Abstract
Oxidative stress including DNA damage, increased lipid and protein oxidation, are important features of aging and neurodegeneration suggesting that endogenous antioxidant protective pathways are inadequate or overwhelmed. Importantly, oxidative protein damage contributes to age-dependent accumulation of dysfunctional mitochondria or protein aggregates. In addition, environmental toxins such as rotenone and paraquat, which are risk factors for the pathogenesis of neurodegenerative diseases, also promote protein oxidation. The obvious approach of supplementing the primary antioxidant systems designed to suppress the initiation of oxidative stress has been tested in animal models and positive results were obtained. However, these findings have not been effectively translated to treating human patients, and clinical trials for antioxidant therapies using radical scavenging molecules such as α-tocopherol, ascorbate and coenzyme Q have met with limited success, highlighting several limitations to this approach. These could include: (1) radical scavenging antioxidants cannot reverse established damage to proteins and organelles; (2) radical scavenging antioxidants are oxidant specific, and can only be effective if the specific mechanism for neurodegeneration involves the reactive species to which they are targeted and (3) since reactive species play an important role in physiological signaling, suppression of endogenous oxidants maybe deleterious. Therefore, alternative approaches that can circumvent these limitations are needed. While not previously considered an antioxidant system we propose that the autophagy-lysosomal activities, may serve this essential function in neurodegenerative diseases by removing damaged or dysfunctional proteins and organelles.
Abbreviations: 6-OHDA, 6-hydroxydopamine; the ADAGIO study, the Attenuation of Disease Progression with Azilect Given Once-daily) study; CBZ, carbamazepine; curcumin, (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; the DATATOP Study, the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism Study; EGCG, epigallocatechin gallate; GSH, glutathione; HIF1α, hypoxia-inducible factor 1-alpha; HNE, 4-hydroxynonenal; iPSC, induced pluripotent stem cells; LRRK2, leucine-rich repeat kinase 2; MDA, malondialdehyde; MnSOD, manganese superoxide dismutase; MitoQ, mitochondrially-targeted coenzyme Q; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine; the NET-PD network, the NINDS Exploratory Trials in Parkinson’s Disease (NET-PD) network; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; PINK1, PTEN-induced putative kinase 1; rasagiline, N-propargyl-1-(R)-aminoindan; ROS/RNS, reactive oxygen and nitrogen species; SOD, superoxide dismutase; Selegiline, N-propargyl-methamphetamine; Sirt1, NAD-dependent deacetylast sirtuin-1; the TEMPO Study, the TVP-1012 in Early Monotherapy for PD Outpatients Study; TFEB, transcription factor EB; UCHL1, ubiquitin carboxyl-terminal hydrolase L1; UPDRS, Unified Parkinson’s Disease Rating Scale
Keywords: Parkinson’s disease, Protein aggregation, Neurons, Mitochondrial dysfunction, Reactive oxygen species, Anti-oxidants, Autophagy, Toxins, Clinical trials, Animal models, Redox signaling
Graphical abstract
Highlights
-
•
Significant oxidative damage occurs in neurodegenerative disease brains.
-
•
Effective in animal models with single toxins, antioxidants are ineffective in clinical trials.
-
•
The failure of antioxidant therapy maybe due to propagation of cellular damage.
-
•
Autophagic clearance of diverse damaged molecules may provide antioxidant mechanisms.
-
•
Further mechanistic and translational studies on autophagy therapy are needed.
Introduction
Neurodegenerative diseases affect millions of people each year and the incidence is increasing with the aging of our populations. It has been projected by the National Institutes of Health that by 2030 about one in five Americans over the age of 65 will be diagnosed with a neurodegenerative disease [1]. Over the last several decades a broad range of studies (summarized in Table 1) have demonstrated that the progression of age-dependent neurodegeneration is associated with decreased antioxidants and increased oxidative damage to proteins, DNA and lipids [2], [3], [4]. Oxidative protein modification occurs at a low and persistent level in diverse cells and tissues, and accumulates in aging and neurodegenerative diseases. Here we will review evidence of oxidative damage in neurodegenerative diseases with a particular focus on Parkinson’s disease, discuss effects and challenges of antioxidant therapies in animal models and clinical trials. Finally, we will propose the hypothesis that the autophagy-lysosomal pathway provides a distinct new class of antioxidant mechanisms which have a therapeutic potential.
Table 1.
Oxidative damage in Parkinson’s disease. Evidence from postmortem studies of aging and neurodegenerative disease and control brains indicate that lipid, protein and DNA damage are higher in aging and disease brains, and supports the hypothesis that oxidative damage promotes neurodegeneration.
| Ref | |
|---|---|
| Evidence of oxidative damage in sporadic Parkinson’s disease | |
| HNE-protein adducts are increased in Alzheimer’s and Parkinson’s disease brains | [15], [16] |
| Protein carbonyl accumulation in Parkinson’s disease brain | [17] |
| Oxidized proteins complex I subunits, ubiquitin carboxyl-terminal hydrolase L1, and DJ-1 | [18], [19], [20] |
| Nitration of α-synuclein in synucleinopathies | [21] |
| Nuclear and mitochondrial DNA damage in aging, Parkinson’s and Alzheimer’s diseases | [23], [24], [25], [26] |
| Lower glutathione in aging and Parkinson’s disease brain | [31], [32], [33] |
| Plasma levels of ascorbate and α-tocopherol are lower in vascular but not non-vascular Parkinson’s disease patients | [39] |
| Coenzyme Q10 redox ratio is decreased in platelets of Parkinson’s disease patients | [40] |
| Increases of thiol-DOPA conjugates in substantia nigra of Parkinson’s disease brains | [41] |
| Evidence of oxidative damage in familial Parkinson’s disease | |
| Increased heme oxygenase 2 and monoamine oxidase expression in differentiated dopaminergic neurons from patient derived induced pluripotent stem cells (iPSC) | [44] |
| Neuronal cells from dominant Parkinson’s disease mutant LRRK2 G2010S iPSCs exhibit higher mitoSOX staining | [45] |
| Fibroblasts from Parkinson’s disease patients with PINK1 mutations exhibit increased mitochondrial ROS | [46] |
| Mitochondrial DNA lesions are increased in neural cells from iPSCs carrying LRRK2 mutations | [47] |
| Lower glutathione in Parkin mutant iPSC neurons | [48] |
Endogenous oxidants in aging and neurodegenerative disease
Mammalian brains have a high propensity to generate reactive oxygen and nitrogen species (ROS/RNS) including hydrogen peroxide, superoxide, nitric oxide and peroxynitrite [5], [6], [7], [8], [9], [10]. The high rate of O2 consumption by mitochondrial metabolism in the brain and the constant supply of oxygen allow for ROS/RNS to be generated from a variety of sources including nitric oxide synthases and from the mitochondrial respiratory chain [7], [8], [9], [10]. These processes are further enhanced by neuroinflammation in which glia cells become an additional source of ROS from NADPH oxidases [10], [11]. The brain also contains high levels of polyunsaturated fatty acids which are targets for the initiation of lipid peroxidation which can propagate the formation of reactive lipid species such as 4-hydroxynonenal (HNE) and malondialdehyde (MDA) which in turn are capable of modifying proteins [12], [13], [14]. These covalent modifications of proteins appear to accumulate in the brain during aging and in Alzheimer’s and Parkinson’s diseases [15], [16]. It should be noted that HNE is only one of several reactive lipid species that are produced during non-specific lipid peroxidation and should be considered as a representative of this class of molecules. Using the less specific protein carbonyl measurement protein oxidation has also been found to be increased in post-mortem Parkinson’s disease brains compared to controls [17]. Specific proteins which have been shown to be oxidized include mitochondrial electron transport complex I subunits [18], ubiquitin carboxyl-terminal hydrolase L1 [19], and DJ-1 [20], all of which have been implicated in familial or sporadic Parkinson’s disease.
A major contributor to the pro-oxidant environment in the brain is thought to be peroxynitrite which is formed from the reaction of superoxide and nitric oxide [7]. Consistent with the formation of peroxynitrite, nitration of α-synuclein is increased in Lewy bodies and Lewy neurites in several synucleinopathies [6], [21], [22]. It is important to note that peroxynitrite can promote protein and lipid oxidation/nitration independently. This finding implies that the targeting of lipid peroxidation by scavengers such as α-tocopherol, ascorbate and coenzyme Q10 would be ineffective if the pathological mechanism of neurodegeneration involves protein oxidation independent of the reactive lipid species. Both nuclear and mitochondrial DNA damage are also associated with aging [23] and neurodegenerative diseases, such as Parkinson’s [24], [25] and Alzheimer’s disease [26]. Therefore, both lipid peroxidation-dependent and independent mechanisms may be involved in cellular damage occurring in neurodegenerative diseases.
Endogenous antioxidants in aging and neurodegenerative disease
The antioxidants produced endogenously, including superoxide dismutases (SOD), catalases, peroxiredoxins, and glutathione, play important roles in preventing endogenous and xenobiotic-induced oxidative damage [27]. Glutathione, the endogenously produced, thiol containing tri-peptide, plays an important role in antioxidant defense and redox homeostasis, and is found at lower levels in neurons compared to astrocytes [28], [29], and is also lower in the substantia nigra compared to cortex [30]. In the brain, decreased glutathione levels have also been reported in aging [31] and sporadic Parkinson’s disease [32], [33], [34], [35], [36], [37], [38]. Plasma levels of ascorbate and α-tocopherol have been reported to be lower in vascular but not non-vascular Parkinson’s disease patients [39]. The coenzyme Q10 is more oxidized in platelets of Parkinson’s disease patients consistent with increased mitochondrial oxidative stress [40]. In support of increased DOPA oxidation and subsequent conjugation with thiol compounds, the specific products 5-S-cysteinyl-dopamine, 5-S-cysteinyl-DOPA and 5-S-cysteinyl-DOPAC, have been found to be increased in substantia nigra of Parkinson’s disease brains [41].
Oxidants and antioxidants in familial Parkinson’s disease
There is considerable evidence that redox-dependent changes also occur in familial Parkinson’s disease. Although most cases are sporadic, about 10% of Parkinson’s disease cases are due to genetic causes [42], [43]. Differentiated dopaminergic neurons from patient-derived induced pluripotent stem cells (iPSCs) with α-synuclein gene triplication exhibited increased heme oxygenase 2 and monoamine oxidase expression compared to control cells [44]. Neuronal cells from iPSC of Parkinson’s disease patients carrying mutant LRRK2 G2010S and fibroblasts carrying PINK1 mutations have been found to exhibit higher mitochondrial superoxide formation [45], [46]. Mitochondrial DNA lesions are increased in neural cells from iPSCs carrying LRRK2 mutations [47]. In Parkin mutant iPSC neurons, glutathione levels have been found to be lower while Nrf2 levels are higher compared to controls, suggesting an impairment in the engagement of the endogenous Keap1-Nrf2 antioxidant protective pathways [14], [48].
Oxidants and antioxidants in neurotoxin-induced Parkinson’s disease
Supporting the hypothesis that oxidants contribute to the pathophysiology of neurodegenerative disease, it has been shown that many of the neurotoxins that increase Parkinson’s disease risk or parkinsonism in humans and animal models also induce oxidant production and promote oxidative damage (Table 2). The neurotoxin MPTP causes parkinsonism in humans by entering astrocytes where it is converted to the active metabolite MPP+ by monoamine oxidase-B. MPP+ is then taken up by the dopaminergic neurons via the dopamine transporter, and causes degeneration of these neurons [49]. It has been reported that MPP+ can bind and inhibit complex I of the mitochondrial respiratory chain, and increase production of superoxide in a complex I-dependent mechanism, although cellular toxicity may be mediated through ROS and complex I-independent mechanisms [50], [51]. MPTP administration in mouse models has been shown to increase mitochondrial and nuclear DNA damage [52], and 2,3-dihydroxbenzoic acid (2,3-DHB), a marker of hydroxyl radical formation. Although there is evidence for an adaptive response such as increased antioxidant enzyme activities, including those of glutathione peroxidase, superoxide dismutase, and catalase, in the midbrain, dopaminergic neurodegeneration still proceeds. This indicates that the increase of antioxidant enzymes is insufficient for neuroprotection during the progression of Parkinson’s disease [53].
Table 2.
Oxidative damage in toxin models of Parkinson’s disease. Evidence of oxidative damage and effects of antioxidant are summarized from studies in toxin models of Parkinson’s disease.
| Toxins | Oxidative damage in animal models | Ref | Protective effects of antioxidants | Ref |
|---|---|---|---|---|
| MPTP | MPTP administration increases mitochondrial and nuclear DNA damage, and markers of hydroxyl radicals | [52], [53] | α-tocopherol, β-carotene, ascorbic acid, selegiline, creatine, ebselen, coenzyme Q, lipoic acid, N-acetyl-cysteine, lipoic acid, EGCG, edaravone | [69], [70], [71], [72], [73], [74], [75], [77], [78] |
| Rotenone | Rotenone administration increased nitric oxide, lipid peroxidation products, and protein thiol modification | [56], [57], [58], [59] | N-acetyl-cysteine-related compound, edaravone | [77], [79] |
| Paraquat | Paraquat exposure decreased glutathione, decreased reduced coenzyme A, and increased 3-nitrotyrosine/tyrosine levels | [64] | ||
| 6-OHDA | 6-OHDA administration increases DNA damage, malonaldialdehyde levels, lipid peroxidation products and protein carbonyls | [66], [67], [68] | N-acetyl-cysteine-related compound, edaravone, resveratrol | [68], [77], [80] |
Other environmental toxins including pesticides, such as rotenone and paraquat, have been associated with increased risk of developing Parkinson’s disease [54]. Rotenone, a complex I inhibitor, administrated in animal models induces dopaminergic neurodegeneration, accumulation of DNA damage [55], increased nitric oxide [56], lipid peroxidation products [56], and protein thiol modification [57], [58], [59], [60]. Although the herbicide paraquat exhibits structural similarity to MPP+, it appears to act through redox cycling rather than inhibition of complex I and induces dopaminergic neurodegeneration [61], [62], [63]. Paraquat exposure in rats has been shown to decrease glutathione, decrease reduced coenzyme A, and increase 3-nitrotyrosine [64].
6-OHDA is a naturally occurring by-product of dopamine synthesis and has been found in human brains and urine [65]. As with MPTP and rotenone, 6-OHDA administration in animals also induces dopaminergic cell death, movement disorders, accumulation of DNA damage [66], increased malonaldialdehyde levels [67], and protein carbonyls [68]. However, the mechanisms appear unrelated to mitochondrial dysfunction and may involve other pro-oxidant mitochondrial-independent pathways [51], [68].
Effects of antioxidants in animal models and human clinical trials
The current treatment of Parkinson’s disease is limited to relieving symptoms, without any significant effects in slowing the neurodegenerative processes. Because oxidative damage may contribute significantly to neurodegeneration in human patients and animal models, the idea of antioxidant therapy has been tested in animal models, followed by clinical trials in Parkinson’s disease patients.
Effects of antioxidants in mammalian models of Parkinson’s disease
Numerous studies using rat, mouse or primates have tested the efficacy of neuroprotective interventions by low molecular weight antioxidants. Here we will highlight some of the antioxidant studies which have shown neuroprotective effects in MPTP, rotenone and 6-OHDA-induced neurodegeneration models (Table 2).
In an acute MPTP administration mouse model, high doses of α-tocopherol, β-carotene, ascorbic acid or N-acetyl-cysteine, all decreased MPTP-induced striatal dopamine loss [69]. Additional studies have shown that a variety of compounds with antioxidant properties such as selegiline [70], creatine [71], ebselen [72], coenzyme Q [73], EGCG [74], and lipoic acid [75] provide protection in MPTP administration-induced neurodegeneration models. It is important to note that many of these compounds have other properties and it should not be assumed that radical scavenging is their primary mode of action. For example, although it has been shown that creatine has antioxidant activity in in vitro models the physiological relevance of these tests is open to question and the concentrations required for a significant effect are higher than those that can be achieved biologically [76].
In addition to MPTP models, effects of antioxidants have also been tested in animal models of rotenone or 6-OHDA-induced neurodegeneration. For example, a small molecular weight N-acetyl-cysteine-related compound AD4 [77], and 3-methyl-1-phenyl-2-pyrazolin-5-one (edaravone) [78], [79], [80] have been shown to decrease nigrostriatal degeneration. Oral N-acetyl-cysteine, which modulates thiol signaling and can act as a glutathione precursor, also attenuates neurodegeneration in alpha-synuclein overexpressing mice [81]. Although all of these toxic inducers of Parkinsonism are associated with changes in non-specific markers of oxidative stress, the detailed mechanisms are not the same.
Clinical trials
Although antioxidant treatments have shown neuroprotective potential in mammalian models of Parkinson’s disease in which it is induced by a defined toxin administered in a pure form, human clinical trials have not been as successful. In the section below we will highlight some of the antioxidant clinical trials (Table 3). The DATATOP (Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism) trial was one of the first large clinical trials assessing the effects of α-tocopherol and selegiline (N-Propargyl-methamphetamine), a monoamine oxidase B (MAO-B) inhibitor, in neuroprotection, with 800 patients randomized with treatment for up to 2 years [82], [83]. In this study, selegiline but not α-tocopherol has improved the initial Unified Parkinson’s Disease Rating Scale (UPDRS) score, which rates both motor and mentation scores by patients and clinicians, and delayed the time of onset of the clinical decision for L-dopa treatment.
Table 3.
Clinical trials of antioxidant therapies in Parkinson’s disease (PD).
| Trial name | Antioxidants | Patients | Type of Clinical Trial | Findings | Citation |
|---|---|---|---|---|---|
| DATATOP | Selegiline: MAO-B inhibitor; Tocopherol: peroxyl radicals and lipid peroxidation products | Stage 1 and 2 PD patients with no treatment for PD (800 patients) | Randomized, double-blind, placebo-controlled, multi-center | Selegiline but not tocopherol slows onset of clinical decisions of needing L-DOPA | [82], [83] |
| TEMPO | Rasagiline: more potent MAO-B inhibitor than selegiline | Early PD patients who did not require treatment (404 patients) | Delayed-start, 6 months placebo versus rasagiline, all followed by rasagiline, double-blind, placebo-controlled clinical trial | Rasagiline is effective in improving UPDRS scores | [84] |
| ADAGIO | Rasagiline: more potent MAO-B inhibitor than selegiline; also found to boost antioxidant production | Untreated PD (1174 patients) | Double blind, delayed-start trial, 36 weeks of placebo versus rasagiline, all followed by rasagiline | Rasagiline is effective in improving UPDRS scores | [85], [86] |
| Creatine | Creatine (increases cellular energy, antioxidant) | PD patients who did not require MAO inhibitors (60 patients) | Placebo controlled randomized pilot study | No change in UPDRS or SPECT scores compared to control subjects after 2 yr treatment. | [87] |
| Creatine | Creatine (increases cellular energy, antioxidant) | PD patients over 30 who did not require medication (200 patients) | Randomized, double blind, futility clinical trial-Phase II | Both creatine and minocycline showed moderate protection of UPDRS, and should be considered for Phase III clinical trials | [88] |
| Creatine LS-1 NET-PD | Creatine (increases cellular energy, antioxidant) | 1741 patients | Phase III | No statistical difference between placebo and creatine, trial terminated | [89] NINDS website |
| Coenzyme Q10--QE2 | Coenzyme Q10 (antioxidant in mitochondria and lipid membranes) | Early PD patients who did not require treatment (80 patients) | Multicenter, randomized, parallel-group, placebo controlled, double-blind, dosage ranging trial | CoQ10 preserved UPDRS and increased time to levodopa treatment, especially in the highest dose of CoQ10 | [90] |
| CoenzymeQ10 | Coenzyme Q10 (antioxidant in mitochondria and lipid membranes) | PD patients over 30 who did not require medication (71 patients each in placebo and CoQ10 groups) | Randomized, double blind, futility clinical trial--Phase II | Although CoQ10 cannot be rejected as futile, the improvement of UPDRS was not significant, Phase III clinical trials was recommended | [91] |
| Coenzyme Q10—QE3 | Coenzyme Q10 (antioxidant in mitochondria and lipid membranes) | 600 patients with early Parkinson’s disease | Phase III trial with 1200mg/day and 2400mg/day for up to 16 months of treatment | Trial terminated due to lack of effect | NINDS website |
| MitoQ | MitoQ (mitochondrial antioxidant) | Newly diagnosed PD patients (128) | Double blind, placebo controlled study | MitoQ did not slow disease progression or preserve UPDRS score | [93] |
A more potent MAO-B inhibitor rasagiline (N-propargyl-1-(R)-aminoindan) has also been shown to have effects in the TEMPO (TVP-1012 in Early Monotherapy for PD Outpatients) study with 404 patients [84]. The patients were either treated with placebo or rasagiline for the first 6 months followed by rasagiline treatment for all subjects for the subsequent 6 months. The early-start rasagline group had an enduring benefit as reflected by improved UPDRS scores compared to the placebo group. Following the TEMPO study, a larger study, the ADAGIO (Attenuation of Disease Progression with Azilect Given Once-daily) study with 1176 patients for an initial 36 weeks rasagiline versus placebo, all followed by rasagiline for subsequent 36 weeks, again supported a positive effect of the drug [85], [86]. It is important to note that improvement of the UPDRS score and clinical measures do not necessarily reflect disease-modification, currently strong evidence supporting disease modification effects of these drugs is still lacking.
Creatine supplementation was also tested in clinical trials, with one trial failing to show a beneficial effect of creatine in improving UPDRS scores or dopamine transporter SPECT variables which characterize dopaminergic cell loss [87], whereas another trial observed improvement in UPDRS [88]. The long-term Study-1 (LS-1) phase III trial of creatine by the NINDS Exploratory Trials in Parkinson’s Disease (NET-PD) network with 1741 patients was recently terminated due to lack of statistically significant difference between creatine and placebo, although a detailed analysis of the complete data set has not been published to our knowledge [89].
Another compound tested in clinical trials is the coenzyme Q10. Coenzyme Q10 is required for mitochondrial bioenergetics and can inhibit lipid peroxidation in mitochondrial membranes. Its effects in delaying the progression of Parkinson’s disease showed promising early results which could be confirmed in later trials [90], [91]. To improve the targeting of coenzyme Q10 to the mitochondrion, the mitochondrially targeted coenzyme (MitoQ) was synthesized, and shown to be protective in the MPTP animal model [92], [93], but ineffective in a human trial [94].
As discussed above clinical trials using antioxidants in neurodegenerative diseases have generally proven ineffective. One critical drawback of this approach is that the interception of initiating oxidants cannot be completely efficient, allowing slow but persistent oxidative damage to occur. It is also important to recognize that the majority of Parkinson’s disease patients do not come to the clinic until there is extensive neurodegeneration. At this point significant damage, including oxidative damage of proteins, DNA and organelles, has already occurred throughout the brain, suggesting that endogenous antioxidant pathways are already overwhelmed and antioxidant therapy alone cannot reverse this damage.
The antioxidant therapies which have been tested have a higher specificity towards lipid peroxidation as the pro-oxidant pathway, but this may not be the most or the only critical initiating mechanism. Antioxidants are generally specific for scavenging specific oxidants. For example, α-tocopherol is effective at removing lipid peroxyl radicals, while deprenyl is more effective at removing free radicals produced from monoamine oxidase. Furthermore, enzymatic antioxidants have even more specific oxidant targets. MnSOD is able to dismutate O2- into H2O2 while glutathione and its associated enzymes remove reactive aldehydes through conjugation and peroxides through reduction. It follows that no single antioxidant therapy can efficiently neutralize all oxidants produced, and it is then perhaps not surprising that efficacy has been limited. Furthermore, the lack of efficacy of compounds, which are effective in animal models, in human subjects could also be due possibility that the mechanisms through which the patients developed the disease were distinct from that produced in the models.
Finally, antioxidant therapies use very high doses of antioxidant which can potentially perturb physiological redox signaling. As many studies demonstrated, redox signaling is important for cellular function. Oxidants can regulate different activities, including Keap1-Nrf2 signaling [95], [96], and cellular differentiation [97]. Furthermore, antioxidant compounds such as the polyphenolics can bind and reduce transition metal ions and then perturb redox signaling within the cell through the formation of superoxide or hydrogen peroxide [98].
Autophagy: a more effective antioxidant pathway for diverse mechanisms of oxidative damage in neurodegenerative diseases
As an alternative approach to antioxidant therapy with low molecular weight compounds targeted at one mechanism we propose that the autophagy pathway, which can clear previously damaged proteins and organelles generated by diverse oxidants without perturbing redox signaling, may achieve a more desirable antioxidant effect in neurodegenerative diseases. Autophagy is taken from the Greek words auto “self” phagy “eating” and was coined by Christian De Duve in the 1960s [2], [4], [99]. The autophagy pathway was first discovered under nutrient starvation conditions associated with mTOR inactivation [2], [4], [99]. Autophagy breaks down macromolecules and recycles their components not only to preserve cellular energy but also to clear damaged proteins and mitochondria [2], [4], [99]. Autophagy of mitochondria can be regulated by Parkin, PINK1 and DJ-1, and importantly mutations in these proteins are thought to cause familial Parkinson’s disease [2], [4], [99]. As discussed earlier, significant accumulation of damaged protein, lipid and DNA has been found in Parkinson’s disease, indicating an insufficient clearance of these modified molecules. Genetic ablation of autophagy genes ATG5 and ATG7 has been shown to cause neurodegeneration and abnormal accumulation of cytoplasmic inclusion bodies [100], [101]. Mouse brains or primary neurons with deficiencies in Parkin, PINK1 and DJ-1 exhibited mitochondrial abnormalities that may be due to insufficient mitophagy [102], [103], [104], increased protein oxidation and lipid peroxidation [105], increased mitochondrial or cytosolic reactive oxygen species and mito-roGFP oxidation [103], [104], [106], [107], [108], and increased sensitivity to neurotoxic or inflammatory insults [109], [110], [111]. The strategy of upregulating autophagy to treat neurodegenerative diseases has been tested in various cell and animal models and shown to decrease protein aggregation and cell death (Table 4).
Table 4.
Evidence that autophagy plays an antioxidant function by mitigating the effects of oxidative stress.
| Model | Evidence that upregulation of autophagy is neuroprotective | Ref |
|---|---|---|
| SH-SY5Y cells | Upregulation of autophagy with rapamycin protects against rotenone toxicity, this protection is dependent on Atg5 | [112] |
| SH-SY5Y cells | Upregulation of autophagy by rapamycin, lithium, CBZ, or valproic acid decreased, while inhibition of autophagy by chloroquine increased cell death and DCFH signals in response to rotenone | [113] |
| PC12 cells | Upregulation of autophagy by trehalose helped clearing mutant huntingtin and α-synuclein | [114] |
| Loss of function in ATG5 or ATG7 in mice | Abnormal accumulation of cytoplasmic inclusion bodies | [100], [101] |
| Loss of function in Parkin, PINK1 or DJ-1 in mice | Accumulation of abnormal mitochondria; Increased protein oxidation and lipid peroxidation; Increased mitochondrial or cytosolic reactive oxygen species and mito-roGFP oxidation; Increased sensitivity to neurotoxic or inflammatory insult | [102], [103], [104], [105],[103], [104],[104], [106], [107], [108],[109], [110], [111] |
| MPTP administration in mice | Rapamycin decreased dopaminergic neurodegeneration | [124] |
| parkin deleted human mutant tau overexpressed mice | Trehalose decreased dopaminergic neurodegeneration | [120] |
| Alpha-synuclein transgenic mice | Beclin delivery is neuroprotective | [126] |
| AAV-alpha-synuclein | AAV-Beclin, AAV-TFEB or CCI-779 a rapamycin derivative, are neuroprotective | [127] |
| MPTP administration in mice | Parkin transgenic overexpression is neuroprotective | [128] |
| 6-OHDA-injected rats, rotenone or MPTP treated mice | DJ-1-binding compounds, or direct delivery of DJ-1 protect against cell death induced by 6-hydroxydopamine, MPTP, or rotenone in rodents | [131], [132], [133] |
Rapamycin, an antifungal macrolide, was found to inhibit mTOR activity and thereby stimulate autophagy. Upregulation of autophagy with rapamycin in differentiated SH-SY5Y cells exposed with rotenone preserved cell viability [112]. The protection by rapamycin was ablated when the essential autophagy protein Atg5 was knocked down [112]. In addition to rapamycin, mTOR independent autophagy induction by lithium, valproic acid, or carbamazepine (CBZ) in SH-SY5Y cells protected against rotenone-induced cell death, whereas autophagy inhibition by chloroquine exacerbated cell death [113]. Upregulation of autophagy by trehalose in PC12 cells expressing either mutant huntingtin or α-synuclein accelerated the clearance of protein aggregates [114]. Nutraceuticals may also modulate autophagy. This has been suggested for the flavonoid polyphenol EGCG, the non-flavonoid polyphenol curcumin ((1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), and resveratrol [115], [116], [117]. These compounds are clearly pleiotropic and have been reported to also increase the levels or activities of HIF1α [118], Nrf2 [119] or Sirt1 [116]-mediated pathways.
Animal studies in neurodegenerative diseases using autophagy upregulators have also shown positive results. Rapamycin and trehalose have been shown to decrease protein aggregation and pathology in animal models of amyotrophic lateral sclerosis, Huntington’s, Alzheimer’s, and Parkinson’s diseases [120], [121], [122], [123], [124], [125]. Trehalose in drinking water, in mice with both deletion of parkin and transgenic overexpression of human mutated tau protein, was able to increase autophagy, decrease the accumulation of Tau and dopaminergic neuron death, and increase glutathione [120]. The ability to upresulate glutathione levels supports the link between autophagy and antioxidant defenses [120]. In contrast to rapamycin, trehalose is free of toxic effects at high concentrations and has a long history of human use, supporting further examination of its effects on Parkinson’s disease therapy.
In addition to pharmacological interventions, genetic activation of autophagy has been tested in animal models and shown to exert a neuroprotective role. In Parkinson’s disease mouse models, gene therapy with Beclin 1, Parkin, DJ-1, and transcription factor EB (TFEB) which is a master transcription activator of many autophagy-lysosomal genes, was reported to protect against Parkinsonian pathology [126], [127], [128]. Parkin transgenic mice are more resistant to MPTP induced neurodegeneration and have decreased proportions of vacuole-containing mitochondria or mitochondria with fragmented cristae [128].
DJ-1 is another multi-functional protein playing an essential role in modulating autophagy [129], as well as a redox sensor through modulatory cysteine residues [130]. Pre-clinical studies of DJ-1-binding compounds, or direct delivery of DJ-1 have been found to protect against oxidative stress-induced death of neuroblastoma cells, primary mesencephalic cells, as well as dopaminergic cells in 6-hydroxydopamine, MPTP, or rotenone-exposed rodents [131], [132], [133]. The potential utility of therapeutic drug screening which targets to proteins modifiable at cysteine residues in response to oxidative stress is important and need to be further explored. Furthermore, although it is hypothesized that autophagy activation decreases the widespread propagation of oxidative damage in neurons, most studies have focused on demonstrating decreased accumulation of aggregation prone proteins and cell death, without any direct examination of cellular redox status or oxidative damage. Future mechanistic investigations of the effects of autophagy upregulation on the oxidative damage of proteins, lipids, DNA or organelles in animal models of Parkinson’s disease are needed.
Conclusions and future directions
In this article we have discussed the finding that despite abundant evidence from both patients and experimental models that oxidative damage occurs in neurodegenerative disease, antioxidant therapy has been largely ineffective in clinical trials in slowing disease progression. There may be several explanations underlying these findings, including the diverse mechanisms of pro-oxidant action in Parkinson’s disease pathology and the advanced nature of the oxidative damage at the time of clinical presentation. As shown in Fig. 1 we propose that rather than direct scavenging oxidants, clearance of oxidative damage may be a more effective strategy for the treatment of neurodegenerative diseases (Fig. 1).
Fig. 1.
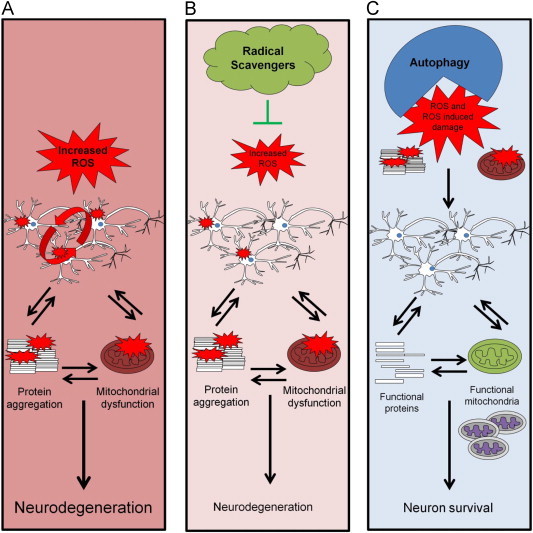
Autophagy as an antioxidant protective pathway. (A) Cells with increased ROS/RNS damage both proteins and mitochondria and propagate damage in neighboring cells. Protein aggregates can cause more mitochondrial damage and damaged mitochondria can further induce protein damage, thus resulting in neurodegeneration. (B) Lipid radical scavenging antioxidants cannot completely suppress lipid peroxidation or clear damaged proteins or organelles, and continued damage of biomolecules occurs. Furthermore, at high levels antioxidants may disrupt normal redox signaling. (C) Through removing the initial damaged mitochondria and aggregated proteins, autophagy can provide an effective antioxidant strategy independent of the initiating mechanism.
Acknowledgments
This work was supported by NIHR01-NS064090 and a VA merit award (to JZ).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Dorsey E.R., George B.P., Leff B., Willis A.W. The coming crisis: obtaining care for the growing burden of neurodegenerative conditions. Neurology. 2013;80:1989–1996. doi: 10.1212/WNL.0b013e318293e2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J., Giordano S., Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohn A., Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1:140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013;1:19–23. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman J.S. Understanding peroxynitrite biochemistry and its potential for treating human diseases. Arch. Biochem. Biophys. 2009;484:114–116. doi: 10.1016/j.abb.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 9.Tieu K., Ischiropoulos H., Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 10.Brown G.C., Neher J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 11.Brown G.C. Nitric oxide and neuronal death. Nitric. Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield D.A., Reed T., Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer's disease. Free Radic. Res. 2011;45:59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- 13.Dexter D.T., Carter C.J., Wells F.R., Javoy-Agid F., Agid Y., Lees A. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J. Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 14.Higdon A., Diers A.R., Oh J.Y., Landar A., rley-Usmar V.M. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E.R., Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterfield D.A., Lauderback C.M. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 17.Alam Z.I., Daniel S.E., Lees A.J., Marsden D.C., Jenner P., Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J. Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 18.Keeney P.M., Xie J., Capaldi R.A., Bennett J.P., Jr. Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J., Levey A.I., Weintraub S.T., Rees H.D., Gearing M., Chin L.S. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J. Biol. Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 20.Choi J., Sullards M.C., Olzmann J.A., Rees H.D., Weintraub S.T., Bostwick D.E. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duda J.E., Giasson B.I., Chen Q., Gur T.L., Hurtig H.I., Stern M.B. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darley-Usmar V.M., Hogg N., O'Leary V.J., Wilson M.T., Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic. Res. Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 23.Mecocci P., MacGarvey U., Kaufman A.E., Koontz D., Shoffner J.M., Wallace D.C. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 24.Alam Z.I., Jenner A., Daniel S.E., Lees A.J., Cairns N., Marsden C.D. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Perry G., Smith M.A., Robertson D., Olson S.J., Graham D.G. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Xiong S., Xie C., Markesbery W.R., Lovell M.A. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J. Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr. Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 28.Raps S.P., Lai J.C., Hertz L., Cooper A.J. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989;493:398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- 29.Slivka A., Mytilineou C., Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987;409:275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y., Viswanath V., Jha N., Qiao X., Mo J.Q., Andersen J.K. Brain gamma-glutamyl cysteine synthetase (GCS) mRNA expression patterns correlate with regional-specific enzyme activities and glutathione levels. J. Neurosci. Res. 1999;58:436–441. [PubMed] [Google Scholar]
- 31.Rebrin I., Kamzalov S., Sohal R.S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce R.K., Owen A., Daniel S., Jenner P., Marsden C.D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J. Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 33.Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 34.Venkateshappa C., Harish G., Mythri R.B., Mahadevan A., Bharath M.M., Shankar S.K. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson's disease. Neurochem. Res. 2012;37:358–369. doi: 10.1007/s11064-011-0619-7. [DOI] [PubMed] [Google Scholar]
- 35.Harish G., Mahadevan A., Srinivas Bharath M.M., Shankar S.K. Alteration in glutathione content and associated enzyme activities in the synaptic terminals but not in the non-synaptic mitochondria from the frontal cortex of Parkinson's disease brains. Neurochem. Res. 2013;38:186–200. doi: 10.1007/s11064-012-0907-x. [DOI] [PubMed] [Google Scholar]
- 36.Mythri R.B., Venkateshappa C., Harish G., Mahadevan A., Muthane U.B., Yasha T.C. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson's disease brains. Neurochem. Res. 2011;36:1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- 37.Krapfenbauer K., Engidawork E., Cairns N., Fountoulakis M., Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 38.Werner C.J., Heyny-von H.R., Mall G., Wolf S. Proteome analysis of human substantia nigra in Parkinson's disease. Proteome. Sci. 2008;6:8. doi: 10.1186/1477-5956-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paraskevas G.P., Kapaki E., Petropoulou O., Anagnostouli M., Vagenas V., Papageorgiou C. Plasma levels of antioxidant vitamins C and E are decreased in vascular parkinsonism. J. Neurol. Sci. 2003;215:51–55. doi: 10.1016/s0022-510x(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 40.Gotz M.E., Gerstner A., Harth R., Dirr A., Janetzky B., Kuhn W. Altered redox state of platelet coenzyme Q10 in Parkinson's disease. J. Neural Transm. 2000;107:41–48. doi: 10.1007/s007020050003. [DOI] [PubMed] [Google Scholar]
- 41.Spencer J.P., Jenner P., Daniel S.E., Lees A.J., Marsden D.C., Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J. Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- 42.Trinh J., Farrer M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013 doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 43.Corti O., Lesage S., Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 44.Byers B., Cord B., Nguyen H.N., Schule B., Fenno L., Lee P.C. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS ONE. 2011;6:e26159. doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Y.C., Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 2013;22:4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]
- 46.Abramov A.Y., Gegg M., Grunewald A., Wood N.W., Klein C., Schapira A.H. Bioenergetic consequences of PINK1 mutations in Parkinson disease. PLoS ONE. 2011;6:e25622. doi: 10.1371/journal.pone.0025622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders L.H., Laganiere J., Cooper O., Mak S.K., Vu B.J., Huang Y.A. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol. Dis. 2013;62C:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imaizumi Y., Okada Y., Akamatsu W., Koike M., Kuzumaki N., Hayakawa H. Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain. 2012;5:35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 50.Dranka B.P., Zielonka J., Kanthasamy A.G., Kalyanaraman B. Alterations in bioenergetic function induced by Parkinson's disease mimetic compounds: lack of correlation with superoxide generation. J. Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giordano S., Lee J., Darley-Usmar V.M., Zhang J. Distinct Effects of Rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on Cellular Bioenergetics and Cell Death. PLoS ONE. 2012;7:e44610. doi: 10.1371/journal.pone.0044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandavilli B.S., Ali S.F., Van H.B. DNA damage in brain mitochondria caused by aging and MPTP treatment. Brain Res. 2000;885:45–52. doi: 10.1016/s0006-8993(00)02926-7. [DOI] [PubMed] [Google Scholar]
- 53.Cassarino D.S., Fall C.P., Swerdlow R.H., Smith T.S., Halvorsen E.M., Miller S.W. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim. Biophys. Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 54.Tanner C.M., Kamel F., Ross G.W., Hoppin J.A., Goldman S.M., Korell M. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marella M., Seo B.B., Nakamaru-Ogiso E., Greenamyre J.T., Matsuno-Yagi A., Yagi T. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. PLoS ONE. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bashkatova V., Alam M., Vanin A., Schmidt W.J. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp. Neurol. 2004;186:235–241. doi: 10.1016/j.expneurol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Mastroberardino P.G., Hoffman E.K., Horowitz M.P., Betarbet R., Taylor G., Cheng D. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol. Dis. 2009;34:417–431. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horowitz M.P., Milanese C., Di M.R., Hu X., Montero L.M., Sanders L.H. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxid. Redox Signal. 2011;15:855–871. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherer T.B., Betarbet R., Testa C.M., Seo B.B., Richardson J.R., Kim J.H. Mechanism of toxicity in rotenone models of Parkinson's disease. J. Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fussell K.C., Udasin R.G., Gray J.P., Mishin V., Smith P.J., Heck D.E. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic. Biol. Med. 2011;50:874–882. doi: 10.1016/j.freeradbiomed.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson J.R., Quan Y., Sherer T.B., Greenamyre J.T., Miller G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 63.Cannon J.R., Greenamyre J.T. Neurotoxic in vivo models of Parkinson's disease recent advances. Prog. Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 64.Liang L.P., Kavanagh T.J., Patel M. Glutathione deficiency in Gclm null mice results in complex I inhibition and dopamine depletion following paraquat administration. Toxicol. Sci. 2013;134:366–373. doi: 10.1093/toxsci/kft112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrew R., Watson D.G., Best S.A., Midgley J.M., Wenlong H., Petty R.K. The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochem. Res. 1993;18:1175–1177. doi: 10.1007/BF00978370. [DOI] [PubMed] [Google Scholar]
- 66.Kikuchi Y., Yasuhara T., Agari T., Kondo A., Kuramoto S., Kameda M. Urinary 8-OHdG elevations in a partial lesion rat model of Parkinson's disease correlate with behavioral symptoms and nigrostriatal dopaminergic depletion. J. Cell Physiol. 2011;226:1390–1398. doi: 10.1002/jcp.22467. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R., Agarwal A.K., Seth P.K. Free radical-generated neurotoxicity of 6-hydroxydopamine. J. Neurochem. 1995;64:1703–1707. doi: 10.1046/j.1471-4159.1995.64041703.x. [DOI] [PubMed] [Google Scholar]
- 68.Khan M.M., Ahmad A., Ishrat T., Khan M.B., Hoda M.N., Khuwaja G. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Perry T.L., Yong V.W., Clavier R.M., Jones K., Wright J.M., Foulks J.G. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by four different antioxidants in the mouse. Neurosci. Lett. 1985;60:109–114. doi: 10.1016/0304-3940(85)90229-0. [DOI] [PubMed] [Google Scholar]
- 70.Cohen G., Pasik P., Cohen B., Leist A., Mytilineou C., Yahr M.D. Pargyline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in monkeys. Eur. J. Pharmacol. 1984;106:209–210. doi: 10.1016/0014-2999(84)90700-3. [DOI] [PubMed] [Google Scholar]
- 71.Matthews R.T., Ferrante R.J., Klivenyi P., Yang L., Klein A.M., Mueller G. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 72.Moussaoui S., Obinu M.C., Daniel N., Reibaud M., Blanchard V., Imperato A. The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson's disease. Exp. Neurol. 2000;166:235–245. doi: 10.1006/exnr.2000.7516. [DOI] [PubMed] [Google Scholar]
- 73.Horvath T.L., Diano S., Leranth C., Garcia-Segura L.M., Cowley M.A., Shanabrough M. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson's disease. Endocrinology. 2003;144:2757–2760. doi: 10.1210/en.2003-0163. [DOI] [PubMed] [Google Scholar]
- 74.Levites Y., Weinreb O., Maor G., Youdim M.B., Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 75.Karunakaran S., Diwakar L., Saeed U., Agarwal V., Ramakrishnan S., Iyengar S. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 76.Lawler J.M., Barnes W.S., Wu G., Song W., Demaree S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 77.Bahat-Stroomza M., Gilgun-Sherki Y., Offen D., Panet H., Saada A., Krool-Galron N. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson's disease. Eur. J. Neurosci. 2005;21:637–646. doi: 10.1111/j.1460-9568.2005.03889.x. [DOI] [PubMed] [Google Scholar]
- 78.Kawasaki T., Ishihara K., Ago Y., Baba A., Matsuda T. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a radical scavenger, prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in the substantia nigra but not the striatum. J. Pharmacol. Exp. Ther. 2007;322:274–281. doi: 10.1124/jpet.106.119206. [DOI] [PubMed] [Google Scholar]
- 79.Xiong N., Xiong J., Khare G., Chen C., Huang J., Zhao Y. Edaravone guards dopamine neurons in a rotenone model for Parkinson's disease. PLoS ONE. 2011;6:e20677. doi: 10.1371/journal.pone.0020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan W.J., Yasuhara T., Shingo T., Muraoka K., Agari T., Kameda M. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark J., Clore E.L., Zheng K., Adame A., Masliah E., Simon D.K. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS ONE. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.1989. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Parkinson Study Group. Arch. Neurol. 46:1052–1060. [DOI] [PubMed]
- 83.Shoulson I. Deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP). Parkinson Study Group. Acta Neurol. Scand. 1989;Suppl 126:171–175. doi: 10.1111/j.1600-0404.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 84.2002. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch. Neurol. 59:1937–1943. [DOI] [PubMed]
- 85.Rascol O., Fitzer-Attas C.J., Hauser R., Jankovic J., Lang A., Langston J.W. A double-blind, delayed-start trial of rasagiline in Parkinson's disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011;10:415–423. doi: 10.1016/S1474-4422(11)70073-4. [DOI] [PubMed] [Google Scholar]
- 86.Olanow C.W., Hauser R.A., Jankovic J., Langston W., Lang A., Poewe W. A randomized, double-blind, placebo-controlled, delayed start study to assess rasagiline as a disease modifying therapy in Parkinson's disease (the ADAGIO study): rationale, design, and baseline characteristics. Mov Disord. 2008;23:2194–2201. doi: 10.1002/mds.22218. [DOI] [PubMed] [Google Scholar]
- 87.Bender A., Koch W., Elstner M., Schombacher Y., Bender J., Moeschl M. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006;67:1262–1264. doi: 10.1212/01.wnl.0000238518.34389.12. [DOI] [PubMed] [Google Scholar]
- 88.2006. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66:664–671. [DOI] [PubMed]
- 89.Elm J.J. Design innovations and baseline findings in a long-term Parkinson's trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson's Disease Long-Term Study-1. Mov. Disord. 2012;27:1513–1521. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shults C.W., Oakes D., Kieburtz K., Beal M.F., Haas R., Plumb S. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 91.2007. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 68:20–28. [DOI] [PubMed]
- 92.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 93.Snow B.J., Rolfe F.L., Lockhart M.M., Frampton C.M., O'Sullivan J.D., Fung V. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov. Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 94.Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A.G. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim. Biophys. Acta. 2013 doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levonen A.L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sauer H., Wartenberg M., Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 98.Yin J.J., Fu P.P., Lutterodt H., Zhou Y.T., Antholine W.E., Wamer W. Dual role of selected antioxidants found in dietary supplements: crossover between anti- and pro-oxidant activities in the presence of copper. J. Agric. Food Chem. 2012;60:2554–2561. doi: 10.1021/jf204724w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dodson M., Darley-Usmar V., Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 101.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J.I., Tanida I. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 102.Mortiboys H., Thomas K.J., Koopman W.J., Klaffke S., bou-Sleiman P., Olpin S. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H.L., Chou A.H., Wu A.S., Chen S.Y., Weng Y.H., Kao Y.C. PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons. Biochim. Biophys. Acta. 2011;1812:674–684. doi: 10.1016/j.bbadis.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Irrcher I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 105.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 106.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andres-Mateos E., Perier C., Zhang L., Blanchard-Fillion B., Greco T.M., Thomas B. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guzman J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P.T. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frank-Cannon T.C., Tran T., Ruhn K.A., Martinez T.N., Hong J., Marvin M. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim R.H., Smith P.D., Aleyasin H., Hayley S., Mount M.P., Pownall S. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manning-Bog A.B., Caudle W.M., Perez X.A., Reaney S.H., Paletzki R., Isla M.Z. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol. Dis. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Pan T., Rawal P., Wu Y., Xie W., Jankovic J., Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 113.Xiong N., Jia M., Chen C., Xiong J., Zhang Z., Huang J. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience. 2011;199:292–302. doi: 10.1016/j.neuroscience.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 114.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 115.Fujita K., Maeda D., Xiao Q., Srinivasula S.M. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1427–1432. doi: 10.1073/pnas.1014156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y., Li X., Zhu J.X., Xie W., Le W., Fan Z. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H., Bosch-Marce M., Shimoda L.A., Tan Y.S., Baek J.H., Wesley J.B. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Mahler A., Mandel S., Lorenz M., Ruegg U., Wanker E.E., Boschmann M. Epigallocatechin-3-gallate: a useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases? EPMA J. 2013;4:5. doi: 10.1186/1878-5085-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang T.F., Zhang Y.J., Zhou H.Y., Wang H.M., Tian L.P., Liu J. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson's disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune. Pharmacol. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- 120.Rodriguez-Navarro J.A., Rodriguez L., Casarejos M.J., Solano R.M., Gomez A., Perucho J. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol. Dis. 2010;39:423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 121.Castillo K., Nassif M., Valenzuela V., Rojas F., Matus S., Mercado G. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9:1308–1320. doi: 10.4161/auto.25188. [DOI] [PubMed] [Google Scholar]
- 122.Du J., Liang Y., Xu F., Sun B., Wang Z. Trehalose rescues Alzheimer's disease phenotypes in APP/PS1 transgenic mice. J. Pharm. Pharmacol. 2013;65:1753–1756. doi: 10.1111/jphp.12108. [DOI] [PubMed] [Google Scholar]
- 123.Ozcelik S., Fraser G., Castets P., Schaeffer V., Skachokova Z., Breu K. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS ONE. 2013;8:e62459. doi: 10.1371/journal.pone.0062459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malagelada C., Jin Z.H., Jackson-Lewis V., Przedborski S., Greene L.A. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J. Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka M., Machida Y., Niu S., Ikeda T., Jana N.R., Doi H. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 2004;10:148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 126.Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J. Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bian M., Liu J., Hong X., Yu M., Huang Y., Sheng Z. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. PLoS ONE. 2012;7:e39953. doi: 10.1371/journal.pone.0039953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomas K.J., McCoy M.K., Blackinton J., Beilina A., van der B.M., Sandebring A. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyazaki S., Yanagida T., Nunome K., Ishikawa S., Inden M., Kitamura Y. DJ-1-binding compounds prevent oxidative stress-induced cell death and movement defect in Parkinson's disease model rats. J. Neurochem. 2008;105:2418–2434. doi: 10.1111/j.1471-4159.2008.05327.x. [DOI] [PubMed] [Google Scholar]
- 132.Kitamura Y., Watanabe S., Taguchi M., Takagi K., Kawata T., Takahashi-Niki K. Neuroprotective effect of a new DJ-1-binding compound against neurodegeneration in Parkinson's disease and stroke model rats. Mol. Neurodegener. 2011;6:48. doi: 10.1186/1750-1326-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jeong H.J., Kim D.W., Woo S.J., Kim H.R., Kim S.M., Jo H.S. Transduced Tat-DJ-1 protein protects against oxidative stress-induced SH-SY5Y cell death and Parkinson disease in a mouse model. Mol. Cells. 2012;33:471–478. doi: 10.1007/s10059-012-2255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


