Abstract
Background
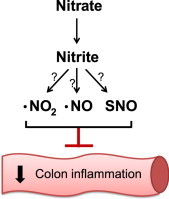
Inorganic nitrate and nitrite have emerged as alternative substrates for nitric oxide (NO) generation in the gastrointestinal tract, and have shown to be protective against drug-induced gastric injury. The aim of this study was to investigate the preventive and therapeutic effects of nitrate and nitrite in a model of experimental colitis.
Methods
Colitis was induced in mice by administrating dextran sulfate sodium (DSS) with concurrent administration of nitrite (1 mM) or nitrate (10 mM) in the drinking water for 7 days. A therapeutic approach was also investigated by initiating nitrite treatment 3 days after DSS-induced colitis. Clinical and inflammatory markers were assessed and the colonic mucus thickness was measured in vivo. The effect of nitrite on wound healing was evaluated using colon epithelial cells.
Results
Concurrent administration of DSS and nitrite (1 mM) alleviated inflammation as determined by reduced disease activity index score (DAI) and increased colon length, while nitrate (10 mM) only reduced the DAI-score. Nitrite also displayed therapeutic effects by ameliorating established colonic inflammation with reduced colonic expression of iNOS and improving histopathology. DSS-induced decrease in colonic mucus thickness was completely prevented by nitrite administration. In addition, goblet cell abundance was lower by DSS treatment, but was increased by addition of nitrite. Further studies using colon epithelial cells revealed an NO-dependent improvement in wound healing with nitrite administration.
Conclusion
Nitrite exerts both preventive and therapeutic effects in colonic inflammation. The protective effects involve preservation of an intact adherent mucus layer and regulation of epithelial cell restitution.
Keywords: Nitrite, Nitrate, DSS, Inflammatory bowel disease, Mucus
Graphical abstract
Highlights
-
•
Inorganic nitrate and nitrite alleviate DSS-induced colitis.
-
•
Dietary nitrite has therapeutic effects in already established colonic inflammation.
-
•
DSS-induced thinning of the colonic mucus layer is prevented by dietary nitrite.
-
•
Nitrite promotes healing of colon epithelial cells.
Introduction
Ulcerative colitis (UC) and Crohn's disease are collectively included in the chronic intestinal disorders of inflammatory bowel diseases (IBDs). The etiology of these diseases remains unknown but the cause is probably multifaceted involving an inappropriate immune response, change in gut microbiota and individual genetic predisposition [1], [2]. A key feature of IBD is the disruption of the protective mucosal barrier, with epithelial damage and a destroyed mucus layer. These changes might result in bacterial penetration of the mucosal barrier, resulting in uncontrolled mucosal inflammation [3], [4], [5].
The most widely used experimental model of IBD is administration of dextran sulfate sodium (DSS) in the drinking water. Colitis is typically induced in mice by administrating 3–5% (DSS) for 5–7 days, and this model exhibits pathophysiological features that resemble human UC [6], [7]. The mechanism of DSS-induced colonic mucosal inflammation is not completely understood. However, recent results indicate that the DSS molecule destabilizes the structure and barrier function of the mucus layer [8] and also exerts a direct toxic effect on the epithelial cells with subsequent infiltration of immune cells [6], [9].
To protect the colonic mucosa from bacterial infiltration, the epithelium is covered by secreted mucus in a two-layer system. The outer loosely adherent layer can be removed by suction while the inner firmly adherent layer is strongly attached to the epithelial cells [10]. In a healthy situation, the outer loosely adherent mucus layer is colonized with commensal bacteria while the inner firmly adherent mucus layer contain substantially fewer bacteria, forming the functional barrier between bacteria and the epithelium [11], [12]. Muc2, the major gel-forming and protective mucin in the mucus layers, is a large glycoprotein secreted by the colonic goblet cells [12], [13], [14]. The loosely adherent mucus layer is formed by regulated protease degradation of the Muc2 mucus gel forming the inner mucus layer, which is maintained at a relatively constant thickness [12]. Secretion of Muc2 mucins is crucial for the protection of the colonic mucosa, as shown in Muc2−/− mice, which lack the protective mucus layer. These mice develop spontaneous colitis and colon cancer with increasing age, and are also more susceptible to pathogens and DSS-induced colitis [8], [14], [15], [16]. Moreover, patients with UC and animal models of experimental colitis demonstrate a thinner mucus layer with increased permeability, allowing bacteria to get in direct contact with the colonic mucosa [4], [8]. Thus, it is suggested that an intact inner adherent mucus layer is an important factor to prevent activation of inflammatory reactions by bacteria [14].
Endogenous production of nitric oxide (NO) by the nitric oxide synthases (NOSs) is essential for physiological regulation of gastrointestinal function [17], and NO also plays a role during inflammatory conditions such as IBD and in DSS-induced colitis [18], [19]. In addition to the NOSs, an alternative pathway for NO generation in mammals has been described [20], [21], involving sequential reduction of dietary-derived nitrate and nitrite [17]. Vegetables, in particular green leafy plants, contain high amounts of nitrate which is efficiently absorbed in the gastrointestinal tract [22]. About 25% of the systemic circulating nitrate is accumulated in the salivary glands [23], [24]. Salivary nitrate is then converted into nitrite by efficient oral commensal bacteria [25], [26], resulting in high concentrations of salivary nitrite [27]. When swallowed into the acidic environment of the stomach, a part of the salivary-derived nitrite is protonated and converted to nitric oxide (NO) and other nitrogen oxides including S-nitrosothiols [20]. In addition, a significant share of the nitrite escapes gastric passage and enters the systemic circulation and tissues where it can be further metabolized to NO and other bioactive nitrogen oxides [27], [28]. A part of the dietary nitrate and nitrite may also proceed into the small intestine and cecum where it can be absorbed or further utilized by bacteria to produce NO [29]. It is believed that nitrate and nitrite are absorbed or consumed before reaching the colon [29].
Dietary nitrate and nitrite have been shown to increase gastric mucus formation and blood flow and to protect the mucosa against NSAID-induced injury [25], [30], [31], [32]. Recently it was also demonstrated that the mucus thickness in colon was decreased after DSS-induced colitis in mice [8] and that high pharmacological doses of nitrite can attenuate experimental colitis [33]. In this study, we investigated potential preventive and therapeutic effects of nitrate and nitrite in experimental colitis and the potential role of nitrite in regulation of colonic mucosal integrity. Furthermore, the protective effect of nitrite in colon epithelial cell wound healing was explored.
Materials and methods
Animals
Female BALB/c mice (Scanbur AB, Sollentuna, Sweden), 7–8 week old and weighing 20–22 g, were housed under temperature- and humidity-controlled conditions with 12-h-light/-dark cycles and with at least 7-d of local acclimatization before starting experiments. Mice were fed a standard pellet diet (R36, Lantmännen, Kimstad, Sweden) and tap water ad libitum. All experimental work on mice was approved by the Ethics Committee for Animal Experiments at the Karolinska Institute and at Uppsala University.
Experimental procedures
Experimental colitis was induced in mice by administrating 2% or 2.5% DSS (45 kDa, TdB Consultancy, Uppsala, Sweden) in the drinking water for 7 d. Sodium nitrite (NaNO2−, 1 mM) (Sigma, St Louis, MO, USA) or sodium nitrate (NaNO3−, 10 mM) (Sigma, St Louis, MO, USA) were also administered in the drinking water. The doses of nitrate and nitrite were chosen to represent a dose readily achievable via the diet (in particular from green leafy vegetables), and compensating for the different pharmacokinetics between mice and humans [31], [34]. In particular, the accumulation of nitrate and nitrite in saliva is considerably greater in humans [35], gradients which are not observed in rodents. Mice were randomly divided into 4 groups: control, DSS, DSS+nitrite and DSS+nitrate. In a first set of experiments, nitrate or nitrite supplementation was initiated simultaneously with 2% DSS administration (Fig. 1A). In the other experimental setup, nitrite supplementation was started 3 d after initiating administration of 2.5% DSS (Fig. 1B), this to slightly increase the severity of the colitis. Control mice received tap water only. The mice were weighed daily and visually inspected for diarrhea and rectal bleeding. At day 7, the Disease Activity Index (DAI) was evaluated as previously described [36]. The mice were anesthetized with isoflurane (Forene®, Abbott Scandinavia AB, Solna, Sweden) and blood was collected by heart puncture, followed by cervical dislocation. The colon was removed and its length was measured. Tissues and plasma samples were frozen and kept at −80 °C until further analysis.
Fig. 1.
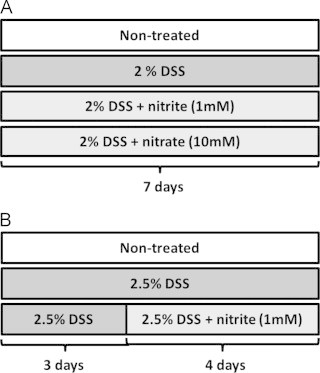
Experimental protocol for DSS-induced colitis in mice. Experimental colitis was induced in mice by administrating 2–2.5% DSS in drinking water ad libtum for 7 d. Sodium nitrite (1 mM) or sodium nitrate (10 mM) was administered orally in drinking water in combination with DSS. (A) In the preventive experimental setup, nitrate or nitrite was supplemented simultaneously with DSS, and (B) in the therapeutic approach, nitrite was added 3 d after DSS administration.
Animal preparation for colonic mucus thickness measurements
Mice pretreated with 2.5% DSS and nitrite (Fig. 1B) were anesthetized by inhalation of ~2.4% isoflurane (Forene®, Abbott Scandinavia AB, Solna, Sweden) in a mixture of air and oxygen (total oxygen 40%) through a breathing mask. Body temperature was maintained by a heating pad underneath the animal during the experimental procedure. The colonic preparation for investigation of the mucus thickness by intravital microscopy has been described extensively elsewhere [8], [37]. Prior to thickness measurements of the firmly adherent mucus, the luminal loosely adherent mucus layer was removed by gentle suction with a thin polyethylene cannula connected to a syringe. This procedure was conducted under supervision through a stereomicroscope to avoid contact with the epithelium. Five different spots in the adherent mucus were chosen and measured after the first suction. The mucosa preparation was allowed to recover during 1 h before a second removal of the loosely adherent mucus by suction, followed by repeated measurements of the same five spots in the adherent mucus layer. Inconsistent values from the same spot of the two separate measurements were excluded to only attain results of a physiological functional mucosa. Mean values from the different spots at the two independent measurements were used as one observation.
Immunohistochemistry
Immunohistochemical staining of frozen colon sections was performed as previously described [38] to asses levels of inducible NO synthase (iNOS), NFκB p65 (Santa Cruz Biotechnology, CA, USA) and mucin 2 (Muc2) (Abcam, Cambridge, UK). Secondary antibodies were biotinylated anti-mouse and anti-rabbit IgG (Jackson Immunoresearch Laboratories, PA, USA). The avidin biotinylated enzyme complex (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA) and 3,3-diaminobenzidine were used to detect the antibody. Quantification of stained sections was done by an investigator blinded to the protocol.
Histological assessment
The colon was removed from mice and fixed in 10% paraformaldehyde by submersion. After 24 h the tissue samples were transferred to 70% ethanol before routine processing and paraffin embedding. Sections were cut at 4 µm thickness on a rotary microtome. Consecutive sections were stained according to standard protocols [39] with hematoxylin and eosin (H&E), and with alcian blue–periodic acid-Schiff (PAS) reagent to visualize neutral and acidic colonic mucin. Histological analysis was performed by an investigator blinded to the protocol. The severity of the disease was graded in the H&E-stained sections on a scale from 0 to 3 by the sum of the scores of epithelial cell damage, crypt destruction and infiltration of inflammatory cells, as previously described [7]. The abundance of goblet cells (mucin) was separately evaluated in the alcian blue–PAS-stained sections, according to previously described protocol [40]. Colon epithelial cell wound repair assay: The human colonocyte cell line SW480 was maintained in DMEM supplemented with 1% penicillin/streptomycin and 10% FBS in a humidified incubator at 37 °C and 0.5% CO2. Cells were grown to confluence in six-well plates, and a straight incision was made on the monolayer using a sterile pipette tip. Nitrite (Sigma, St Louis, MO, USA) (1 µM–1 mM) and 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) (Cayman, Ann Arbor, MI, USA) (100 µM) treatment was initiated after wound incision. The administration of the inhibitor was performed 1 h before the treatment with nitrite. Wound closure was monitored by microscopy (Axiovert 40 CFL, Zeiss), and the distance of cell healing was measured at 24 h compared to the initial wound area, quantified using NIH ImageJ software. Each experiment was repeated three times and treatments were performed in duplicates, each well was measured at 3 independent positions which were calculated to a mean of distance.
Statistical analysis
Data are presented as mean±SEM. Statistical analysis was performed using 1-way ANOVA with Tukey's post hoc test. Differences of P<0.05 were considered significant using GraphPad Prism 5.02 (Graph Pad Software, San Diego, CA, USA).
Results
Nitrate and nitrite supplementation attenuates DSS-induced colitis in mice
Shortening of the colon is a distinctive hallmark of colon inflammation in rodents [41]. Administration of 2% DSS for 7 days caused a 30% reduction of colon length compared with the control group (Fig. 2A), and concurrent supplementation with nitrite reduced the colon shortening. Furthermore, DSS administration significantly increased the DAI-score while nitrite supplementation mediated a 70% lower score compared with the DSS-group (Fig. 2B). Nitrate supplementation did not affect colon length after DSS administration, however, the DAI-score was reduced (Supplemental Fig. S1A and B). Further anti-inflammatory effects of nitrate were demonstrated by lower colonic expression of iNOS and the NF-κB subunit p65 (Supplemental Fig. S1C–F). No differences in water consumption between the groups were observed (Supplemental Fig. S2A and B).
Fig. 2.
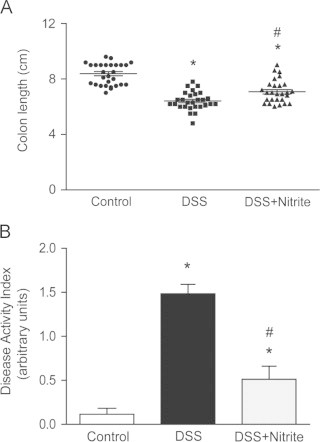
Preventive effects of nitrite with concurrent DSS administration. (A) Colon length and (B) disease activity index (DAI) in untreated mice (n=29) and those treated with 2% DSS (n=28) or mice with DSS+nitrite supplementation (1 mM) (n=27), measured at 7 d after treatment (P<0.05, ⁎ vs. control, # vs. DSS-group).
Therapeutic effects of nitrite supplementation
To explore whether nitrite has a therapeutic effect on an already established inflammation, mice were treated with 2.5% DSS during 3 d before nitrite administration (Fig. 1B). The time point for onset of nitrite supplementation was chosen based on initial observation of sickness onset (data not shown). Administration of 2.5% DSS for 7 days mediated a reduction in colon length and increased DAI-score (Fig. 3A and B). Nitrite supplementation started once colitis was initiated (day3), demonstrated a protective effect with a reduced colon shortening compared to the DSS-group at day 7. Further, the DAI-score was reduced with 60% compared with the DSS-treated mice. Investigation of the anti-inflammatory effects demonstrated an increased colonic expression of iNOS after DSS treatment, which was completely prevented by nitrite (Fig. 4A and B). Microscopic examination of H&E stained colonic sections revealed significant histopathological injuries (including colon crypt loss, immune cell influx, and ulceration of the epithelial surface) in DSS-treated mice compared to controls (Fig. 4C and D). Nitrite supplementation initiated when the colonic inflammation was already established strongly reduced the clinical symptoms as well as markers of colonic inflammation. No differences in water consumption between the groups were observed (Supplemental Fig. S2C).
Fig. 3.
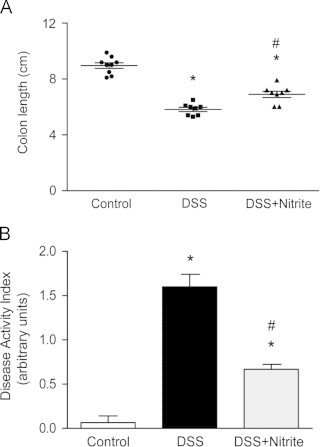
Therapeutic effects of delayed nitrite supplementation after DSS administration. (A) Colon length and (B) disease activity index (DAI) in untreated mice and those treated with 2.5% DSS or with DSS+nitrite supplementation (1 mM), measured at 7 d after treatment (P<0.01, ⁎ vs. control, # vs. DSS-group).
Fig. 4.
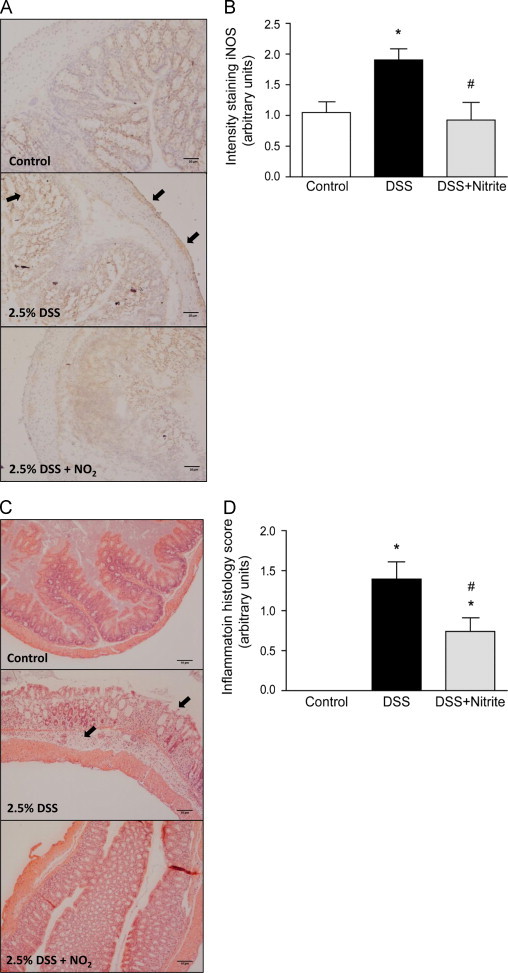
Nitrite attenuates DSS-induced colonic inflammation. (A) Representative pictures of colonic iNOS expression by immunohistochemical staining, and (B) quantification of iNOS staining (a.u.) (n=10 in each individual group). (C) Representative histology pictures with hematoxylin and eosin staining (scale bar 10 µm) and (D) summary of the colonic histology score (n=8 in each individual group) (P<0.05, ⁎ vs. control, # vs. DSS-group).
Nitrite supplementation increases the adherent colonic mucus layer after DSS treatment
Since the colonic mucus layer has an important barrier function protecting from invading bacteria [4], [12] and is reduced with DSS administration [8], we next investigated the effect of nitrite on the adherent colonic mucus layer with DSS-induced colitis. The firmly adherent mucus layer was significantly thinner after treatment with 2.5% DSS for 7 days compared with control mice (38±1 and 42±1 µm, respectively, Fig. 5A). Nitrite supplementation starting day 3 after induction of colitis prevented the decrease in mucus thickness, and maintained the thickness at the same levels as control animals (43±1 µm). In addition, the goblet cells in the colonic mucosa were quantified. The abundance of goblet cells was decreased in DSS-treated mice compared with control group (Fig. 5B and C), while nitrite supplementation had a protective effect on goblet cells. In contrast, an increased Muc2-expression in colon tissue was observed with DSS-treatment (Fig. 5D), while nitrite supplementation indicated a trend towards lower Muc2-expression in the disease group.
Fig. 5.
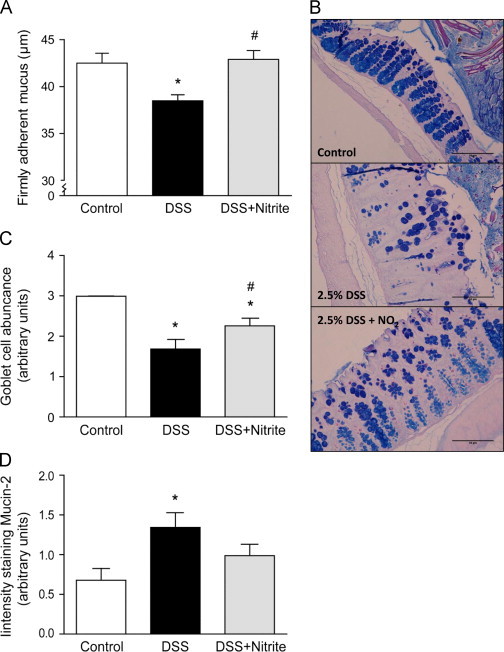
Analysis of colonic mucus thickness and goblet cell abundance with nitrite supplementation. (A) Firmly adherent mucus thickness (μm) in untreated mice (n=6), DSS-treated mice (n=5) and mice with DSS+nitrite supplementation (n=5). (B) Representative pictures of histological colon samples stained with Periodic Acid Schiff/Alcian Blue (PAS/AB) (scale bar 50 µm) and (C) quantification of the number of goblet cells in colon samples of untreated mice, DSS-treated mice and mice with DSS+nitrite supplementation (n=8 in each individual group). (D) Quantification (a.u.) of colonic Muc2 expression by immunohistochemistry staining (n=10 in each individual group) (P<0.05, ⁎ vs. control, # vs. DSS-group).
Nitrite promotes colon epithelial cell wound healing
One proposed mechanism of DSS-induced colonic injury is direct epithelial cell injury with increased apoptosis and erosions with subsequent breakdown of the epithelial barrier function [7], [9]. We therefore assessed the effect of sodium nitrite (1 µM–1 mM) on colonic epithelial wound repair in vitro. Increased healing 24 h after a mechanically made incision in confluent monolayers of SW480 cells was observed with 100 µM nitrite compared to untreated cells (Fig. 6A). To further explore whether the effects of improved epithelial healing was due to NO formation, the cells were pretreated with the NO scavenger cPTIO (100 µM) before initiation of nitrite treatment (10 and 100 µM). The protective effect of increased healing with nitrite was lost in the presence of cPTIO, and cPTIO alone did not have any effect on wound healing (Fig. 6B).
Fig. 6.
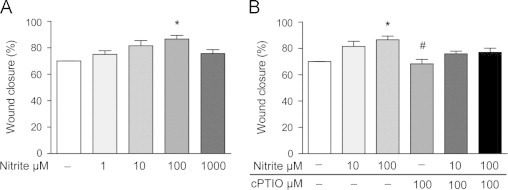
Colon epithelial cell wound healing with nitrite. (A) SW480 colon epithelial cells were treated with varying concentrations of sodium nitrite (1 µM–1 mM), and (B) with or without cPTIO (100 µM) after wound incision. Healing was measured after 24 h and expressed as healing vs. 0 h (%) (P<0.01, ⁎ vs. untreated, # vs. Nitrite 100 µM).
Discussion
Although the precise etiology of UC still is unknown, an intact barrier function with the colonic epithelium and a protective mucus layer are crucial to avoid aggravation of the disease [4], [5]. We now demonstrate that supplementation with sodium nitrite alleviates colitis in mice, both as preventive and therapeutic regiments. The protective effects by nitrite in the therapeutic approach involve a restored thickness of the colonic firmly adherent mucus layer demonstrated in vivo, and in vitro observations of increased healing of colon epithelial cells. These results provide important insights of the therapeutic effect by nitrite on already initiated colonic inflammation.
Nitrite and nitrate originating from dietary sources are important alternative substrates for NO formation and complement the endogenous production by the NOS pathway [17]. Nitrogen oxides have over the last decade been discovered to perform important effects on gastrointestinal integrity [42], [43]. Björne et al. [31] demonstrated that human salivary nitrite, collected after a dietary nitrate load, could increase rat gastric mucus generation and mucosal blood flow. Also, dietary nitrate has been shown to increase gastric mucus thickness and mucosal blood flow, thereby inducing protection against NSAID-induced injury [25], [30]. However, the effect on colonic mucosal integrity is less well studied. In order to investigate the effects of nitrite supplementation on colonic injury, the widespread model of DSS-induced colitis was used. The classical signs of tissue damage with continuous DSS-treatment can be seen by increased crypt disruption, infiltration of inflammatory cells, weight loss, colon shortening and rectal bleeding [7], [44]. In our study, we chose to use 2–2.5% of DSS in order to induce mild colitis and be able to study the anti-inflammatory effects of a dietary supplementation with sodium nitrite [45]. Even with this relatively low dose of DSS, robust increases in DAI score and reduction in colon length were seen after 7 days. Simultaneous supplementation with nitrite resulted in lower DAI score and an increased colon length compared to the disease group. Administration of nitrate also revealed some anti-inflammatory effects, with lower DAI-score and reduced expression of colonic iNOS and p65 after DSS-administration. However, no effect was seen on the length of the colon. The reasons for the discrepancy between nitrate and nitrite treatment are unclear but could be related to differences in pharmacokinetics, with nitrite giving sharper and shorter nitrite peaks in plasma and nitrate resulting in modest but sustained nitrite increases. However, a single measurement of nitrite after the 7 days of treatment did not reveal any significant differences in plasma nitrite between the different treatments (data not shown). Since nitrite supplementation demonstrated stronger clinical anti-inflammatory results, we decided to continue with this supplementation for further experiments. Our results are in line with other recent studies showing anti-inflammatory effects of nitrite administration [33], [46], and suggest that nitrite and to some extent dietary nitrate, can attenuate acute inflammation in experimental colitis.
The few studies published looking at anti-inflammatory effects of nitrite and nitrate have been designed in a preventive manner; thus the administration was initiated before or simultaneously as the inflammatory agent [33], [46]. In order to also study the therapeutic effects, we initiated DSS-administration before the onset of nitrite supplementation. Common clinical symptoms of DSS-treated mice were observed, such as rectal bleeding and diarrhea, which have shown good correlation to the inflammatory status in the acute phase of colitis [47]. It has been well described that histological changes with inflammation and clinical signs of colitis are present at day 3 of DSS-administration [7]. Similar observations on clinical status were made with mice in our study (data not shown), therefore administration of nitrite was initiated at day 3. Interestingly, even with this delay in nitrite supplementation a strongly reduced DAI score as well as improvements of colon shortening was seen. Both the preventive and the therapeutic approach of nitrite supplementation indicated similar clinical results, demonstrating a strong protective effect of nitrite even on established disease. This was further confirmed by closer investigations of the inflammatory status in colonic tissue, which indicated a reduced colonic expression of iNOS and lower histopathological scores in nitrite-supplemented mice. Othake et al. [33] have previously shown protective effects of nitrite, where the treatment was initiated before the onset of DSS-induced colitis. However, our results demonstrate that nitrite also have therapeutic effects with a much lower dose than previously studied, which is clearly closer to what is achieved from a normal diet. Other studies demonstrating anti-inflammatory effects of dietary nitrate and nitrite are emerging in different experimental inflammatory diseases, e.g. by reducing vascular pathology [43]. Dietary nitrite supplementation has been demonstrated to prevent inflammation induced by hypercholesterolemia, with reduced leukocyte adhesion and emigration [46]. In line with these results, we have recently shown that also dietary nitrate prevents leukocyte activation in acute microvascular inflammation, and further reduces expression of P-selectin leukocyte infiltration in small-intestinal tissue with NSAID-injury [48]. Moreover, Carlström et al. [49] recently demonstrated potent anti-inflammatory and antioxidant effects of dietary nitrate in a rat model of chronic hypertension with concomitant renal and cardiac inflammation. Inflammatory responses with enhanced leukocyte and platelet aggregation, leading to defects in the microcirculation have been postulated to play a central pathogenic role in development of IBD and in DSS-induced colitis [50], [51]. Our observation is in line with these previous studies, suggesting that oral therapeutic nitrite supplementation may influence the inflammatory response. A reduced immune cell infiltration was seen on the histological sections of colons after DSS-administration, as well as lower levels of iNOS, indicating a systemic effect of oral nitrite supplementation on immune cell activation.
An altered colonic mucus layer is prominent in patients with UC and in DSS-treated mice [4], [8], [52], [53]. Furthermore, it has been indicated that bacteria may penetrate the inner mucus layer in patients with UC and in several different animal models of experimental bowel disease [4], [11], [12], [53], [54]. This demonstrates the importance of an intact mucus layer for protection against bacterial-induced inflammation. In agreement with previous results, our studies show a reduced thickness of the adherent mucus layer after DSS-induced colitis [8]. Interestingly, nitrite supplementation normalized the colonic adherent mucus thickness to similar levels as the control group. In addition to our results showing lower inflammatory status after nitrite supplementation, this data suggest a potential therapeutic approach that may increase the mucus barrier to prevent bacterial penetration and maintain colon homeostasis. Goblet cell depletion is another morphological characteristic to assess disease activity in experimental colitis and UC, since these cells produce and release the major colonic mucin, Muc2 [55], [56]. Our results of increased mucus thickness with nitrite supplementation corresponded to an induced abundance of goblet cells in the colonic mucosa compared to DSS-treatment alone. This might indicate a direct effect of nitrite on mucus production and release. Despite this preservation of goblet cells by nitrite, the opposite result was surprisingly seen in colonic stainings of Muc2. As DSS-administration degraded mucus thickness and lowered the abundance of goblet cells, Muc2 expression was increased. The reason for this can only be speculated upon at this stage. In this setting, the production of Muc2 per goblet cell might be enhanced to compensate for the loss of colonic mucus. In accordance with our findings, Renes et al. [57] demonstrated a decreased number of goblet cells during DSS colitis in rats, with maintained and even increased Muc2 synthesis and total Muc2 secretion. Further studies are needed to investigate the regulatory mechanism of nitrite- and NO-mediated mucus secretion.
The initial damage of DSS is loss of crypts and thinning of the epithelium, without obvious signs of inflammation. Only later stages of DSS-induced colitis involve infiltration of inflammatory cells [7]. Therefore, by maintaining epithelial cell homeostasis in the initial state of disease, the onset of an inflammatory response might be reduced and thereby alleviate the severity of the disease. Our in vitro studies clearly show that nitrite can improve colon epithelial cell healing of a disrupted cell layer. These results are in line with modern treatment strategies for UC where stimulation of mucosal healing has attained increased attention among clinicians and is emerging as an important therapeutic endpoint in clinical trials of IBD [5]. There is growing evidence that mucosal healing is important to predict probability of disease relapse, risk of a future colectomy and of colorectal cancer development [2], [5], [58]. Thus, therapies with the ability to improve epithelial restitution of mild-to-moderate UC can have a significant impact on the quality of life of affected persons [58]. In agreement with our results, Wang et al. [59] recently showed that sodium nitrite enhances cell proliferation and wound healing of human airway epithelial cells, at similar concentrations of nitrite. The fact that cPTIO inhibited nitrite-induced wound healing suggests an important role of NO in these cells, an effect also observed by Wang et al. and by earlier studies on airway cells [60]. Although NO is a likely mediator of the observed effects, one should note that cPTIO also scavenges ·NO2 [61], another bioactive radical that might be formed from nitrite. More studies on the effect of nitrite in other inflammatory models of colitis, e.g. genetically modified or by cell transfer, are crucial to evaluate if these pre-clinical data have potential to translate into a new clinically effective agent [62].
In the present study, we demonstrate that dietary nitrite supplementation significantly alleviates DSS-induced colitis using both preventive and therapeutic approaches. A likely mechanism of protection by nitrite is NO-dependent improvement of the colonic mucosal integrity. This includes effects on the colonic epithelium, as revealed by less epithelial cell damage and crypt destruction in histology sections after DSS-administration and increased epithelial cell wound healing. Furthermore, nitrite supplementation during DSS-colitis resulted in a maintained thickness of the colonic adherent mucus layer, as well as increased abundance of mucus generating goblet cells. Further studies will reveal if this novel approach could lead to new dietary recommendations and approaches for therapy of IBD.
Acknowledgments
We thank Raoul Kuiper, Margareta Stensdotter and Annika Jägare for excellent technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Work supported by The Ruth and Richard Juhlin's Foundation, Dr. P Håkanssons/Druvan Stiftelse, Karolinska Institutet Foundation (S.B.), Vinnova (CIDaT) (J.O.L.), Vetenskapsrådet -08646 (LH) and K2012-99x (MP), Ragnar Söderberg Foundation (MP), Nanna Svartz Foundation (MP).
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2013.12.012.
Appendix A. Supporting materials
Supplementary data
References
- 1.Abraham C., Cho J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. (November 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford A.C., Moayyedi P., Hanauer S.B. Ulcerative colitis. Br. Med. J. 2013;346:f432. doi: 10.1136/bmj.f432. [DOI] [PubMed] [Google Scholar]
- 3.Salim S.Y., Soderholm J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011;17(1):362–381. doi: 10.1002/ibd.21403. (January) [DOI] [PubMed] [Google Scholar]
- 4.Johansson M.E., Gustafsson J.K., Holmen-Larsson J. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2012-303207. (February 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neurath M.F., Travis S.P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61(11):1619–1635. doi: 10.1136/gutjnl-2012-302830. (November) [DOI] [PubMed] [Google Scholar]
- 6.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. (March) [DOI] [PubMed] [Google Scholar]
- 7.Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;69(2):238–249. (August) [PubMed] [Google Scholar]
- 8.Petersson J., Schreiber O., Hansson G.C. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300(2):G327–G333. doi: 10.1152/ajpgi.00422.2010. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki Y., Mukaisyo K., Sugihara H., Fujiyama Y., Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol. Rep. 2010;24(4):869–874. doi: 10.3892/or.2010.869. (October) [DOI] [PubMed] [Google Scholar]
- 10.Atuma C., Strugala V., Allen A., Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(5):G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. (May) [DOI] [PubMed] [Google Scholar]
- 11.Dicksved J., Schreiber O., Willing B. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS One. 2012;7(9):e46399. doi: 10.1371/journal.pone.0046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U.S.A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. (September 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gum J.R., Jr., Hicks J.W., Toribara N.W., Siddiki B., Kim Y.S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269(4):2440–2446. (January 28) [PubMed] [Google Scholar]
- 14.Bergstrom K.S., Kissoon-Singh V., Gibson D.L. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Sluis M., De Koning B.A., De Bruijn A.C. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. (July) [DOI] [PubMed] [Google Scholar]
- 16.Velcich A., Yang W., Heyer J. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. (March 1) [DOI] [PubMed] [Google Scholar]
- 17.Weitzberg E., Lundberg J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013;33:129–159. doi: 10.1146/annurev-nutr-071812-161159. (July 17) [DOI] [PubMed] [Google Scholar]
- 18.Lundberg J.O., Hellstrom P.M., Fagerhol M.K., Weitzberg E., Roseth A.G. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2(2):96–102. doi: 10.1038/ncpgasthep0094. (February) [DOI] [PubMed] [Google Scholar]
- 19.Petersson J., Schreiber O., Steege A. eNOS involved in colitis-induced mucosal blood flow increase. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(6):G1281–G1287. doi: 10.1152/ajpgi.00357.2007. (December) [DOI] [PubMed] [Google Scholar]
- 20.Lundberg J.O., Weitzberg E., Lundberg J.M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35(11):1543–1546. doi: 10.1136/gut.35.11.1543. (November) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweier J.L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1(8):804–809. doi: 10.1038/nm0895-804. (August) [DOI] [PubMed] [Google Scholar]
- 22.Auth E.F.S. Nitrate in vegetables: scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;689:1–79. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976;14(6):545–548. doi: 10.1016/s0015-6264(76)80005-3. (December) [DOI] [PubMed] [Google Scholar]
- 24.Qin L., Liu X., Sun Q. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 2012;109(33):13434–13439. doi: 10.1073/pnas.1116633109. (August 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersson J., Carlstrom M., Schreiber O. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 2009;46(8):1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. (April 15) [DOI] [PubMed] [Google Scholar]
- 26.Li H., Duncan C., Townend J. Nitrate-reducing bacteria on rat tongues. Appl. Environ. Microbiol. 1997;63(3):924–930. doi: 10.1128/aem.63.3.924-930.1997. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundberg J.O., Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37(3):395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. (August 1) [DOI] [PubMed] [Google Scholar]
- 28.Jansson E.A., Huang L., Malkey R. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008;4(7):411–417. doi: 10.1038/nchembio.92. (July) [DOI] [PubMed] [Google Scholar]
- 29.Sobko T., Huang L., Midtvedt T. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006;41(6):985–991. doi: 10.1016/j.freeradbiomed.2006.06.020. (September 15) [DOI] [PubMed] [Google Scholar]
- 30.Petersson J., Phillipson M., Jansson E.A., Patzak A., Lundberg J.O., Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(3):G718–G724. doi: 10.1152/ajpgi.00435.2006. (March) [DOI] [PubMed] [Google Scholar]
- 31.Bjorne H.H., Petersson J., Phillipson M., Weitzberg E., Holm L., Lundberg J.O. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J. Clin. Invest. 2004;113(1):106–114. doi: 10.1172/JCI200419019. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson E.A., Petersson J., Reinders C. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic. Biol. Med. 2007;42(4):510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. (February 15) [DOI] [PubMed] [Google Scholar]
- 33.Ohtake K., Koga M., Uchida H. Oral nitrite ameliorates dextran sulfate sodium-induced acute experimental colitis in mice. Nitric Oxide. 2010;23(1):65–73. doi: 10.1016/j.niox.2010.04.004. (August 1) [DOI] [PubMed] [Google Scholar]
- 34.Bjorne H., Govoni M., Tornberg D.C., Lundberg J.O., Weitzberg E. Intragastric nitric oxide is abolished in intubated patients and restored by nitrite. Crit. Care Med. 2005;33(8):1722–1727. doi: 10.1097/01.ccm.0000171204.59502.aa. (August) [DOI] [PubMed] [Google Scholar]
- 35.Tannenbaum S.R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet. Toxicol. 1976;14(6):549–552. doi: 10.1016/s0015-6264(76)80006-5. (December) [DOI] [PubMed] [Google Scholar]
- 36.Ito R., Shin-Ya M., Kishida T. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 2006;146(2):330–338. doi: 10.1111/j.1365-2249.2006.03214.x. (November) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holm L., Phillipson M. Assessment of mucus thickness and production in situ. Methods Mol. Biol. 2012;842:217–227. doi: 10.1007/978-1-61779-513-8_12. [DOI] [PubMed] [Google Scholar]
- 38.Borniquel S., Jadert C., Lundberg J.O. Dietary conjugated linoleic acid activates PPARgamma and the intestinal trefoil factor in SW480 cells and mice with dextran sulfate sodium-induced colitis. J. Nutr. 2012;142(12):2135–2140. doi: 10.3945/jn.112.163931. (December) [DOI] [PubMed] [Google Scholar]
- 39.Kim S., Suvarna C.L., John D. 7th ed. Elsevier; 2013. Bancroft. Bancroft's Theory and Practice of Histological Techniques. [Google Scholar]
- 40.Linden S.K., Florin T.H., McGuckin M.A. Mucin dynamics in intestinal bacterial infection. PLoS One. 2008;3(12):e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz-Granados N., Howe K., Lu J., McKay D.M. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am. J. Pathol. 2000;156(6):2169–2177. doi: 10.1016/S0002-9440(10)65087-0. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundberg J.O., Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62(4):616–629. doi: 10.1136/gutjnl-2011-301649. (April) [DOI] [PubMed] [Google Scholar]
- 43.Kevil C.G., Kolluru G.K., Pattillo C.B., Giordano T. Inorganic nitrite therapy: historical perspective and future directions. Free Radic. Biol. Med. 2011;51(3):576–593. doi: 10.1016/j.freeradbiomed.2011.04.042. (August 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y., Kolachala V., Dalmasso G. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4(6):e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger B., Bajaj-Elliott M., MacDonald T.T., Inglin R., Eysselein V.E., Buchler M.W. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62(4):240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 46.Stokes K.Y., Dugas T.R., Tang Y., Garg H., Guidry E., Bryan N.S. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2009;296(5):H1281–H1288. doi: 10.1152/ajpheart.01291.2008. (May) [DOI] [PubMed] [Google Scholar]
- 47.Melgar S., Karlsson A., Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(6):G1328–G1338. doi: 10.1152/ajpgi.00467.2004. (June) [DOI] [PubMed] [Google Scholar]
- 48.Jadert C., Petersson J., Massena S. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic. Biol. Med. 2012;52(3):683–692. doi: 10.1016/j.freeradbiomed.2011.11.018. (February 1) [DOI] [PubMed] [Google Scholar]
- 49.Carlstrom M., Persson A.E., Larsson E. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011;89(3):574–585. doi: 10.1093/cvr/cvq366. (February 15) [DOI] [PubMed] [Google Scholar]
- 50.Vowinkel T., Wood K.C., Stokes K.Y. Mechanisms of platelet and leukocyte recruitment in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(5):G1054–G1060. doi: 10.1152/ajpgi.00350.2007. (November) [DOI] [PubMed] [Google Scholar]
- 51.Irving P.M., Macey M.G., Shah U., Webb L., Langmead L., Rampton D.S. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm. Bowel Dis. 2004;10(4):361–372. doi: 10.1097/00054725-200407000-00007. (July) [DOI] [PubMed] [Google Scholar]
- 52.Pullan R.D., Thomas G.A., Rhodes M. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35(3):353–359. doi: 10.1136/gut.35.3.353. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson M.E., Gustafsson J.K., Sjoberg K.E. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5(8):e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swidsinski A., Loening-Baucke V., Theissig F. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56(3):343–350. doi: 10.1136/gut.2006.098160. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCormick D.A., Horton L.W., Mee A.S. Mucin depletion in inflammatory bowel disease. J. Clin. Pathol. 1990;43(2):143–146. doi: 10.1136/jcp.43.2.143. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizoguchi A., Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J. Gastroenterol. 2008;43(1):1–17. doi: 10.1007/s00535-007-2111-3. [DOI] [PubMed] [Google Scholar]
- 57.Renes I.B., Boshuizen J.A., Van Nispen D.J. Alterations in Muc2 biosynthesis and secretion during dextran sulfate sodium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(2):G382–G389. doi: 10.1152/ajpgi.00229.2001. (February) [DOI] [PubMed] [Google Scholar]
- 58.Seidelin J.B., Coskun M., Nielsen O.H. Mucosal healing in ulcerative colitis: pathophysiology and pharmacology. Adv. Clin. Chem. 2013;59:101–123. doi: 10.1016/b978-0-12-405211-6.00004-8. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Frizzell S.A., Zhao X., Gladwin M.T. Normoxic cyclic GMP-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing. Nitric Oxide. 2012;26(4):203–210. doi: 10.1016/j.niox.2012.03.002. (May 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bove P.F., Wesley U.V., Greul A.K., Hristova M., Dostmann W.R., van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am. J. Respir. Cell Mol. Biol. 2007;36(2):138–146. doi: 10.1165/rcmb.2006-0253SM. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldstein S., Russo A., Samuni A. Reactions of PTIO and carboxy-PTIO with *NO, *NO2, and O2–*. J. Biol. Chem. 2003;278(51):50949–50955. doi: 10.1074/jbc.M308317200. (December 19) [DOI] [PubMed] [Google Scholar]
- 62.Koboziev I., Karlsson F., Zhang S., Grisham M.B. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm. Bowel Dis. 2011;17(5):1229–1245. doi: 10.1002/ibd.21557. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


