;10:1122–1129. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Orimo H, Hosoi T, Miyao M, Yoshida H, Watanabe S et al. Association of bone mineral density with polymorphism of the human matrix Gla protein locus in elderly women. J Bone Miner Metab 2000;18:27–30. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Hisa I, Seino S, Kaji H. Alendronate induces mineralization in mouse osteoblastic MC3T3-E1 cells: regulation of mineralization-related genes. Exp Clin Endocrinol Diabetes 2010;118:719–723. [DOI] [PubMed] [Google Scholar]
- Gamsjaeger S, Buchinger B, Zwettler E, Recker R, Black D, Gasser JA et al. Bone material properties in actively bone-forming trabeculae in postmenopausal women with osteoporosis after three years of treatment with once-yearly Zoledronic acid. J Bone Miner Res 2011;26:12–18. [DOI] [PubMed] [Google Scholar]
- Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis G. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem 2012;287:33926–33933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 1998;20:78–82. [DOI] [PubMed] [Google Scholar]
- Forlino A, Tani C, Rossi A, Lupi A, Campari E, Gualeni B et al. Differential expression of both extracellular and intracellular proteins is involved in the lethal or nonlethal phenotypic variation of BrtlIV, a murine model for osteogenesis imperfecta. Proteomics 2007;7:1877–1891. [DOI] [PubMed] [Google Scholar]
- Duncan EL, Danoy P, Kemp JP, Leo PJ, McCloskey E, Nicholson GC et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet 2011;7:e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Simmer JP. Hereditary dentin defects. J Dent Res 2007;86:392–399. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. J Dent Res 2012;91:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama M, Kawanami M, Tamura M. Wasf2: a novel target of intermittent parathyroid hormone administration. Int J Mol Med 2013;31:1243–1247. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Zaidi S, Peng Y, Zhou H, Moonga BS, Blesius A et al. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem Biophys Res Commun 2007;355:307–311. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Amizuka N, Oda K, Li M, Yoshie H, Ohshima H et al. Histological evidence of the altered distribution of osteocytes and bone matrix synthesis in klotho-deficient mice. Arch Histol Cytol 2005;68:371–381. [DOI] [PubMed] [Google Scholar]
- Welldon KJ, Findlay DM, Evdokiou A, Ormsby RT, Atkins GJ. Calcium induces pro-anabolic effects on human primary osteoblasts associated with acquisition of mature osteocyte markers. Mol Cell Endocrinol 2013;376:85–92. [DOI] [PubMed] [Google Scholar]
- Jemtland R, Holden M, Reppe S, Olstad OK, Reinholt FP, Gautvik VT et al. Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. J Bone Miner Res 2011;26:1793–1801. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 2009;41:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén A, Hultenby K, Reinholt FP, Onnerfjord P, Heinegård D. Altered osteoclast development and function in osteopontin deficient mice. J Orthop Res 2008;26:721–728. [DOI] [PubMed] [Google Scholar]
- Chiang TI, Chang IC, Lee HS, Lee H, Huang CH, Cheng YW. Osteopontin regulates anabolic effect in human menopausal osteoporosis with intermittent parathyroid hormone treatment. Osteoporos Int 2011;22:577–585. [DOI] [PubMed] [Google Scholar]
- Mansergh FC, Wells T, Elford C, Evans SL, Perry MJ, Evans MJ et al. Osteopenia in Sparc (osteonectin)-deficient mice: characterization of phenotypic determinants of femoral strength and changes in gene expression. Physiol Genomics 2007;32:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany AM, McMahon DJ, Powell JS, Greenberg DA, Kurland ES. Osteonectin/SPARC polymorphisms in Caucasian men with idiopathic osteoporosis. Osteoporos Int 2008;19:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Mori S, Kou I, Fuku N, Naka Mieno M, Honma N et al. Association of the formiminotransferase N-terminalsub-domain containing gene and thrombospondin, type 1, domain-containing 7 A gene with the prevalence of vertebral fracture in 2427 consecutive autopsy cases. J Hum Genet 2013;58:109–112. [DOI] [PubMed] [Google Scholar]
- Mori S, Kou I, Sato H, Emi M, Ito H, Hosoi T et al. Association of genetic variations of genes encoding thrombospondin, type 1, domain-containing 4 and 7 A with low bone mineral density in Japanese women with osteoporosis. J Hum Genet 2008;53:694–697. [DOI] [PubMed] [Google Scholar]
- Sarathchandra P, Cassella JP, Ali SY. Enzyme histochemical localisation of alkaline phosphatase activity in osteogenesis imperfecta bone and growth plate: a preliminary study. Micron 2005;36:715–720. [DOI] [PubMed] [Google Scholar]
- Ebert R, Zeck S, Krug R, Meissner-Weigl J, Schneider D, Seefried L et al. Pulse treatment with zoledronic acid causes sustained commitment of bone marrow derived mesenchymal stem cells for osteogenic differentiation. Bone 2009;44:858–864. [DOI] [PubMed] [Google Scholar]
- Fan W, Bouwense SA, Crawford R, Xiao Y. Structural and cellular features in metaphyseal and diaphyseal periosteum of osteoporotic rats. J Mol Histol 2010;41:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong DH, Liu XG, Guo YF, Tan LJ, Wang L, Sha BY et al. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet 2009;84:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Doty SB, Hughes C, Dempster D, Camacho NP. Increased resorptive activity and accompanying morphological alterations in osteoclasts derived from the oim/oim mouse model of osteogenesis imperfecta. J Cell Biochem 2007;102:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EM, Patsch J, Muschitz C, Becker S, Resch H. Severe osteoporosis with multiple vertebral fractures after gender reassignment therapy—is it male or female osteoporosis? Gynecol Endocrinol 2011;27:341–344. [DOI] [PubMed] [Google Scholar]
- Pienkowski D, Doers TM, Monier-Faugere MC, Geng Z, Camacho NP, Boskey AL et al. Calcitonin alters bone quality in beagle dogs. J Bone Miner Res 1997;12:1936–1943. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Geng Z, Paschalis EP, Qi Q, Arnala I, Bauss F et al. Intermittent and continuous administration of the bisphosphonate ibandronate in ovariohysterectomized beagle dogs: effects on bone morphometry and mineral properties. J Bone Miner Res 1999;14:1768–1778. [DOI] [PubMed] [Google Scholar]
- Bock O, Börst H, Beller G, Armbrecht G, Degner C, Martus P et al. Impact of oral ibandronate 150 mg once monthly on bone structure and density in post-menopausal osteoporosis or osteopenia derived from in vivo μCT. Bone 2012;50:317–324. [DOI] [PubMed] [Google Scholar]
- Misof BM, Patsch JM, Roschger P, Muschitz C, Gamsjaeger S, Paschalis EP et al. Intravenus treatment with ibandronate normalizes bone matrix mineralization and reduces cortical porosity after two years in male osteoporosis: a paired biopsy study. J Bone Miner Res 2013; e-pub ahead of print; 10.1002/jbmr.2035. [DOI] [PubMed] [Google Scholar]
- Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 2010;25:2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl-Zelman N, Roschger P, Fisher JE, Duong le T, Klaushofer K. Effects of Odanacatib on bone mineralization density distribution in thoracic spine and femora of ovariectomized adult rhesus monkeys: a quantitative backscattered electron imaging study. Calcif Tissue Int 2013;92:261–269. [DOI] [PubMed] [Google Scholar]
- Durchschlag E, Paschalis EP, Zoehrer R, Roschger P, Fratzl P, Recker R et al. Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res 2006;21:1581–1590. [DOI] [PubMed] [Google Scholar]
- Zoehrer R, Roschger P, Paschalis EP, Hofstaetter JG, Durchschlag E, Fratzl P et al. Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res 2006;21:1106–1112. [DOI] [PubMed] [Google Scholar]
- Recker RR, Delmas PD, Halse J, Reid IR, Boonen S, García-Hernandez PA et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res 2008;23:6–16. [DOI] [PubMed] [Google Scholar]
- Gamsjaeger S, Hofstetter B, Zwettler E, Recker R, Gasser JA, Eriksen EF et al. Effects of 3 years treatment with once-yearly zoledronic acid on the kinetics of bone matrix maturation in osteoporotic patients. Osteoporos Int 2013;24:339–347. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 2001;16:1846–1853. [DOI] [PubMed] [Google Scholar]
- Doublier A, Farlay D, Khebbab MT, Jaurand X, Meunier PJ, Boivin G. Distribution of strontium and mineralization in iliac bone biopsies from osteoporotic women treated long-term with strontium ranelate. Eur J Endocrinol 2011;165:469–476. [DOI] [PubMed] [Google Scholar]
- Roschger P, Manjubala I, Zoeger N, Meirer F, Simon R, Li C et al. Bone material quality in transiliac bone biopsies of postmenopausal osteoporotic women after 3 years of strontium ranelate treatment. J Bone Miner Res 2010;25:891–900. [DOI] [PubMed] [Google Scholar]
- Wu Y, Adeeb SM, Duke MJ, Munoz-Paniagua D, Doschak MR. Compositional and material properties of rat bone after bisphosphonate and/or strontium ranelate drug treatment. J Pharm Pharm Sci 2013;16:52–64. [DOI] [PubMed] [Google Scholar]
- Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res 2013;28:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]