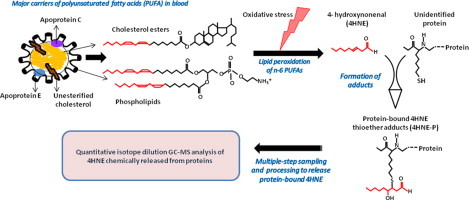
Abstract
Objective
Measurements of oxidative stress biomarkers in patients with heart failure (HF) have yielded controversial results. This study aimed at testing the hypothesis that circulating levels of the lipid peroxidation product 4-hydroxynonenal bound to thiol proteins (4HNE-P) are strongly associated with those of its potential precursors, namely n-6 polyunsaturated fatty acids (PUFA).
Methods and results
Circulating levels of 4HNE-P were evaluated by gas chromatography-mass spectrometry in 71 control subjects and 61 ambulatory symptomatic HF patients along with various other clinically- and biochemically-relevant parameters, including other oxidative stress markers, and total levels of fatty acids from all classes, which reflect both free and bound to cholesterol, phospholipids and triglycerides. All HF patients had severe systolic functional impairment despite receiving optimal evidence-based therapies. Compared to controls, HF patients displayed markedly lower circulating levels of HDL- and LDL-cholesterol, which are major PUFA carriers, as well as of PUFA of the n-6 series, specifically linoleic acid (LA; P=0.001). Circulating 4HNE-P in HF patients was similar to controls, albeit multiple regression analysis revealed that LA was the only factor that was significantly associated with circulating 4HNE-P in the entire population (R2=0.086; P=0.02). In HF patients only, 4HNE-P was even more strongly associated with LA (P=0.003) and HDL-cholesterol (p<0.0002). Our results demonstrate that 4HNE-P levels, expressed relative to HDL-cholesterol, increase as HDL-cholesterol plasma levels decrease in the HF group only.
Conclusion
Results from this study emphasize the importance of considering changes in lipids and lipoproteins in the interpretation of measurements of lipid peroxidation products. Further studies appear warranted to explore the possibility that HDL-cholesterol particles may be a carrier of 4HNE adducts.
Abbreviations: 4HNE, 4-hydroxynonenal; 4HNE-P, 4-hydroxynonenal bound to circulating thiol proteins; AA, arachidonic acid; CRP, C-reactive protein; DHA, docosahexanaenoic acid; eGFR, estimated glomerular filtration rate; EPA, eicosapentaenoic acid; GSH, reduced glutathione; GSSG, oxidized glutathione; HF, heart failure; HFC-MHI, heart failure clinic of the Montreal Heart Institute; HOMA-IR, homeostatic model assessment of insulin resistance; LA, linoleic acid; MDA, malondialdehyde; MPO, myeloperoxidase; NT-pro-BNP, N-terminal proB-type natriuretic peptide; NYHA, New York Heart Association; PUFA, polyunsaturated fatty acids; RAS, renin-angiotensin system; TBARS, thiobarbituric acid-reactive substances; TNF, tumor necrosis factor
Keywords: 4-Hydroxynnonenal, Oxidative stress, Lipid peroxidation, Linoleic acid, Polyunsaturated fatty acids, Heart failure patients
Graphical abstract
Highlights
-
•
Peroxidation of n-6 polyunsaturated fatty acids (PUFA) forms 4-hydroxynonenal (4HNE).
-
•
Heart failure (HF) patients have lower plasma levels of n-6 PUFA linoleic acid (LA).
-
•
Blood levels of 4HNE bound to proteins (4HNE-P) in HF patients are similar to controls.
-
•
4HNE-P levels are associated with those of LA in the entire population (p<0.02).
-
•
4HNE-P levels are strongly associated with LA and HDL-cholesterol in HF patients.
Introduction
While considerable evidence supports the role of oxidative stress in the development and progression of chronic diseases such as heart failure (HF) [1], [2], [3], [4], clinical trials with natural antioxidant interventions conducted in the last two decades have yielded controversial results [5], [6], [7], [8], [9]. However, until 2000, the few clinical studies that documented circulating biomarkers of oxidative stress predominantly measured the plasma level of malondialdehyde (MDA) using the highly criticized thiobarbituric acid-reactive substances (TBARS) method without chromatographic separation [3], [10], [11], [12], [13]. More recently, urinary and plasma isoprostanes, which arise from the peroxidation of the n-6 polyunsaturated fatty acid (PUFA) arachidonic acid (AA), have been advocated for the in vivo assessment of oxidative stress status for human studies [14], [15], [16]. However, it appears unlikely that the measurement of a single biomarker will provide a comprehensive picture of the various oxidative stress-related events that may contribute to progression of cardiovascular diseases [17].
Another peroxidation product of the n-6 PUFAs AA and linoleic acid (LA) that has generated considerable research interest for its potential as a biomarker of oxidative stress is 4-hydroxynonenal (4HNE) [18]. This aldehyde readily binds covalently to nucleophilic residues of proteins, peptides, phospholipids, and nucleic acids and thereby exhibits cytotoxic effects. 4HNE can also modulate signaling pathways involved in cell proliferation, fibrosis, apoptosis and inflammation, which are all hallmarks of cardiovascular diseases, especially HF [19], [20], [21]. Interestingly, Nakamura et al. showed that the myocardial levels of protein-bound 4HNE were decreased after treatment with carvedilol in patients with HF [22], and were also correlated with left ventricular dilatation and systolic dysfunction in patients with hypertrophic cardiomyopathy [23]. Recently, we developed and validated a method using gas chromatography-mass spectrometry to quantify, with precision, blood levels of total protein-bound 4HNE thioether adducts (4HNE-P) [24]. Using this method, we reported a higher accumulation of blood 4HNE-P in spontaneously hypertensive rats than in normal rats, which correlated positively with left ventricular diastolic dysfunction [24], [25], and in hypercholesterolemic rabbits [26]. These findings support a role for this biomarker in the pathophysiological events linked to heart disease progression, although it has not yet been documented in humans.
Measurements of oxidative stress in cohorts of patients with HF have, however, yielded controversial results, with some studies [13], [27], [28] reporting an increase in these biomarkers. This discrepancy has been attributed in part to current drug therapies [29] that include β-blockers [22], [28], [30], renin-angiotensin system (RAS) inhibitor, and statins [31], [32], which exert indirect antioxidant effects through regulation of free radical-producing processes or of antioxidant defenses. However, since commonly measured biomarkers of oxidative stress are predominantly lipid peroxidation products, one other factor that may contribute to this discrepancy is the circulating level of PUFAs, which are the precursors of these biomarkers and are affected by disease [33] and/or by pharmacological treatment such as statins [34]. In their recent review, Halliwell and Lee [35] recognized that this issue should be further explored.
Therefore, in this study we tested the hypothesis that circulating levels of the lipid peroxidation product 4-hydroxynonenal bound to proteins (4HNE-P) are strongly associated with those of its potential precursors. Hence, we documented circulating levels of (i) biomarkers of oxidative stress, including 4HNE-P but also the commonly assessed malondialdehyde, as well as (ii) total fatty acids, which include all classes of fatty acids and reflect both free and bound fatty acids, either to albumin or esterified to cholesterol or as triglycerides and phospholipids in lipoproteins, in a population of 61 symptomatic ambulatory HF patients receiving evidence-based therapies and 71 control subjects.
Patients and methods
Study population and design
This study involved a total of 132 subjects. A group of 61 symptomatic patients followed at the Heart Failure Clinic of the Montreal Heart Institute (HFC-MHI) who were older than 45 years and had a left ventricular ejection fraction ≤40%, as determined by echocardiography, was studied. The HFC-MHI is a multidisciplinary specialized outpatient disease management program. Symptomatic HF patients are referred to the clinic by a MHI cardiologist after an emergency department visit or a hospital admission for documented HF. Thus, this clinic is restricted to patients at high risk of decompensation, needing a close medical follow-up [36]. For the purpose of this analysis, patients with recent (<3 months) cardiac surgery were excluded. We also studied 71 control subjects who were older than 45 years and free of significant cardiovascular disease or risk factors such as diabetes mellitus, untreated dyslipidemia or hypertension, severe obesity (Body mass index ≥32 kg/m2) or cigarette smoking (≥25/day). Complete medical assessment, including history and physical examination, was performed and a 20-ml blood sample was drawn from an antecubital vein after overnight fast. Blood and plasma samples were collected in EDTA tube and immediately frozen in liquid nitrogen and stored at −80 °C until assayed. The investigation conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the MHI research ethics committee and all subjects gave written informed consent.
Biochemical determinations and calculations
The following assays on plasma samples were performed according to the manufacturer's instructions: glucose, creatinine, blood urea, HDL-cholesterol and triglycerides were determined by spectrophotometric methods, using respective reagent Flex on a multianalyzer Dimension RxL Max (Dade Behring Diagnostics, Marburg, Germany), N-terminal proB-type natriuretic peptide (NT-proBNP) and troponin T were assessed by electrochemiluminescence immunoassay on a Elecsys 2010 analyzer using Roche assay kits (Roche Diagnostic, Mannheim, Germany), and C-reactive protein (CRP) was quantified using the Dade Behring N High Sensitivity assay (Dade Behring Diagnostics, Marburg, Germany) on the BN ProSpec Nephelometer. Insulin was quantified by a double-antibody radioimmunoassay (Bi-Ins-IRMA kit, CIS Bio International, Gif-sur-Yvette, France). ELISA kits were used to assess levels of Tumor Necrosis Factor-alpha (TNF-α) (R&D Systems, Minneapolis, USA) and myeloperoxidase (MPO) (Oxis International Inc., Foster City, USA). Levels of reduced (GSH) and oxidized (GSSG) glutathione were determined in 200 µl of whole blood by capillary electrophoresis according to Serru et al. [37]. The amount of free MDA formed during the TBARS reaction was determined by HPLC, and the plasma fatty acid composition was assessed as previously described with some minor modifications [38], [39]. The estimated glomerular filtration rate (eGFR) and the homeostatic model assessment of insulin resistance (HOMA-IR) were calculated by the Cockcroft and Gault [40] and Matthews [41] formulas, respectively. The cholesterol ratio was calculated from the total-cholesterol value divided by the HDL-cholesterol value.
Statistical analysis
Patient characteristics are expressed as mean±SD, median [min−max], or frequencies and percentages when appropriate. Because of significant differences in age and gender between the control and HF patients, comparisons between these two groups of subjects for clinical and biochemical characteristics were conducted using an analysis of covariance (ANCOVA) adjusting for age and sex as covariates. For parameters exhibiting a severely skewed distribution, a logarithmic transformation was used. To test for NYHA classes and type 2 diabetes among the HF group, categorical variables were analyzed using Pearson chi-square tests and continuous variables with t-tests or Mann–Whitney–Wilcoxon tests (according to the distribution of the variable). Supplementary analyses were conducted for (i) primary outcomes measures, namely the oxidative stress marker 4HNE-P and its potential precursors the n-6 PUFA LA, as well as for the commonly assessed oxidative stress marker MDA. For LA, covariance analysis was conducted for further adjusting values to plasma levels of the various cholesterol classes (total, LDL- and HDL-cholesterol) as covariates, in addition to age and gender. For 4HNE-P, Pearson correlation analyses were conducted to test its association with its potential precursors. We assessed also risk factors and parameters associated with its circulating levels using multiple linear regression analysis. This analysis was performed (i) in the entire population and subsequently (ii) in HF patients only using the following risk factors and parameters, which were selected for their high clinical and biochemical relevance: (i) group, age, body mass index, sex, log NT-proBNP, malondialdehyde (MDA), reduced-to-oxidized glutathione ratio, myeloperoxidase, lymphocytes, tumor necrosis factor-α, C-reactive protein, HDL-cholesterol, LDL-cholesterol, triglycerides, insulin, glucose, uric acid, estimated glomerular filtration rate, total bilirubin, alkaline phosphatase, linoleic acid and arachidonic acid, and (ii) all parameters listed in (i) except group, plus systolic pressure, NYHA class, left ventricular ejection fraction, etiology, non-insulin dependent diabetes mellitus, hypertension, myocardial infarction, medication (β-blocker, RAS inhibitor, calcium channel inhibitor, chronic nitrate, acute nitrate, amiodarone, digoxin, warfarin, salicylates, diuretics, hypolipidemic agents, hypoglycemic agents, allopurinol). For MDA, we have also conducted a multiple linear regression analysis in HF patients only using the same risk factors and parameters listed above for 4HNE-P, except that MDA was replaced with 4HNE-P. All these parameters were entered into a stepwise selection procedure with a 0.1 significance level for staying in the model. Parameters with a P-value between 0.05 and 0.1 were kept in the model to indicate potential trends. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.1 or higher (Cary, NC, USA).
Results
Clinical and biochemical characteristics of HF patients and controls, including medications used, are summarized in Table 1, Table 2; these tables include parameters routinely assessed in clinical laboratories. Circulating levels of lipid peroxidation markers and of their potential n-6 PUFA precursors, as well as other fatty acids can be found in Table 3, Table 4, respectively. Because the HF patient cohort was slightly older and had a greater proportion of men than controls, all tables report only statistical differences which remained significant after adjustment for age- and gender-differences.
Table 1.
Clinical characteristics of heart failure (HF) patients and controls.
| Parameters | Control | HF |
|---|---|---|
| N | 71 | 61 |
| Subjects characteristics | ||
| Gender (male, %) | 37 (52%) | 47 (77%)⁎⁎⁎ |
| Age (years) | 59±9 | 67±10⁎⁎⁎⁎ |
| Body mass index (kg/m2) | 25.9±2.9 | 26.9±4.3 |
| Clinical parameters | ||
| Left ventricular ejection fraction (%) | n.d. | 26.3±7.1 |
| NYHA Classa | ||
| I | n.a. | 1 (2%) |
| II | 33 (54%) | |
| III | 27 (44%) | |
| Systolic blood pressure (mmHg) | 119±13 | 106±19⁎⁎⁎⁎ |
| Diastolic blood pressure (mmHg) | 73±8 | 58±9⁎⁎⁎⁎ |
| Heart rate (bpm) | 68±7 | 68±10 |
| Medical history(etiology/comorbidities) | ||
| Ischemic etiology | 0 | 39 (64%)⁎⁎⁎⁎ |
| Dyslipidemia | 19 (27%) | 55 (90%)⁎⁎⁎⁎ |
| Hypertension | 0 | 30 (49%)⁎⁎⁎⁎ |
| Type 2 diabetes | 0 | 29 (48%)⁎⁎⁎⁎ |
| History of myocardial infarction | 0 | 33 (54%)⁎⁎⁎⁎ |
| Medications | ||
| RAS inhibitor | 7 (10%) | 52 (85%)⁎⁎⁎⁎ |
| β-blocker | 3 (4%) | 48 (79%)⁎⁎⁎⁎ |
| Digoxin | 0 | 42 (69%)⁎⁎⁎⁎ |
| Lipid-lowering agentsb | 17 (24%) | 46 (75%)⁎⁎⁎⁎ |
| Diureticsc | 5 (7%) | 59 (97%)⁎⁎⁎⁎ |
| Aspirin | 1 (1%) | 29 (48%)⁎⁎⁎⁎ |
| Salicylates | 9 (13%) | 39 (65%)⁎⁎⁎⁎ |
| Chronic nitrate | 0 | 18 (30%)⁎⁎⁎⁎ |
| Spironolactone | 0 | 42 (62%)⁎⁎⁎⁎ |
| Acute nitrate | 0 | 22 (36%)⁎⁎⁎⁎ |
| Allopurinol | 1 (1%) | 19 (31%)⁎⁎⁎⁎ |
| Amiodarone | 0 | 17 (28%)⁎⁎⁎⁎ |
| Warfarin | 1 (1%) | 29 (48%)⁎⁎⁎⁎ |
| Calcium channel antagonists | 3 (4%) | 3 (5%) |
| Hypoglycemic agents | 0 | 21 (35%)⁎⁎⁎⁎ |
Data are means±SD, median [min−max] or number of patients (percentage). NYHA, New York Heart Association, n.a., not applicable, n.d., not determined, RAS, renin-angiotensin system.
This classification applies for HF patients only.
Lipid-lowering agents include statins and fibrates.
Diuretics include loop diuretic, thiazides and potassium sparing diuretic.
P<0.05, ⁎⁎P<0.01, ⁎⁎⁎P<0.001 and ⁎⁎⁎⁎P<0.0001: HF vs. controls, using ANCOVA adjusting for age and sex as covariates.
Table 2.
Standard laboratory parameters assessed in HF patients and controls.
|
Parameters |
Control |
HF |
|---|---|---|
| N | 71 | 61 |
| NT-Pro-BNP (ng/L)a | 57 | 2641⁎⁎⁎⁎ |
| [12–559] | [101–35,000] | |
| Increased troponin T (≥0.03 µg/L) | 0 | 11 (16%) |
| Creatinine (μM) | 83±18 | 160±119⁎⁎⁎⁎ |
| eGFR (ml/min) | 83.1±21.0 | 51.7±28⁎⁎⁎⁎ |
| Blood urea (mM) | 6.0±1.4 | 12.1±5.7⁎⁎⁎⁎ |
| Uric acid (μM) | 282±77 | 431±139⁎⁎⁎⁎ |
| Total bilirubin (μM) | 10.6 ±4.9 | 11.9±7.3 |
| C-reactive protein (μg/ml)a | 2.02±3.22 | 7.08±9.27⁎⁎⁎⁎ |
| Tumor necrosis factor-α (pg/ml) | 1.13±0.36 | 2.26±0.85⁎⁎⁎⁎ |
| Myeloperoxidase (ng/ml)a | 16.9±6.7 | 28.8±44.0⁎⁎⁎ |
| Leukocytes (×109/L) | 5.80±1.34 | 7.65±1.98⁎⁎⁎⁎ |
| Lymphocytes (×109/L) | 0.30±0.08 | 0.22±0.07⁎⁎⁎⁎ |
| Hemoglobin (g/L) | 142±10 | 133±16⁎⁎⁎⁎ |
| Platelets (×109/L) | 240±52 | 213±67 |
| Total cholesterol (mM) | 5.10±0.79 | 4.07±1.09⁎⁎⁎⁎ |
| HDL-Cholesterol (mM) | 1.45±0.43 | 0.90±0.27⁎⁎⁎⁎ |
| LDL-Cholesterol (mM) | 3.15±0.69 | 2.42±0.92⁎⁎⁎⁎ |
| Total cholesterol/HDL ratio | 3.73±0.93 | 4.74±1.38⁎⁎⁎ |
| Triglycerides (mM) | 1.11±0.53 | 1.68±1.06⁎⁎ |
| Glucose (mM) | 4.60±0.47 | 7.12±2.51⁎⁎⁎⁎ |
| Insulin (mU/L) | 6.33±2.86 | 9.85±5.97⁎⁎⁎ |
| HOMA-IR | 26.7±11.9 | 55.7±35.1⁎⁎⁎⁎ |
Data are means±SD, median [min−max] or number of patients (percentage). NT-ProBNP, N-terminal proB-type natriuretic peptide, eGFR, estimated glomerular filtration rate, HOMA-IR: homeostatic model assessment of insulin resistance, calculated from Ref. [41].
Parameters exhibiting a severely skewed distribution and for which a logarithmic transformation was used.
P<0.05, ⁎⁎P<0.01, ⁎⁎⁎P<0.001 and ⁎⁎⁎⁎P<0.0001: HF vs. controls, using ANCOVA adjusting for age and sex as covariates.
Table 3.
Circulating levels of oxidative stress-related parameters in HF patients and controls.
| Parameters |
Control |
HF |
|---|---|---|
| N | 71 | 61 |
| GSH (μM) | 561.9±154.9 | 555.6±168.4 |
| GSSG (μM) | 42.9±21.9 | 33.3±22.2 |
| GSH-to-GSSG ratio | 15.7±7.7 | 24.3±18.7⁎⁎ |
| Malondialdehyde (μM) | 967±313 | 1143±473 |
| 4HNE-P (μM) | 223±97 | 213±113 |
Data are means±SD. GSH: reduced glutathione; GSSG: oxidized glutathione.
P<0.01: HF vs. controls, using ANCOVA adjusting for age and sex as covariates.
Table 4.
Total plasma fatty acid concentration in HF patients and controls.
| Fatty acids/Concentration | Control | HF |
|---|---|---|
| µM | µM | |
| TOTAL | 15,169±2951 | 15,030±5433 |
| (A)PUFA | 6202±1012 | 5298±1526⁎⁎⁎⁎ |
| n-6 | 5518±926 | 4826±1377⁎⁎⁎ |
| C18:2n-6 (linoleic acid) | 4226±804 | 3592±1192⁎⁎ |
| C18:3n-6 (gamma linolenic) | 70.6±28.1 | 69.9±36.3 |
| C20:3n-6 (dihomogamma acid) | 203±61 | 183±67 |
| C20:4n-6 (arachidonic acid) | 976.2±235.8 | 930.2±267.0 |
| n-3 | 664±238 | 446±194⁎⁎⁎⁎ |
| C18:3n-3 (α-linolenic acid) | 96.3±32.4 | 97.0±45.6 |
| C20:5n-3 (EPA) | 158±112 | 81.4±45.2⁎⁎⁎⁎ |
| C22:5n-3 (DPA) | 67.4±18.2 | 61.2±28.2 |
| C22:6n-3 (DHA) | 338±123 | 202±109⁎⁎⁎⁎ |
| (B)Mono-unsaturated fatty acids | 3808±1039 | 4513±2006⁎ |
| n-9 | 3169±821 | 3788±1587⁎⁎ |
| C18:1n-9 (oleic acid) | 2924±808 | 3556±1566⁎ |
| C18:1n-9T (elaidic acid) | 33.9±16.5 | 45.6±24.5⁎ |
| C20:3n-9 (eicosatrienoic acid) | 19.7±10.1 | 26.1±14.8⁎ |
| C22:1n-9 (erucic acid) | 51.7±10.0 | 53.6±17.1 |
| C24:1n-9 (nervonic acid) | 149±38 | 126±36⁎ |
| n-7 | 604±241 | 680±431 |
| C16:1n-7 (palmitoleic acid) | 361±185 | 399±336 |
| C18:1n-7 (vaccenic acid) | 243±63 | 281±108 |
| Trans fatty acids | 50.3±19.9 | 64.4±29.2⁎⁎ |
| Saturated fatty acids | 5214±1261 | 5291±2240 |
| C12:0 (lauric acid) | 19.8±10.3 | 26.0±21.5 |
| C14:0 (myristic acid) | 197±95 | 195±123 |
| C16:0 (palmitic acid) | 3809±973 | 3932±1867 |
| C18:0 (stearic acid) | 890±208 | 894±249 |
| C20:0 (arachidic acid) | 30.0±5.4 | 27.2±5.3⁎ |
| C22:0 (behenic acid) | 90.1±19.8 | 67.2±22.4⁎⁎⁎⁎ |
| C24:0 (lignoceric acid) | 67.7±21.9 | 37.9±15.8⁎⁎⁎⁎ |
Data are means±SD. PUFA, polyunsaturated fatty acid, EPA, eicosapentaenoic acid, DPA, Docosapentaenoic acid, DHA, docosahexanaenoic acid.
P<0.05, ⁎⁎P<0.01, ⁎⁎⁎P<0.001 and ⁎⁎⁎⁎P<0.0001: HF vs. controls, using ANCOVA adjusting for age and sex as covariates.
Clinical and biochemical characteristics of the study population
Our population of HF patients had a markedly depressed left ventricular ejection fraction with a mean of 26.3±7.1% and moderate to severe functional impairment, being mostly in New York Heart Association (NYHA) classes II (54%) and III (44%). These patients were mostly of ischemic etiology (66%) and the vast majority of them presented 2 or more comorbidities, such as type 2 diabetes (48%) and hypertension (49%). Finally, as per guidelines recommendation, HF patients received multiple medications compared to controls (7.4±2.0 vs. 0.8±1.2 medications/subject, P<0.0001), which included diuretics (97%), RAS inhibitors (85%), β-blockers (79%), lipid-lowering agents (75%), warfarin (48%) and salicylates (65%).
With respect to circulating parameters routinely assessed in the clinical laboratory, as expected, HF patients exhibited markedly increased plasma levels of NT-ProBNP, uric acid, urea and creatine; some degree of renal dysfunction was evidenced by a lower eGFR. While 16% of them had an elevated plasma troponin T level (≥0.03 μg/L), their plasma levels of inflammation markers namely TNF-α (50%), CRP (71%) and MPO (41%), were also significantly higher.
At the metabolic levels, HF patients displayed markedly lower levels of HDL-cholesterol (38%) and LDL-cholesterol (23%), but a greater [total cholesterol]/[HDL-cholesterol] ratio (21%), and higher levels of triglycerides (51%). They also showed significantly higher plasma levels of glucose (55%) and insulin (56%), as well as HOMA-IR scores (135%).
It is noteworthy that none of the reported parameters in Table 1, Table 2 were found to differ significantly between NYHA classes I–II vs. III of HF patients (data not shown) and only a few of them differed significantly between HF patients with or without type 2 diabetes (diastolic blood pressure: p<0.01, glucose p<0.0001 and HOMA-IR p<0.03).
Circulating levels of lipid peroxidation markers and their potential fatty acid precursors
HF patients depicted significantly elevated levels of blood GSH-to-GSSG ratio (55%; Table 3). This higher ratio was due to a significantly decreased blood level of GSSG (22%), while those of GSH and total glutathione were similar in both groups. Similarly, compared to controls, HF patients displayed similar circulating levels of MDA, a commonly assessed lipid peroxidation marker, as well 4HNE-P (Table 3).
With regards to the plasma level of total fatty acids, which reflect both free and bound fatty acids, either to albumin or esterified to cholesterol or as triglycerides and phospholipids in lipoproteins, this did not differ between groups. However, concurring with literature data [33], HF displayed a markedly altered fatty acid profile compared to controls (Table 4), including decreased level of potential 4HNE-P precursors the n-6 PUFA, specifically LA (by 15%) that represents ~75% of all n-6 PUFA, while that of arachidonic acid was unchanged. There were also changes in the plasma levels of other fatty acid classes, namely a decrease in the level of n-3 PUFAs, with an increased n-6/n-3 PUFA ratio (11.7±3.4 vs. 9.1±3.0, P<0.0001), while that of mono-unsaturated fatty acids (MUFA) was higher and saturated fatty acids remained unchanged. In the n-3 series, the levels of both EPA and DHA were lower by almost 50%. It is noteworthy that, despite their lower levels of n-6 and n-3 PUFAs, HF patients do not depict essential fatty acid deficiency as a result of undernutrition or fat malabsorption, since the ratio (20:3n-9/20:4n-6) and (C16:1n-7/18:2n-6) remained within the normal range [42].
Circulating levels of 4HNE-P, but not MDA, are associated to that of its potential PUFA precursors
Additional statistical analyses, both univariate correlation Pearson analysis as well as multivariate regression analysis (Table 5), which included both age and gender as variables, were conducted in the entire population and revealed a strong association between circulating 4HNE-P and its potential precursor LA. In fact, using multiple regression analysis, LA was found to be the only parameter that was positively associated with 4HNE-P in the entire population (β-coefficient 0.0139, P=0.0183).
Table 5.
Blood level of 4-hydroxynonenal-protein thioether adducts (4HNE-P) and its association with that of its potential precursors in the entire population of HF patients and control subjects.
|
HF+Control |
HF |
Control |
|
|---|---|---|---|
| Parameters | RCoefficient;P | R Coefficient;P | RCoefficient;P |
| Total n-6 PUFA | R=0.262;P=0.003 | R=0.396;P=0.002 | R=0,093; P=0.483 |
| Linoleic acid | R=0.252;P=0.004 | R=0.366;P=0.004 | R=0.106; P=0.378 |
| Arachidonic acid | R=0.119; P=0.176 | R=0.316;P=0.013 | R=−0.058; P=0.627 |
| HDL-cholesterol | R=0.121; P=0.166 | R=0.323;P=0.011 | R=−0.014; P=0.909 |
| LDL-cholesterol | R=0.087; P=0.329 | R=0.222; P=0.085 | R=−0.112; P=0.358 |
| Triglycerides | R=0.099; P=0.254 | R=0.128; P=0.324 | R=0.162; P=0.177 |
Results from the univariate analysis conducted in the two groups of subjects separately revealed, however, that only the HF group showed a significant association between 4HNE-P and LA (Table 5). The relationship between 4HNE-P and LA for the two groups is depicted in Fig. 1.
Fig. 1.
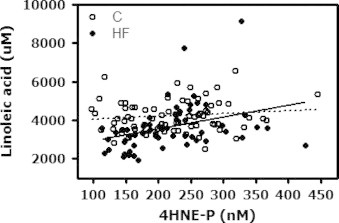
Relationship between circulating levels of 4-hydroxynonenal bound to circulating thiol proteins (4HNE-P) and its precursor the n-6 polyunsaturated fatty acid linoleic acid in control and heart failure (HF) subjects. The dotted lines show the linear regression lines with 95% confidence intervals for all subjects in the control (C) and HF group. Control: Linoleic acid=1.2×4HNE-P+3691, P=0.38 (NS), HF: Linoleic acid=6.5×4HNE-P+2207, P=0.004.
When multiple regression analyses were conducted in the HF group only, LA remained strongly associated with 4HNE-P (Table 6). The multiple regression analysis suggested, however, additional factors that could explain variations in blood levels of 4HNE-P in HF patients (R2=0.5692) and the strongest association was found for HDL-cholesterol (P<0.0002). Interestingly, 4HNE-P in HF patients was found to be also associated positively with disease-related parameters such as the NYHA class. The aforementioned associations appear specific to 4HNE-P, since MDA, which is another product of peroxidation of n-6 PUFA, was not found to be significantly associated with either LA or arachidonic acid in an univariate Pearson correlation analysis (data not shown) nor in multiple regression analysis conducted in the entire population neither in HF patients using MDA as the dependent variable (Table 6). In fact, MDA showed significant positive associations with plasma glucose only, as well as MPO and hypertension.
Table 6.
Parameters associated with blood 4HNE-P and MDA levels in HF patients (multiple regression analysis).
| Parameters | β Coefficient | P |
|---|---|---|
| 4HNE-P (R2=0.5692) | ||
| Lipid profile-potential precursors | ||
| Linoleic acid | 0.0184 | 0.0031 |
| HDL-Cholesterol | 102.4 | 0.0002 |
| Disease-related parameters | ||
| NYHA | 43.4 | 0.0015 |
| History of myocardial infarction | 35.5 | 0.0122 |
| Various factors | ||
| Body mass index | 4.11 | 0.0194 |
| Total bilirubin | −2,06 | 0.0268 |
| Ratio GSH/GSSG | −0.758 | 0.0462 |
| Medication | ||
| RAS inhibitors | 63.8 | 0.0034 |
| Acute nitrate | −31.7 | 0.0034 |
| Hypoglycemic agents | −29.1 | 0.0485 |
| MDA (R2=0.4074) | ||
| Disease-related parameters | ||
| History of myocardial infarction | −244 | 0.0228 |
| Risk factors | ||
| Hypertension | 233 | 0.0286 |
| Biochemical parameters | ||
| Glucose | 48.2 | 0.0202 |
| Alkaline phosphatase | 2.57 | 0.0379 |
| Myeloperoxidase | 4.36 | 0.0005 |
| Medication | ||
| Amiodarone | −299 | 0.0151 |
Multivariate analysis was performed in HF patients only using risk factors and parameters selected for their high clinical and biochemical relevance, which are listed in the Material and Methods. A P-value<0.05 was considered statistically significant.
Further to results of our multiple regression analysis, supplemental analyses were conducted in the entire population to explore the relationship between LA and the specific cholesterol fractions, and more specifically HDL-cholesterol. Using Pearson correlation tests, we found a strong positive association between LA and each of the cholesterol fraction (P<0.0001): total cholesterol (R=0.771, P<0.0001)>LDL-cholesterol (R=0.674, P<0.0001)>HDL-cholesterol (R=0.276, P=0.0013) (Fig. 2A). In addition, covariance analysis was conducted using age, gender and the plasma levels of the various cholesterol classes (total, LDL- and HDL-cholesterol) as covariates. After adjustment, the difference between HF patients and controls was no longer significantly different for LA (3895±97 vs. 3998±90 μM; p=0.40; Fig. 2B), suggesting that the difference in plasma level of LA between HF patients and controls is explained by that of cholesterol, total, HDL- and LDL-cholesterol. The relationship between 4HNE-P and HDL-cholesterol in HF patients and control subjects is depicted in Fig. 3: HNE-P levels are expressed either as absolute values (Fig. 3A), and as a molar ratio relative to HDL cholesterol, as an index of the content of 4HNE-P per unit of HDL-cholesterol molecule (Fig. 3B). Collectively, these results suggest that the HNE-P level expressed per unit of HDL-cholesterol increases as the plasma level of HDL-cholesterol decreases in the HF group.
Fig. 2.
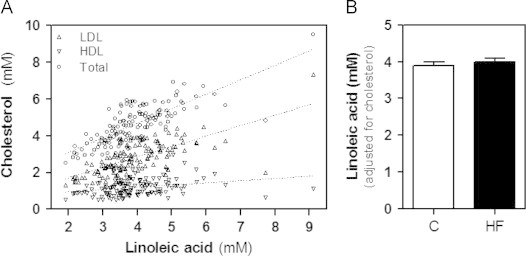
Relationship between circulating levels of linoleic acid and cholesterol fractions in the entire population. (A) Correlation between levels of linoleic acid (LA) and total cholesterol, LDL- and LDL-cholesterol in the entire population. The dotted lines show the regression lines: (i) total cholesterol: R2=0.595, P<0.0001, total cholesterol=0.79×LA+1.52; (ii) LDL-cholesterol: R2=0.455, P<0.0001, LDL-cholesterol=0.567×LA+0.583; and (iii) HDL: R2=0.076, P=0.001, HDL-cholesterol=0.120×LA+0.726. (B) Circulating levels of LA adjusted for age, gender and lipoproteins variables assessed by ANCOVA. Values are means±SD.
Fig. 3.
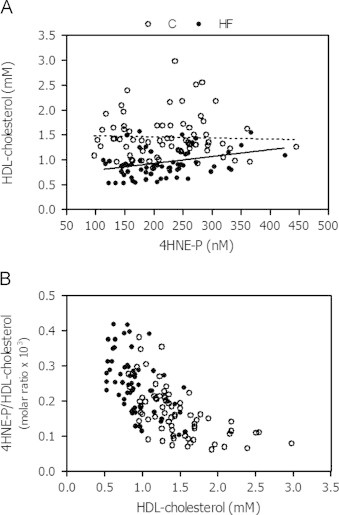
Relationship between 4HNE-P and HDL-cholesterol expressed in absolute values or relative to HDL-cholesterol in heart failure (HF) and control (C) subjects. The lines in Fig. 2A show the linear regression lines for each group: (i) HF: R2=0.086, P=0.016, regression equation: HDL-cholesterol=0.0014×4HNE-P+0.651, and (ii) controls: R2=0.0149, P=0.768, regression equation: HDL-cholesterol=−0.00022×4HNE-P+1.51.
Discussion
In support of our initial working hypothesis, this study documents a significant strong association between circulating levels of 4HNE-P and its potential precursors, particularly the n-6 PUFA LA and HDL-cholesterol. Given that the levels of these precursors are reduced significantly in HF patients compared to controls, this raises an important question about data interpretation of measurements of absolute values for plasma levels of 4HNE-P, and thereby emphasizes the importance of measuring changes in the levels of 4HNE-P with its potential precursors.
In fact, our results highlight significant positive associations between circulating levels of 4HNE-P and LA, a n-6 PUFA, using univariate correlation and multiple regression analyses in the entire population. However, multiple regression analysis conducted in the HF group only revealed a stronger association with HDL-cholesterol than LA. Concurring with the notion that lipoproteins are the predominant PUFA carrier in plasma, plasma levels of LA were strongly correlated with those of HDL-cholesterol, and were no longer significantly different between the control and HF groups, when adjusted for the levels of total cholesterol, HDL- and LDL-cholesterol. Hence, on this basis, supplemental analyses were focused on the relationship between 4HNE-P and HDL-cholesterol.
Interestingly, these supplementary analyses demonstrate that the level of 4HNE-P, expressed relative to that of HDL-cholesterol, increases as the level of HDL-cholesterol decreases in the HF population. This may appear surprising given that HDL-cholesterol has been shown to exert a protective role against cardiovascular disease through multiple mechanisms [43], [44]. However, a number of studies have shown that HDL, and not LDL, particles may well be the circulating lipoprotein that carries the greatest oxidized lipid load, and emphasized the consequences of oxidative damage to proteins comprised in HDL particles, which would make them dysfunctional and thereby contribute to disease outcome [45], [46], [47], [48], [49], [50]. Consistent with this notion, Woodward et al. recently reported a strong positive correlation between plasma levels of the lipid peroxidation product F2-isoprostanes and HDL-cholesterol in a cohort of coronary patients and control subjects, although levels of F2-isoprostanes were not different between groups [51]. Our finding of a positive association between HDL-cholesterol and circulating levels of total protein-bound 4HNE in HF patients in this study raise the possibility that this specific lipid peroxidation product may contribute to protein modification in HDL particles.
Beyond HDL-cholesterol, our multiple regression analysis revealed that circulating levels of 4HNE-P were also negatively associated with the GSH-to-GSSG ratio [52], [53], which was elevated in HF patients suggesting an adaptive up-regulation of enzymes involved in glutathione synthesis and recycling in response to the increased oxidative stress in these patients. In support of this notion, abnormalities in GSH cycling were previously reported to be linked with increased lipid peroxidation in HF patients [54]. It remains, however, unclear whether circulating sulfhydryl groups represent the first line of defense against reactive species that can initiate lipid peroxidation in blood of HF patients, which display high levels of MPO and leukocytes. Indeed, on the one hand, the relative abundance of circulating 4HNE-P levels has been shown to depend on their removal by phase II metabolic pathways such as gluthathione-S-transferases [21]. On the other hand, Frei et al. [55] showed that the formation of hydroperoxides from plasma cholesterol and phospholipid esters in the presence of activated leukocytes occurs following depletion of plasma ascorbate (known to scavenge the MPO oxidant hypochlorous acid) even though sulfhydryl groups are still present at high concentrations.
The participation of additional antioxidative mechanisms such as heme oygenase (HO) or paraoxonase in HDL-cholesterol particles [56], [57], cannot be excluded. While we did not directly assess these mechanisms, indirect evidence supports a role for HO. Indeed, our multiple regression analysis in HF patients only revealed also a negative association between circulating levels of 4HNE-P and those of bilirubin. Interestingly, plasma bilirubin levels of HF patients were not significantly different from controls. Bilirubin, which is the end product of heme catabolism, has been shown to depict chain-breaking antioxidant properties [58] and to inhibit peroxidation of lipids in lipoproteins [59]. HO catalyzes heme oxidation and the induction of cardiac isoform (HO-1) under pathophysiological stresses has also been linked to inhibition of lipid peroxidation [60], [61]. One potential source of non-protein-bound heme – considered a particularly deleterious species – is oxidation of hemoglobin released following erythrocyte lysis, which can be induced by lipid peroxidation end-products such as 4HNE [62] and constitutes a well known mechanism that induces HO-1 [63]. Interestingly, in this regard, is our finding of significantly lower hemoglobin levels in our HF patient cohort vs. controls.
Finally other factors identified by the multiple regression analyses include the NYHA class. Two small studies reported a link between NYHA classes and oxidative stress markers [27], [64]. However, it should be noted that the characteristics of our HF patient population, which is typical for a HF clinic, did not provide adequate discrimination of the role of some clinically-relevant parameters in the multiple regression analysis. Additional studies are needed to substantiate this association using a larger HF and control cohort, which would be randomized for age and gender.
Interestingly, in contrast to 4HNE-P, plasma levels of MDA did not show any associations with LA or HDL-cholesterol or any other lipid parameters. In our study, MDA levels were not significantly elevated in HF patients, a finding that concurs with that of Tingberg et al. [29], but contrasts with other studies [13], [27], [65], [66]. It is noteworthy that while both 4HNE and MDA are lipid peroxidation by-products, MDA can also be generated by cyclooxygenases in thromboxane metabolism [67] and from free radical attack on sialic acid and deoxyribose [68]. Thus, these two biomarkers may reflect different pathogenic events linked to HF. This possibility is supported by the results of supplemental statistical analyses conducted in HF patients, which revealed that 4HNE-P and MDA were associated with different disease-related parameters and metabolites. Another factor that should also be considered is that we assessed free MDA levels in plasma, while 4HNE-P reflects 4HNE bound to all circulating proteins, which may be albumin or within lipoproteins, as it is suggested from by our correlation an multiple regression analyses. Nevertheless, the relationship depicted between 4HNE-P and its lipid precursors appears to be specific to 4HNE-P, and not MDA. Further studies are needed, however, to identify unambiguously circulating protein(s) that bind 4HNE, as well as factors that could explain the reduction in the circulating levels of lipoproteins as well as essential PUFAs, which include the n-6 series, but also the n-3 series. In this regard, other mass spectrometric methods combining enrichment strategies with targeted analysis such as neutral loss scanning should be used to enable detection and identification of 4HNE-modified proteins in complex biological matrices, including HDL particles [18].
In conclusion, results from this study document a strong association between the lipid peroxidation product 4HNE-P, but not MDA, and its potential precursors the n-6 PUFA LA and HDL-cholesterol, which is a major plasma PUFA carrier, in HF patients. This relationship suggests that in relative term, the level of 4HNE-P increases markedly as the level of HDL cholesterol decreases in these patients. Results from this study emphasize the importance of measuring the levels of these PUFA when assessing their lipid peroxidation by-products such as 4HNE-P. Based on our findings, it appears also warranted to test the possibility that 4HNE-P contributes to oxidative stress-induced HDL protein modification and dysfunction in HF patients.
Acknowledgments
This work was presented at the 5th Canadian Consortium of Oxidative Stress, May 3–6 2007, Montreal, Canada. This study was supported by the Canadian Institutes of Health Research (CIHR Grant 10920 to C.D.R.) and by the Montreal Heart Institute Foundation. Dr. Ducharme was supported by the Fonds de Recherche en Santé du Québec (FRSQ). Dr. Tardif holds the Canadian Institutes of Health Research and Pfizer chair in atherosclerosis. The authors gratefully acknowledge the technical and secretarial assistance of Karine Tétreault, Jeanne Millette, Luce Cayouyette, Marie Cournoyer and Antoinette Paolitto.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Wolfram R., Oguogho A., Palumbo B., Sinzinger H. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF(2alpha) Eur. J. Heart Fail. 2005;7:167–172. doi: 10.1016/j.ejheart.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Polidori M.C., Pratico D., Savino K., Rokach J., Stahl W., Mecocci P. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J. Card. Fail. 2004;10:334–338. doi: 10.1016/j.cardfail.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 3.McMurray J., Chopra M., Abdullah I., Smith W.E., Dargie H.J. Evidence of oxidative stress in chronic heart failure in humans. Eur. Heart J. 1993;14:1493–1498. doi: 10.1093/eurheartj/14.11.1493. [DOI] [PubMed] [Google Scholar]
- 4.Mak S., Newton G.E. The oxidative stress hypothesis of congestive heart failure: radical thoughts. Chest. 2001;120:2035–2046. doi: 10.1378/chest.120.6.2035. [DOI] [PubMed] [Google Scholar]
- 5.Boaz M., Smetana S., Weinstein T., Matas Z., Gafter U., Iaina A. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 6.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 7.Hopper L., Ness A., Higgins J.P., Moore T., Ebrahim S. GISSI-Prevenzione trial. Lancet. 1999;354:1557. doi: 10.1016/s0140-6736(05)76587-9. [DOI] [PubMed] [Google Scholar]
- 8.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J.M. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 9.Stephens N.G., Parsons A., Schofield P.M., Kelly F., Cheeseman K., Mitchinson M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 10.Kukin M.L., Kalman J., Charney R.H., Levy D.K., Buchholz-Varley C., Ocampo O.N. Prospective, randomized comparison of effect of long-term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction, and oxidative stress in heart failure. Circulation. 1999;99:2645–2651. doi: 10.1161/01.cir.99.20.2645. [DOI] [PubMed] [Google Scholar]
- 11.Serdar A., Yesilbursa D., Serdar Z., Dirican M., Turel B., Cordan J. Relation of functional capacity with the oxidative stress and antioxidants in chronic heart failure. Congest. Heart Fail. 2001;7:309–311. doi: 10.1111/j.1527-5299.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 12.Belardinelli R., Solenghi M., Volpe L., Purcaro A. Trimetazidine improves endothelial dysfunction in chronic heart failure: an antioxidant effect. Eur Heart J. 2007;28:1102–1108. doi: 10.1093/eurheartj/ehm071. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Velez C.R., Garcia-Castineiras S., Mendoza-Ramos E., Hernandez-Lopez E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J. 1996;131:146–152. doi: 10.1016/s0002-8703(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 14.Galano J.M., Mas E., Barden A., Mori T.A., Signorini C., De F.C. Isoprostanes and neuroprostanes: total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostaglandins Other Lipid Mediat. 2013 doi: 10.1016/j.prostaglandins.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Miller E., Mrowicka M., Saluk-Juszczak J., Ireneusz M. The level of isoprostanes as a non-invasive marker for in vivo lipid peroxidation in secondary progressive multiple sclerosis. Neurochem. Res. 2011;36:1012–1016. doi: 10.1007/s11064-011-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies S.S., Roberts L.J. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veglia F., Cighetti G., De F.M., Zingaro L., Boccotti L., Tremoli E. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers. 2006;11:562–573. doi: 10.1080/13547500600898623. [DOI] [PubMed] [Google Scholar]
- 18.Spickett C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 20.Poli G., Schaur R.J. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 21.Chapple S.J., Cheng X., Mann G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1:319–331. doi: 10.1016/j.redox.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K., Kusano K., Nakamura Y., Kakishita M., Ohta K., Nagase S. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105:2867–2871. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K., Kusano K.F., Matsubara H., Nakamura Y., Miura A., Nishii N. Relationship between oxidative stress and systolic dysfunction in patients with hypertrophic cardiomyopathy. J. Card. Fail. 2005;11:117–123. doi: 10.1016/j.cardfail.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Asselin C., Bouchard B., Tardif J.C., Des Rosiers C. Circulating 4-hydroxynonenal-protein thioether adducts assessed by gas chromatography-mass spectrometry are increased with disease progression and aging in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006;41:97–105. doi: 10.1016/j.freeradbiomed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Asselin C., Shi Y., Clement R., Tardif J.C., Des Rosiers C. Higher circulating 4-hydroxynonenal-protein thioether adducts correlate with more severe diastolic dysfunction in spontaneously hypertensive rats. Redox Rep. 2007;12:68–72. doi: 10.1179/135100007X162202. [DOI] [PubMed] [Google Scholar]
- 26.Lesgards J.F., Frayne I.R., Comte B., Busseuil D., Rheaume E., Tardif J.C. Differential distribution of 4-hydroxynonenal adducts to sulfur and nitrogen residues in blood proteins as revealed using Raney nickel and gas chromatography-mass spectrometry. Free Radic. Biol. Med. 2009;47:1375–1385. doi: 10.1016/j.freeradbiomed.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Keith M., Geranmayegan A., Sole M.J., Kurian R., Robinson A., Omran A.S. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 28.Kono Y., Nakamura K., Kimura H., Nishii N., Watanabe A., Banba K. Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure. Circ. J. 2006;70:1001–1005. doi: 10.1253/circj.70.1001. [DOI] [PubMed] [Google Scholar]
- 29.Tingberg E., Ohlin A.K., Gottsater A., Ohlin H. Lipid peroxidation is not increased in heart failure patients on modern pharmacological therapy. Int. J. Cardiol. 2006;112:275–281. doi: 10.1016/j.ijcard.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Arumanayagam M., Chan S., Tong S., Sanderson J.E. Antioxidant properties of carvedilol and metoprolol in heart failure: a double-blind randomized controlled trial. J. Cardiovasc. Pharmacol. 2001;37:48–54. doi: 10.1097/00005344-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Davignon J., Jacob R.F., Mason R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004;15:251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 32.Shishehbor M.H., Aviles R.J., Brennan M.L., Fu X., Goormastic M., Pearce G.L. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 33.Rupp H., Rupp T.P., Alter P., Maisch B. Mechanisms involved in the differential reduction of omega-3 and omega-6 highly unsaturated fatty acids by structural heart disease resulting in “HUFA deficiency”. Can. J. Physiol. Pharmacol. 2012;90:55–73. doi: 10.1139/y11-101. [DOI] [PubMed] [Google Scholar]
- 34.Harris J.I., Hibbeln J.R., Mackey R.H., Muldoon M.F. Statin treatment alters serum n-3 and n-6 fatty acids in hypercholesterolemic patients. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:263–269. doi: 10.1016/j.plefa.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B., Lee C.Y. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid. Redox Signal. 2010;13:145–156. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- 36.Ducharme A., Doyon O., White M., Rouleau J.L., Brophy J.M. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ. 2005;173:40–45. doi: 10.1503/cmaj.1041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serru V., Baudin B., Ziegler F., David J.P., Cals M.J., Vaubourdolle M. Quantification of reduced and oxidized glutathione in whole blood samples by capillary electrophoresis. Clin. Chem. 2001;47:1321–1324. [PubMed] [Google Scholar]
- 38.Lepage G., Roy C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 39.Trudel K., Sinnett D., James R.W., Delvin E., Amre D., Seidman E. Iron-ascorbic acid-induced oxidant stress and its quenching by paraoxonase 1 in HDL and the liver: comparison between humans and rats. J. Cell Biochem. 2005;96:404–411. doi: 10.1002/jcb.20542. [DOI] [PubMed] [Google Scholar]
- 40.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 41.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 42.Das U.N. A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:251–268. doi: 10.1016/j.plefa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Assmann G., Gotto A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 44.Kontush A., Chantepie S., Chapman M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 45.Newman J.W., Kaysen G.A., Hammock B.D., Shearer G.C. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J. Lipid Res. 2007;48:1792–1800. doi: 10.1194/jlr.M700146-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Pruzanski W., Stefanski E., de Beer F.C., de Beer M.C., Ravandi A., Kuksis A. Comparative analysis of lipid composition of normal and acute-phase high density lipoproteins. J. Lipid Res. 2000;41:1035–1047. [PubMed] [Google Scholar]
- 47.Heinecke J.W. The HDL proteome: a marker – and perhaps mediator – of coronary artery disease. J. Lipid Res. 2009;50(Suppl):S167–S171. doi: 10.1194/jlr.R800097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navab M., Shechter I., Anantharamaiah G.M., Reddy S.T., Van Lenten B.J., Fogelman A.M. Structure and Function of HDL Mimetics. Arterioscler. Thromb. Vasc. Biol. 2009 doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowry V.W., Stanley K.K., Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsunaga T., Koyama I., Hokari S., Komoda T. Detection of oxidized high-density lipoprotein. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;781:331–343. doi: 10.1016/s1570-0232(02)00556-1. [DOI] [PubMed] [Google Scholar]
- 51.Woodward M., Croft K.D., Mori T.A., Headlam H., Wang X.S., Suarna C. Association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clin. Sci. (Lond.) 2009;116:53–60. doi: 10.1042/CS20070404. [DOI] [PubMed] [Google Scholar]
- 52.Maclean P.D., Drake E.C., Ross L., Barclay C. Bilirubin as an antioxidant in micelles and lipid bilayers: its contribution to the total antioxidant capacity of human blood plasma. Free Radic. Biol. Med. 2007;43:600–609. doi: 10.1016/j.freeradbiomed.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Stocker R., McDonagh A.F., Glazer A.N., Ames B.N. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- 54.Campolo J., Caruso R., De M.R., Parolini M., Oliva F., Roubina E. Aminothiol redox alterations in patients with chronic heart failure of ischaemic or non-ischaemic origin. J. Cardiovasc. Med. (Hagerstown) 2007;8:1024–1028. doi: 10.2459/JCM.0b013e3281053a63. [DOI] [PubMed] [Google Scholar]
- 55.Frei B., Stocker R., Ames B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stocker R., Perrella M.A. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.B., Hama S., Hough G., Navab M., Fogelman A.M., Maclellan W.R. Heart failure is associated with impaired anti-inflammatory and antioxidant properties of high-density lipoproteins. Am. J. Cardiol. 2013 doi: 10.1016/j.amjcard.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 58.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 59.Neuzil J., Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J. Biol. Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 60.Ishikawa K., Navab M., Vasculitis Lusis A.J. Atherosclerosis, and altered HDL composition in heme-oxygenase-1-knockout mice. Int. J. Hypertens. 2012;2012:948203. doi: 10.1155/2012/948203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collino M., Pini A., Mugelli N., Mastroianni R., Bani D., Fantozzi R. Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis. Model Mech. 2013;6:1012–1020. doi: 10.1242/dmm.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagy E., Eaton J.W., Jeney V., Soares M.P., Varga Z., Galajda Z. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2010;30:1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belcher J.D., Beckman J.D., Balla G., Balla J., Vercellotti G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 65.White M., Ducharme A., Ibrahim R., Whittom L., Lavoie J., Guertin M.C. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin. Sci. (Lond.) 2006;110:483–489. doi: 10.1042/CS20050317. [DOI] [PubMed] [Google Scholar]
- 66.Belch J.J., Bridges A.B., Scott N., Chopra M. Oxygen free radicals and congestive heart failure. Br. Heart J. 1991;65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadiiska M.B., Gladen B.C., Baird D.D., Graham L.B., Parker C.E., Ames B.N. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic. Biol. Med. 2005;38:711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 68.Halliwell B., Gutteridge J.M.C. Oxford University Press; New York: 1999. Free Radical in Biology and Medicine. [Google Scholar]


