Abstract
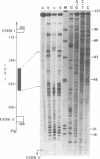
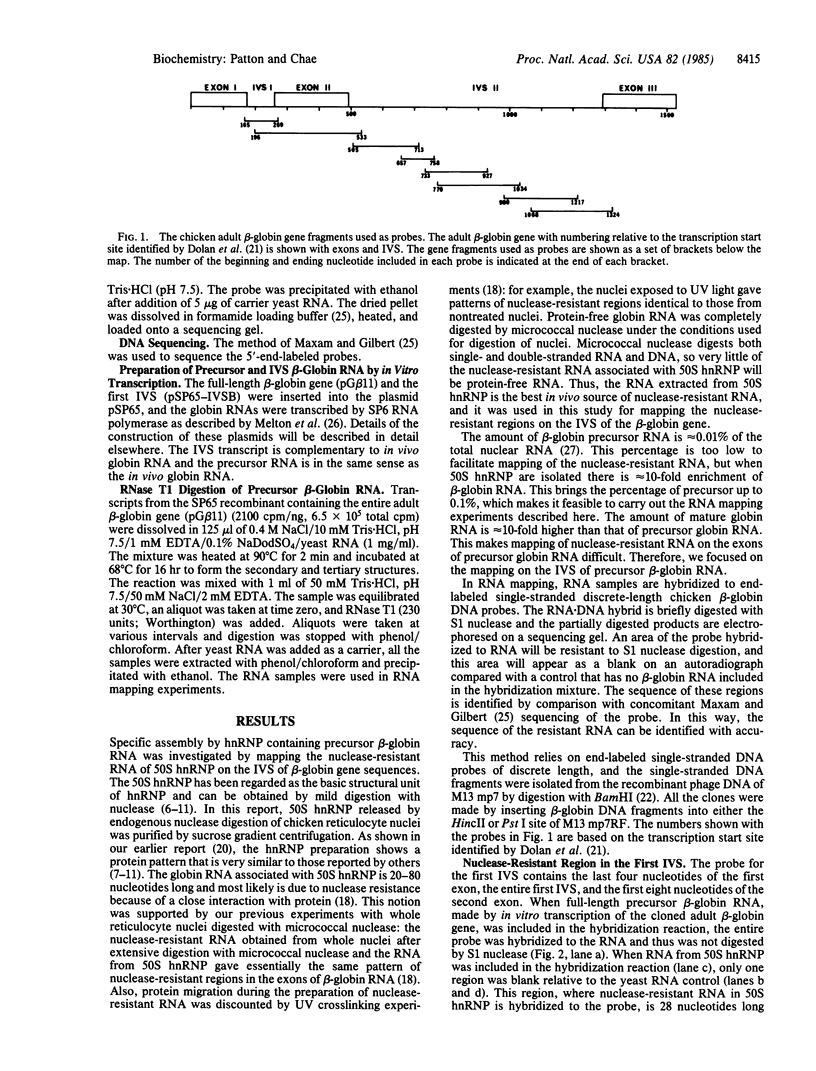
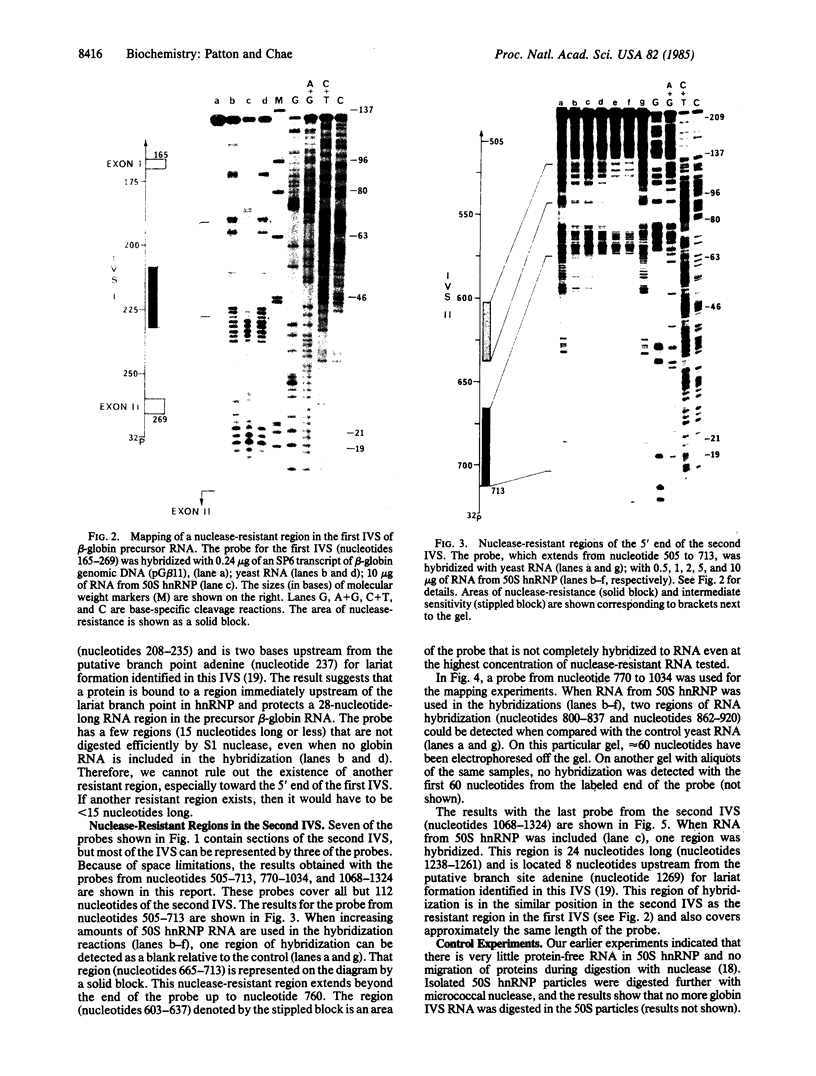
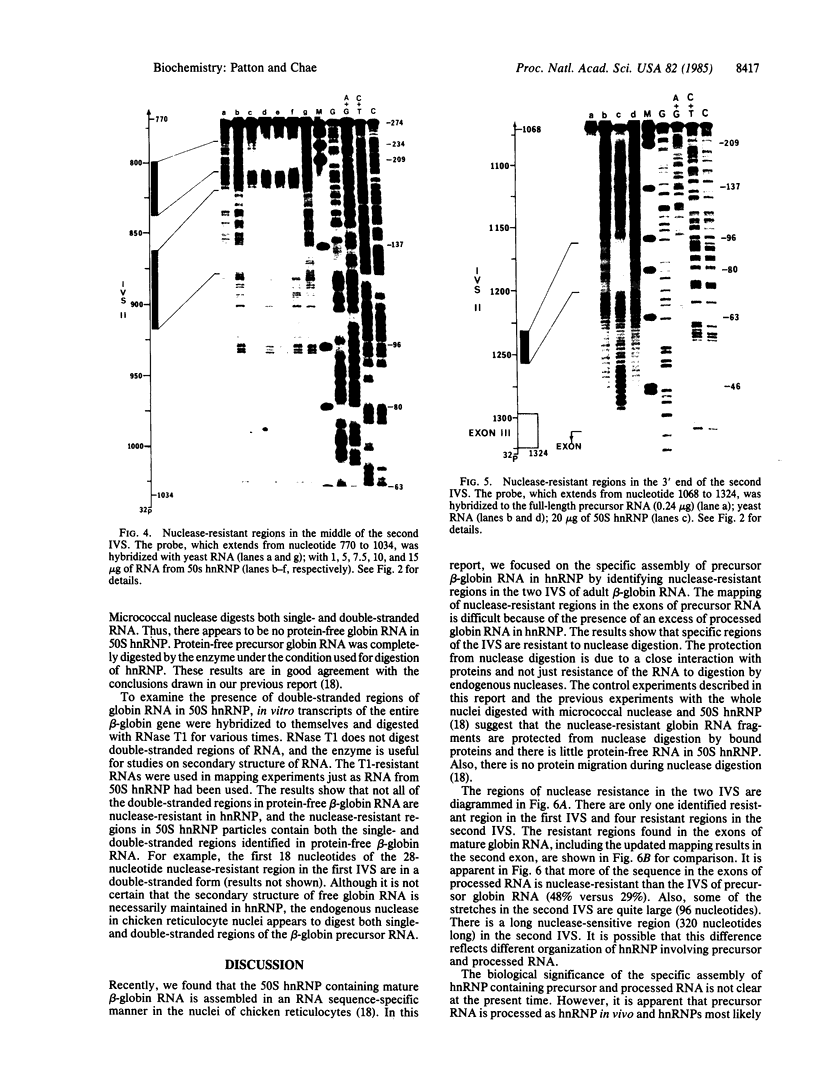
The specific assembly of heterogeneous nuclear RNA-protein complexes (hnRNPs) containing precursor beta-globin RNA was investigated by using the 50S hnRNP released from chicken reticulocyte nuclei by endogenous nuclease. The nuclease-resistant regions were mapped on adult beta-globin intervening sequences (IVS) at the resolution of nucleotides with an RNA mapping method [Patton, J. R. and Chae, C.-B. (1983) J. Biol. Chem. 258, 3991-3995]. We found that there is one 28-nucleotide-long nuclease-resistant region in the first IVS and there are four nuclease-resistant regions in the second IVS. Of particular interest is the presence in 50S hnRNP of a nuclease-resistant region (24-28 nucleotides long) in both IVS immediately upstream from the putative lariat branch site in an RNA splicing intermediate. Our results demonstrate that hnRNPs containing precursor beta-globin RNA are, like those containing mature beta-globin RNA, assembled in a site-specific manner.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Brody E., Abelson J. The "spliceosome": yeast pre-messenger RNA associates with a 40S complex in a splicing-dependent reaction. Science. 1985 May 24;228(4702):963–967. doi: 10.1126/science.3890181. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7471–7475. doi: 10.1073/pnas.81.23.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. M., Chae C. B. Different RNA patterns of globin and non-globin 40S heterogeneous nuclear RNA-protein complexes in chicken reticulocyte nuclei. Nucleic Acids Res. 1983 Oct 25;11(20):7057–7068. doi: 10.1093/nar/11.20.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Daneholt B. Characterization of active transcription units in Balbiani rings of Chironomus tentans. Cell. 1979 Aug;17(4):835–848. doi: 10.1016/0092-8674(79)90324-6. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Gallwitz D. Evidence for an intron-contained sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983 Jun;33(2):519–527. doi: 10.1016/0092-8674(83)90433-6. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Malcolm D. B., Sommerville J. The structure of chromosome-derived ribonucleoprotein in oocytes of Triturus cristatus carnifex (Laurenti). Chromosoma. 1974;48(2):137–158. doi: 10.1007/BF00283960. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson R. I., van Eekelen C., Philipson L. Non-random localization of ribonucleoprotein (RNP) structures within an adenovirus mRNA precursor. Nucleic Acids Res. 1982 May 25;10(10):3053–3068. doi: 10.1093/nar/10.10.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Patton J. R., Chae C. B. A method for isolation of a large amount of a single-stranded DNA fragment. Anal Biochem. 1982 Oct;126(1):231–234. doi: 10.1016/0003-2697(82)90134-8. [DOI] [PubMed] [Google Scholar]
- Patton J. R., Chae C. B. A method for mapping RNA initiation, termination, splice, and protein binding sites. Ribosome binding sites on beta-globin messenger RNA. J Biol Chem. 1983 Mar 25;258(6):3991–3995. [PubMed] [Google Scholar]
- Patton J. R., Ross D. A., Chae C. B. Specific regions of beta-globin RNA are resistant to nuclease digestion in RNA-protein complexes in chicken reticulocyte nuclei. Mol Cell Biol. 1985 Jun;5(6):1220–1228. doi: 10.1128/mcb.5.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Munroe S. H. Ribonucleoprotein organization of eukaryotic RNA. XV. Different nucleoprotein structures of globin messenger RNA sequences in nuclear and polyribosomal ribonucleoprotein particles. J Mol Biol. 1981 Aug 25;150(4):509–524. doi: 10.1016/0022-2836(81)90377-6. [DOI] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Teem J. L., Rosbash M. Evidence for the biochemical role of an internal sequence in yeast nuclear mRNA introns: implications for U1 RNA and metazoan mRNA splicing. Cell. 1983 Sep;34(2):395–403. doi: 10.1016/0092-8674(83)90373-2. [DOI] [PubMed] [Google Scholar]
- Rautmann G., Breathnach R. A role for branchpoints in splicing in vivo. 1985 May 30-Jun 5Nature. 315(6018):430–432. doi: 10.1038/315430a0. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Brunel C., Jeanteur P. Interaction of snRNAs with rapidly sedimenting nuclear sub-structures (hnRNPs) from HeLa cells. Nucleic Acids Res. 1983 Oct 11;11(19):6631–6646. doi: 10.1093/nar/11.19.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Kamen R. Arrangement of 30S heterogeneous nuclear ribonucleoprotein on polyoma virus late nuclear transcripts. Mol Cell Biol. 1981 Jan;1(1):21–34. doi: 10.1128/mcb.1.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Divilliers G., Jacob M. Rearrangements in the course of ribonuclease hydrolysis of pre-messenger ribonucleoproteins. A warning. Eur J Biochem. 1979 Apr;95(3):593–606. doi: 10.1111/j.1432-1033.1979.tb13000.x. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Keohavong P., Jacob M. Mild nuclease treatment as a probe for a non-random distribution of adenovirus-specific RNA sequences and of cellular RNA in nuclear ribonucleoprotein fibrils. J Mol Biol. 1982 Mar 5;155(3):185–205. doi: 10.1016/0022-2836(82)90001-8. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Efstratiadis A. In vivo splicing products of the rabbit beta-globin pre-mRNA. Cell. 1984 Dec;39(3 Pt 2):589–602. doi: 10.1016/0092-8674(84)90466-5. [DOI] [PubMed] [Google Scholar]