Abstract
Objective
To describe the pharmacoepidemiology of rituximab use in children and to estimate the frequency of infectious events within a 1-year period after rituximab exposure.
Study design
This is a retrospective cohort study of patients who received rituximab at 1 of 42 children’s hospitals contributing data to the Pediatric Health Information System between January 1999 and June 2011. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes were analyzed to categorize underlying diseases (hematologic malignancies, primary immunodeficiencies, autoimmune diseases, and transplant recipients) and to estimate inpatient infectious complication rates within each category.
Results
A total of 2875 patients with 4639 rituximab admissions were identified. The median age at index admission was 11 years (IQR, 5–15 years). The rate of rituximab admissions increased from 3 to 185 per 100 000 admissions per year over the study interval. During the 1-year follow-up period, 463 patients (16%) died. Infectious events were assessed in 2246 of the rituximab-exposed patients; 6.1% were diagnosed with sepsis and 2.0% with septic shock. The frequency of sepsis ranged from 2.4% in patients with autoimmune diseases to 12.2% in those with primary immunodeficiencies. Three patients were assigned an ICD-9-CM discharge diagnosis code for Pneumocystis joroveci pneumonia, 1 patient was assigned an ICD-9-CM discharge diagnosis code for hepatitis B, and 1 patient was assigned an ICD-9-CM discharge diagnosis code for progressive multifocal leukoencephalopathy.
Conclusion
The use of rituximab has increased significantly in children with a variety of underlying diseases. Based on ICD-9-CM code data, the rates of sepsis and other life-threatening infections after rituximab exposure vary depending on the underlying condition. Based on surveillance of infection using ICD-9-CM diagnosis codes, the rates of opportunistic infections appear to be low.
Rituximab, first approved by the US Food and Drug Administration in 1997 for use in adult CD20+ B-cell lymphomas, is currently used to treat various malignant and immune disorders. Despite the lack of an approved pediatric indication, rituximab use in children is increasing for a number of oncologic, hematologic, autoimmune, and primary immunodeficiency disorders. However, pediatric data describing the use of rituximab are scarce, and thus the risks of rituximab in children have not been well characterized. Limited data show relative safety, highlighting primarily mild infusion reactions that typically resolve with subsequent administrations.1 Concerns about serious adverse infectious events associated with rituximab exist, but so far have been documented only in the form of case reports and small case series.2–7
By causing B-cell depletion, rituximab interferes with humoral immunity, with suppression lasting up to several months and possibly even years after treatment.8 Based on rituximab’s inhibition of humoral immunity, rituximab exposure has been linked to episodes of bacteremia, sepsis, and sinopulmonary infections.2–4 Other opportunistic infections generally considered to be associated with T-cell immunosuppression have been associated with rituximab, including reactivation of herpes viruses; progression of latent viral infections, such as hepatitis B virus; and development of progressive multifocal leukoencephalopathy (PML).5,6 Rituximab also has been associated with impaired immunity against nonviral pathogens, such as Babesia microti and Pneumocystis jirovecii.7 Some of these reports have prompted safety warnings from the US Food and Drug Administration.9
The role of rituximab in opportunistic infections is not known. Data from patients with systemic lupus erythematosus (SLE) suggest that B-cell depletion affects downstream T-cell activation and regulation.10 Furthermore, in mouse models, B-cells may play a vital role in the generation of memory T-cells in response to P jirovecii infection.11 Finally, rituximab may cause immunosuppression through such mechanisms as delayed cytopenia12 and late-onset neutropenia. However, patients receiving rituximab often have an underlying immunocompromising condition and thus are at increased risk for opportunistic infections regardless of rituximab exposure, complicating the attribution of opportunistic infections to rituximab.
We used the Pediatric Health Information System (PHIS) database to describe inpatient rituximab prescribing patterns between 1999 and 2011 and to report infections present within 1 year of rituximab administration. The PHIS captures approximately 85% of pediatric inpatient care provided at freestanding US pediatric hospitals and includes every major US metropolitan area. We hypothesized that rituximab use would increase significantly over time across a variety of underlying diseases, and that PHIS discharge diagnosis data could be leveraged to estimate specific inpatient infectious complication rates after recent rituximab exposure.
Methods
We performed a retrospective cohort study to describe the use of rituximab between 1999 and 2011 in children with a variety of underlying diseases and to evaluate the incidence of hospitalized infections within a 1-year period after the first exposure to rituximab. Children entered the cohort on the first day of the hospital admission on which rituximab billing was first recorded. Patients were then followed for up to 1 year from the day of initial rituximab exposure. Patients who died were censored at the time of death. In addition, patients without 1 year of follow-up owing to inclusion after June 30, 2010, were excluded from the infectious events analysis.
The PHIS database is an administrative database containing comprehensive inpatient data from nonprofit tertiary care children’s hospitals throughout the US affiliated with the Child Health Corporation of America (Shawnee Mission, Kansas). Forty-three hospitals contributed to the PHIS database between January 1, 1999, and June 31, 2011. Each hospital contributed data for varying durations during the study period. PHIS hospitals account for 85% of freestanding US children’s hospitals registered by the National Association of Children’s Hospitals and Related Institutions, and represent 17 of the 20 US major metropolitan areas. Each patient is assigned a deidentified medical record number that allows tracking across multiple admissions at a particular hospital.
PHIS data content come from 2 primary sources. The hospital’s medical record system provides a blinded unique patient identifier, demographic information, dates of service, discharge disposition, payer information, and up to 41 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis procedure codes per admission. The hospital’s billing system provides resource utilization data, including all medications, laboratory tests (without results), imaging procedures (without results), and supplies. Oversight of PHIS data quality methods is a joint responsibility of the Child Health Corporation of America (data management center), Thomson Reuters Healthcare (data processing partner), and participating hospitals. After file submission to Thomson Reuters, data quality audits are performed. Reports are generated identifying errors needing correction by the respective hospital. Error rates above threshold values require that the hospital review its data and resubmit until error rates fall below these values.
The source population for the present study included all patients who were admitted to PHIS-contributing hospitals between January 1, 1999, and June 31, 2011. Patients who received rituximab during inpatient admissions were identified through pharmacy billing codes. In this study, hospital admissions during which rituximab was administered are referred to as “rituximab admissions”; all admissions within 1 year of rituximab admission are referred to as “study admissions.” Each patient was followed forward from the first rituximab exposure date until 1 year after exposure, death, or completion of the study.
Demographic data (age, sex, and race) were extracted for each patient at the first rituximab admission. Sex was coded as a dichotomous variable, and age was analyzed as a categorical variable (<1 year, 1 to <5 years, 5 to <10 years, 10 to <15 years, and 15 to <19 years). Race (white, black, Asian, Native American, other, and missing) was also analyzed as a categorical variable. Data on Hispanic ethnicity were missing for a large percentage of patients (54.2%), and thus were not used in our analysis. Information on length of hospital stay, timing and frequency of rituximab exposure, and inhospital mortality within 1 year after the first observed rituximab dose were recorded as well.
Underlying Disease Categorization
All patients included in the final rituximab-exposed cohort were then categorized by their underlying disease using a classification system based on ICD-9-CM discharge diagnosis codes reported during the first rituximab admission. Patients were grouped into 1 of the following categories: transplant (solid organ or hematopoietic stem cell transplantation), hematologic malignancy, primary immunodeficiency conditions, and autoimmune disease. Patients with an ICD-9-CM discharge diagnosis code that mapped to more than 1 category were classified by the following a priori defined hierarchical order: transplant, malignancy, primary immunodeficiency, and autoimmunity. Patients with a diagnosis code that did not fall into any of our diagnostic categories based on our clinical classification were classified as “other.” These diagnoses could not be matched with any published indication for rituximab use.
Infection-Related Adverse Events
For each patient, all ICD-9-CM discharge diagnosis codes were reviewed for every admission within 1 year of rituximab exposure to identify the presence of an infectious event. The identified ICD-9-CM discharge diagnosis codes are reported in the Appendix (available at www.jpeds.com) and are categorized as follows: bacteremia, sepsis, septic shock, cytomegalovirus infection, adenovirus infection, herpes virus infection, herpes zoster, candidiasis, aspergillosis, unspecified mycoses, and pneumocystis pneumonia. To avoid infectious events that might have occurred before the first rituximab exposure, discharge ICD-9-CM diagnosis codes from the first admission were included only if the rituximab was billed for within the first 3 days of that admission.
Statistical Analyses
An increasing linear trend in rituximab use over time and a decrease in rituximab use from 2006 to 2007 was tested using Poisson regression, with the number of rituximab admissions in each year as the outcome and the number of total admissions in the year as the offset. Separate regression models were constructed for overall rituximab admissions and then for each of the 5 disease categories. Patient demographics, rituximab use during the 1-year observation period, and in-hospital mortality rate were summarized by standard descriptive statistics for the overall population and each patient category. In the infectious event analysis, for each disease category the proportion of patients with at least 1 infectious complication in the predefined event categories was calculated (Appendix).
Results
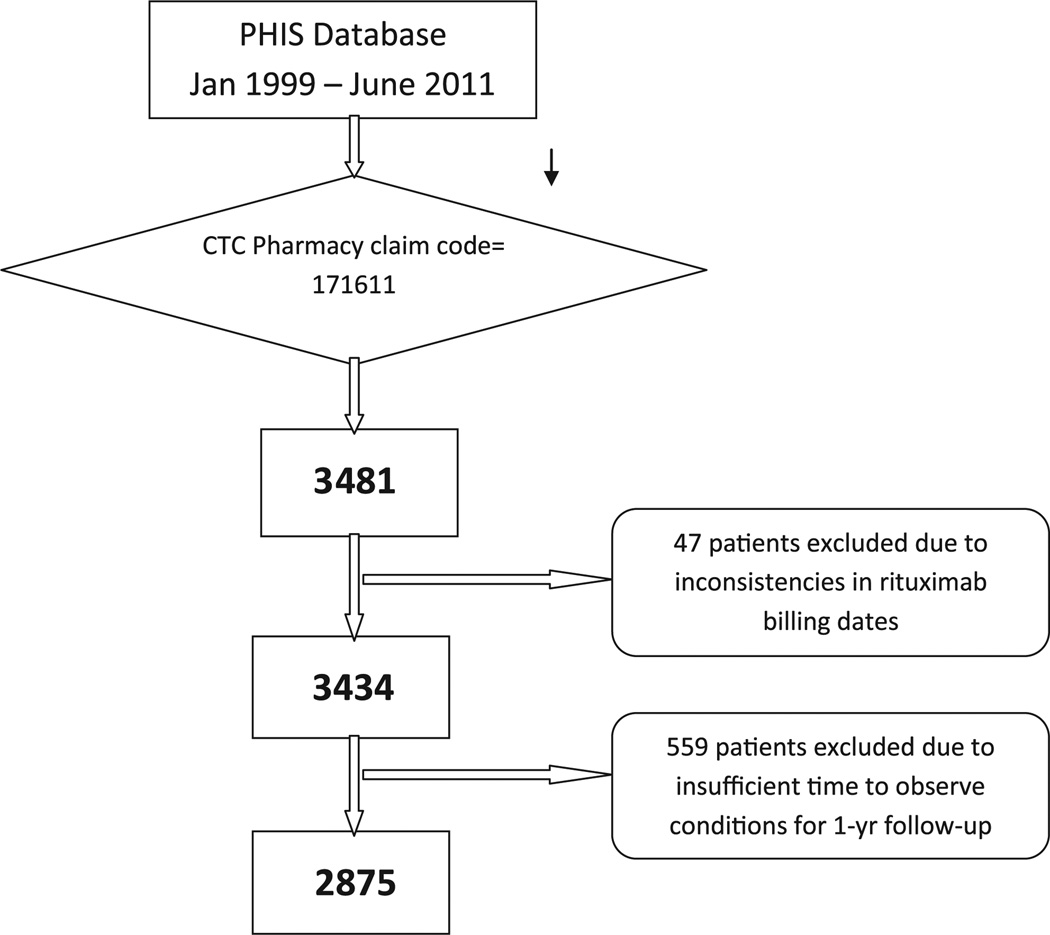
A total of 5 126 861 admissions occurred during the study period of January 1, 1999, to June 31, 2011. We identified 3481 patients from 42 of the 43 PHIS institutions who had a billing code for rituximab. Of these patients, 47 were excluded owing to poor data quality of the rituximab billing date, and 559 were excluded owing to insufficient observation time for the 1-year follow-up. This resulted in a final study population of 2875 patients with 4639 rituximab admissions, and a total of 10 510 study admissions during the 1-year observation period (Figure 1; available at www.jpeds.com).
Figure 1.
Identification of the rituximab study cohort. CTC, Clinical Transaction Classification.
Figure 2 illustrates the increase in rituximab admissions during the study period. The rate of rituximab use increased substantially between 1999 to 2011, from 3 to 185 per 100 000 admissions (P < .0001). However, a significant decrease in this rate occurred from 2006 to 2007, from 148 per 100 000 admissions in 2006 to 133 per 100 000 admissions in 2007 (P = .04). This decrease was noted in all 5 patient categories and was most pronounced in the “other” category (P = .06). After 2007, the trend of increasing rituximab admissions resumed.
Figure 2.
Rate of rituximab use over time.
Table I illustrates the frequency of each ICD-9-CM discharge diagnosis code within each assigned category during each patient’s initial rituximab admission. The 3 most commonly identified ICD-9-CM discharge diagnosis codes were complication of a transplanted organ (996.8X), Burkitt lymphoma (202.X), and SLE (710.0). Patients in the “other” group (n = 271) did not present with ICD-9-CM discharge codes that could be matched with any of published indications for rituximab use. The most commonly identified discharge diagnoses in that group were unspecific codes, including unspecified essential hypertension (401.9) in 70 patients and acute respiratory failure (518.81) in 49 patients.
Table I.
Frequency of ICD-9-CM diagnoses in the index rituximab admission (a total of 2875 patients, with up to 41 diagnostic codes per patient)
| Underlying disease category | ICD-9-CM code | Frequency, n |
|---|---|---|
| Transplant category | ||
| Complications of transplanted organ | 996.8X | 852 |
| Surgical operation with transplant of whole organ causing abnormal patient reaction, or later complication | E878.0 | 417 |
| Bone marrow replaced by transplant | V42.81 | 130 |
| Posttransplantation lymphoproliferative disorder | 238.77 | 96 |
| Liver replaced by transplant | V42.7 | 72 |
| Heart replaced by transplant | V42.1 | 60 |
| Peripheral stem cells replaced by transplant | V42.82 | 58 |
| Kidney replaced by transplant | V42.0 | 41 |
| Acute graft-versus-host disease | 279.51 | 25 |
| Chronic graft-versus-host disease | 279.52 | 22 |
| Intestines replaced by transplant | V42.84 | 21 |
| Lung replaced by transplant | V42.6 | 9 |
| Pancreas replaced by transplant | V42.83 | 7 |
| Graft-versus-host disease, unspecified | 279.53 | 2 |
| Malignancy category | ||
| Burkitt lymphoma | 200.2X | 235 |
| Other lymphomas | 202.8X | 198 |
| Lymphoid leukemia | 204.XX | 144 |
| Neoplasm of uncertain behavior of other lymphatic and hematopoietic tissues | 238.79 | 90 |
| Reticulosarcoma | 200.0X | 41 |
| Hodgkin disease | 201.XX | 31 |
| Large-cell lymphoma | 200.7X | 16 |
| Other specified leukemia | 207.8X | 8 |
| Lymphosarcoma | 200.1X | 6 |
| Leukemia of unspecified cell type | 208.XX | 5 |
| Other and unspecified malignant neoplasms of lymphoid and histiocytic tissue | 202.9X | 4 |
| Anaplastic large-cell lymphoma | 200.6X | 2 |
| Marginal zone lymphoma | 200.3X | 1 |
| Primary central nervous system lymphoma | 200.5X | 1 |
| Primary immunodeficiency category | ||
| Deficiency of humoral immunity | 279.0X | 107 |
| Deficiency of cell-mediated immunity | 279.1X | 69 |
| Combined immunity deficiency | 279.2 | 63 |
| Unspecified immunity deficiency | 279.3 | 34 |
| Unspecified disorder of immune mechanism | 279.9 | 11 |
| Autoimmune category | ||
| SLE | 710.0 | 303 |
| Autoimmune hemolytic anemias | 283.0 | 178 |
| Immune thrombocytopenic purpura | 287.31 | 133 |
| Nephrotic syndrome | 581.XX | 76 |
| Primary thrombocytopenia | 287.3 | 62 |
| Acquired hemolytic anemia, unspecified | 283.9 | 56 |
| Evans syndrome | 287.32 | 48 |
| Wegener granulomatosis | 446.4 | 38 |
| Juvenile chronic polyarthritis | 714.3X | 30 |
| Arteritis, unspecified | 447.6 | 25 |
| Congenital factor VIII disorder | 286.0 | 23 |
| Neuromyelitis optica | 341.0 | 19 |
| Acquired coagulation factor deficiency | 286.7 | 16 |
| Polyarteritis nodosa | 446.0 | 14 |
| Cerebral arteritis | 437.4 | 14 |
| Optic neuritis, unspecified | 377.30 | 6 |
| Rheumatoid arthritis | 714.0 | 5 |
| Myasthenia gravis | 358.0X | 5 |
| Other optic neuritis | 377.39 | 3 |
| Congenital factor IX disorder | 286.1 | 2 |
| Giant cell arteritis | 446.5 | 1 |
Table II provides summary data for patients during the first admission in which rituximab was billed, as well as details on additional rituximab exposure during the 1-year follow-up period. The median age at index admission was 11 years (IQR, 5–15 years), and 52% of the cohort was male. White patients composed 63% of the study population, and 46% had public insurance as their primary mode of insurance at their admission into the cohort. The majority of patients (68.5%) had only 1 rituximab admission during the 1-year observation period, whereas 16.3% had 2 admissions and 15.2% had 3 or more rituximab admissions. Of the patients in the malignancy category, 35% had only 1 rituximab admission, 17% had 2 rituximab admissions, 19% had 3 rituximab admissions, and 20% had 4 rituximab admissions during the 1-year follow-up period. The inhospital case fatality rate for patients within 1 year of initial rituximab exposure was 16% (463 of 2875), with the highest rate in the transplant category (25%; 288 of 1163) and the lowest rate in the autoimmune category (5%; 39 of 764).
Table II.
Characteristics of the study population
| Underlying disease category | ||||||
|---|---|---|---|---|---|---|
| All patients (n = 2875) |
Transplant (n = 1163) |
Malignancy (n = 479) |
Primary immunodeficiency (n = 105) |
Autoimmune (n = 764) |
Other (n = 364) |
|
| Age group, years | ||||||
| <1 | 145 (5.0) | 60 (5.2) | 2 (0.4) | 21 (20.0) | 23 (3.0) | 39 (10.7) |
| ≥1 and <5 | 536 (18.6) | 307 (26.4) | 47 (9.8) | 33 (31.4) | 59 (7.7) | 90 (24.7) |
| ≥5 and <10 | 510 (17.7) | 229 (19.7) | 101 (21.1) | 16 (15.2) | 98 (12.8) | 66 (18.1) |
| ≥10 and <15 | 735 (25.6) | 237 (20.4) | 150 (31.3) | 20 (19.1) | 247 (32.3) | 81 (22.3) |
| ≥15 | 949 (33.0) | 330 (28.4) | 179 (37.4) | 15 (14.3) | 337 (44.1) | 88 (24.2) |
| Sex | ||||||
| Male | 1496 (52.0) | 659 (56.7) | 338 (70.6) | 58 (55.2) | 251 (32.9) | 190 (52.2) |
| Female | 1379 (48.0) | 504 | 141 | 47 | 513 | 174 |
| Race | ||||||
| White | 1816 (63.2) | 793 (68.2) | 339 (70.8) | 72 (68.7) | 384 (50.3) | 228 (62.7) |
| Black | 558 (19.4) | 192 (16.5) | 64 (13.4) | 10 (9.5) | 231 (30.2) | 61 (16.8) |
| Asian | 89 (3.1) | 31 (2.7) | 14 (2.9) | 3 (2.9) | 31 (4.1) | 10 (2.8) |
| Native American | 32 (1.1) | 12 (1.0) | 2 (0.4) | 2 (1.9) | 8 (1.1) | 8 (2.2) |
| Other | 324 (11.3) | 119 (10.2) | 54 (11.3) | 13 (12.4) | 90 (11.8) | 48 (13.2) |
| Unknown | 56 (2.0) | 16 (1.4) | 6 (1.2) | 5 (4.8) | 20 (2.6) | 9 (2.5) |
| Inpatient days per patient in 1 year | ||||||
| Mean (SD) | 45.9 (56.1) | 55.2 (64.0) | 57.4 (47.2) | 68.7 (71.8) | 22.0 (34.8) | 44.7 (55.1) |
| Median (range) | 27 (1–365) | 31 (1–365) | 46 (1–324) | 51 (1–334) | 9 (1–329) | 25 (1–295) |
| Patients with 1–5 or more rituximab admissions | ||||||
| 1 | 1968 (68.5) | 872 (75.0) | 168 (35.1) | 70 (66.7) | 565 (74.0) | 293 (80.5) |
| 2 | 469 (16.3) | 194 (16.7) | 81 (16.9) | 13 (12.4) | 137 (17.90\) | 44 (12.1) |
| 3 | 201 (7.0) | 53 (4.6) | 92 (19.2) | 9 (8.6) | 31 (4.1) | 16 (4.4) |
| 4 | 151 (5.3) | 22 (1.9) | 97 (20.3) | 4 (3.8) | 22 (2.9) | 6 (1.7) |
| 5–13 | 86 (3.0) | 22 (1.9) | 41 (8.6) | 9 (8.6) | 9 (1.2) | 5 (1.4) |
| Patients with 1–5 or more rituximab days | ||||||
| 1 | 1159 (40.3) | 445 (38.3) | 94 (19.6) | 41 (39.1) | 403 (52.8) | 176 (48.4) |
| 2 | 599 (20.8) | 244 (21.0) | 79 (16.5) | 11 (10.5) | 193 (25.3) | 72 (19.8) |
| 3 | 346 (12.0) | 157 (13.5) | 64 (13.4) | 10 (9.5) | 80 (10.5) | 35 (9.6) |
| 4 | 350 (12.2) | 142 (12.2) | 102 (21.3) | 8 (7.6) | 59 (7.7) | 39 (10.7) |
| 5–46 | 421 (14.7) | 175 (15.1) | 140 (29.2) | 35 (33.3) | 29 (3.8) | 42 (11.5) |
| Time between first 2 rituximab days (if ≥2 rituximab days; n = 1716) | ||||||
| Mean (SD) | 20.5 (49.7) | 18.8 (49.3) | 16.8 (30.2) | 23.1 (62.4) | 27.0 (61.8) | 21.0 (51.7) |
| Median (range) | 7 (6–14) | 7 (6–8) | 7 (2–23) | 7 (4.5–13.5) | 7 (7–14) | 7 (7–12.5) |
| Deceased | 463 (16.1) | 288 (24.8) | 77 (16.1) | 18 (17.1) | 39 (5.1) | 41 (11.3) |
In an effort to establish a temporal association between rituximab exposure and infectious events, 629 patients (22%) were excluded from the infectious event analysis because they had only 1 identified admission during which the rituximab exposure occurred more than 3 days after admission. Of the remaining patients, 277 had only 1 admission in which the rituximab exposure was identified during the first 3 days of hospitalization, and 1969 had at least 1 subsequent admission in the 1-year period after the index rituximab admission. Table III displays the proportions of these patients with at least 1 infectious complication within 1 year after rituximab exposure. Infectious complications, including bacterial, viral, and invasive fungal infections, were distributed similarly across the transplant and malignant categories. Patients in the primary immunodeficiency category had higher rates of sepsis, cytomegalovirus, and adenovirus infection compared with those in the other categories. Overall, patients in the autoimmune category had lower rates of infectious complications. In total, 3 patients with an ICD-9-CM discharge diagnosis code for P joroveci pneumonia (all in the transplant category), 1 patient with hepatitis B, and 1 with PML (both in the malignant category) were detected.
Table III.
Proportion of patients with at least 1 infectious complication (n = 2246)
| Transplant (n = 886) |
Malignancy (n = 434) |
Primary immunodeficiency (n = 82) |
Autoimmune (n = 573) |
Other (n = 271) |
|
|---|---|---|---|---|---|
| Bacteremia | 47 (5.3) | 25 (5.8) | 4 (4.9) | 9 (1.6) | 4 (1.5) |
| Sepsis | 65 (7.3) | 41 (9.5) | 10 (12.2) | 14 (2.4) | 8 (3.0) |
| Septic shock | 18 (2.0) | 16 (3.7) | 4 (4.9) | 4 (0.7) | 4 (1.5) |
| Cytomegalovirus infection | 30 (3.4) | 8 (1.8) | 6 (7.3) | 2 (0.4) | 9 (3.3) |
| Adenovirus infection | 23 (2.6) | 8 (1.8) | 12 (14.6) | 0 (0) | 6 (2.2) |
| Herpes simplex virus infection | 8 (0.9) | 8 (1.8) | 6 (7.3) | 6 (1.1) | 2 (0.7) |
| Herpes zoster virus infection | 2 (0.2) | 4 (0.9) | 0 (0) | 0 (0) | 1 (0.4) |
| Candidiasis | 16 (1.8) | 7 (1.6) | 2 (2.4) | 6 (1.1) | 2 (0.6) |
| Aspergillosis | 11 (1.2) | 5 (1.2) | 1 (1.2) | 0 (0) | 0 (0) |
| Unspecified mycoses | 13 (1.5) | 7 (1.6) | 1 (1.2) | 0 (0) | 0 (0) |
| Pneumocystis pneumonia | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Infectious complications detected with ICD-9-CM discharge codes of first rituximab admission (if rituximab was billed on the first 3 days, n = 277) or any subsequent admission within 1 year after first rituximab exposure (n = 1969) are displayed by patient category.
Discussion
Rituximab use has increased significantly since 1999. Unexpectedly, there was a significant decline in the rate of rituximab use in 2007. Although the reason for this decline is difficult to determine, it may reflect concerns resulting from published data on rituximab safety. Specifically, the Food and Drug Administration issued a safety report in December 2006 describing 2 fatal cases of PML in adults receiving rituximab for SLE.9 The etiology of PML in these 2 patients remains unknown. In our cohort, 1 patient was assigned an ICD-9-CM discharge diagnosis code of PML: a 10-year-old female diagnosed with hematologic malignancy, who received 4 once-weekly doses of rituximab. This patient did not suffer inpatient death during the observation period.
Although rituximab is considered a risk factor for opportunistic infections, the role of the underlying condition in determining the risk of infectious complications is not known. Adult clinical trials have produced conflicting results and substantial variability regarding the association of rituximab with infections.13 A 2009 meta-analysis of randomized controlled studies showed that rituximab use significantly increased the risk of infection in adult patients with follicular lymphoma; the risk was more pronounced when only grade 3 or 4 infections were included in the analysis.14 In contrast, an open-label extension analysis of 3 double-blind trials of adult patients with rheumatoid arthritis revealed no significant increase in the risk of infections after rituximab use.3 Kelesidis et al13 reported that the infectious safety profile of rituximab is better in adult patients with autoimmune diseases than in those with lymphoma.
Pediatric data on the association of rituximab with the risk of infection are limited to case reports and small case series. Willems et al2 reported on 11 female children given rituximab for SLE, 2 of whom developed bacterial sepsis within 2 months after rituximab infusion. El-Hallak et al15 reported on 10 patients given rituximab for refractory autoimmune diseases, 3 of whom developed serious infectious complications (Staphylococcus aureus central line–associated infection, S. aureus sepsis, and grade 3 pneumonia).15 Results from the first phase II study of rituximab use in pediatric mature B-cell non-Hodgkin lymphoma suggested that a single dose of rituximab can be safely added to the standard chemotherapy backbone.16 Similarly, in a prospective study of 36 children with severe chronic immune thrombocytopenic purpura, rituximab was well tolerated, with only 1 patient developing primary varicella after the first rituximab infusion.17
Given the paucity of pediatric data regarding rituximab use and safety, the use of administrative data represents a unique approach to active surveillance for signs of increased risk of infectious complications associated with rituximab use. These data demonstrate that the risk of infectious complications in children treated with rituximab varies based on the underlying diagnosis. Although these data cannot assign attributable risk for infection from rituximab, they do provide context for the rate of these infections across different patient groups. Specifically, patients in the primary immunodeficiency category had the highest rates of infection, and those in the autoimmune category had the lowest. In addition, these data can better define the risk of certain infections in patient populations in which rituximab is more commonly used. For instance, based on scattered case report data, one might infer that the risk for infections such as P jirovecii pneumonia and hepatitis B in rituximab-exposed individuals is high.6,13,18 However, in our cohort, only 3 patients had an ICD-9-CM discharge diagnosis code for P jirovecii pneumonia, and 1 patient had an ICD-9-CM code for hepatitis B. These results suggest that the breakthrough infection rates for P jirovecii pneumonia and hepatitis B in patients receiving rituximab are not increased.
Other noninfectious negative implications have also been reported following rituximab exposure. Tarella et al19 identified rituximab as a possible risk factor for secondary solid tumor development after high-dose chemotherapy in adults.19 The actual function of B-cell–derived immunity in cancer development is largely unknown; however, the profound immunosuppression induced by rituximab B-cell depletion and subsequent disruption of the T-cell activation pathway may allow for malignant cell proliferation.20,21 Because of the difficulty in mapping ICD-9-CM discharge diagnosis codes to specific solid tumors, this concern could not be adequately addressed in our 1-year follow-up cohort. Long-term surveillance for solid tumor development in rituximab-exposed patients is important and merits further investigation.
The use of PHIS data to assemble the study cohort has some well-characterized limitations. First, all diagnoses are based on ICD-9-CM codes assigned at discharge; thus, the actual rate of infectious complications may be higher, given that ICD-9-CM discharge diagnosis codes typically underreport infectious events.22 In addition, our process of classifying patients in our cohort into disease categories has not been validated and likely misclassifies some patients. Furthermore, the category classification system does not identify patients with multiple diseases. The relative infection rates among disease categories are consistent with a priori expectations, however. Finally, the attributable risk of rituximab for infection could not be determined, because we were not able to establish a comparable group of patients without rituximab exposure. Thus, our analysis is unable to assess the additional risk of infectious complications caused by rituximab use. Nonetheless, our data do provide an estimate of infection rates in patients with rituximab exposure within a specific disease category, and also provide benchmark estimates that should be applicable to children receiving care at children’s hospitals across the US.
Acknowledgments
Supported by the National Institutes of Health (1R01 CA133881) and the Slovenian Ministry of Education, Science, Culture, and Sport (grant J3-4220).
Glossary
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PHIS
Pediatric Health Information System
- PML
Progressive multifocal leukoencephalopathy
- SLE
Systemic lupus erythematosus
Appendix
ICD-9-CM discharge diagnosis codes for infection-related adverse events
| Bacteremia | 790.7 |
| Sepsis | |
| Streptococcal septicemia | 038.0 |
| Staphylococcal septicemia | 038.1X |
| Staphylococcal septicemia, unspecified | 038.10 |
| Staphylococcus aureus septicemia | 038.11 |
| Methicillin-resistant S aureus septicemia | 038.12 |
| Other staphylococcal septicemia | 038.19 |
| Pneumococcal septicemia | 038.2 |
| Septicemia due to anaerobes | 038.3 |
| Septicemia due to other gram-negative organisms | 038.4X |
| Other specified septicemias | 038.8 |
| Unspecified septicemia | 038.9 |
| Systemic inflammatory response syndrome due to infectious process without organ dysfunction | 995.91 |
| Systemic inflammatory response syndrome due to infectious process with organ dysfunction | 995.92 |
| Septic shock | 785.52 |
| Cytomegalovirus infection | 078.5 |
| Adenovirus infection | |
| Enteritis due to adenovirus | 008.62 |
| Pneumonia due to adenovirus | 480.0 |
| Meningitis due to adenovirus | 049.1 |
| Adenovirus infection in conditions classified elsewhere and of unspecified site | 079.0 |
| Herpes simplex | 054.XX |
| Herpes zoster | 053.XX |
| Hepatitis B infection | 070.2X |
| 070.3X | |
| Aspergillosis | |
| Infection by Aspergillus spp | 117.3 |
| Pneumonia in aspergillosis | 484.6 |
| Other and unspecified mycoses | 117.9 |
| Candidiasis | |
| Disseminated candidiasis | 112.5 |
| Candidiasis of lung | 112.4 |
| Candidiasis of other specified sites | 112.8X |
| Candidiasis of unspecified site | 112.9 |
| Pneumocystosis (pneumonia due to P jiroveci) | 136.3 |
| PML | 046.3 |
Footnotes
The authors declare no conflicts of interest.
References
- 1.Solal-Céligny P. Safety of rituximab maintenance therapy in follicular lymphomas. Leuk Res. 2006;30(Suppl 1):S16–S21. doi: 10.1016/s0145-2126(06)80004-4. [DOI] [PubMed] [Google Scholar]
- 2.Willems M, Haddad E, Niaudet P. Rituximab therapy for childhood-onset systemic lupus erythematosus. J Pediatr. 2006;148:623–627. doi: 10.1016/j.jpeds.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56:3896–3908. doi: 10.1002/art.23059. [DOI] [PubMed] [Google Scholar]
- 4.Grim SA, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, et al. Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant. 2007;21:628–632. doi: 10.1111/j.1399-0012.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 5.Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–1312. doi: 10.1080/10428190701411441. [DOI] [PubMed] [Google Scholar]
- 6.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teichmann LL, Woenckhaus M, Vogel C, Salzberger B, Schölmerich J, Fleck M. Fatal Pneumocystis pneumonia following rituximab administration for rheumatoid arthritis. Rheumatology. 2008;47:1256–1257. doi: 10.1093/rheumatology/ken234. [DOI] [PubMed] [Google Scholar]
- 8.van Vollenhoven RF, Ezery P, Bingham CO, Keystone EC, Fleischmann R, Furst DE, et al. Long-term safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010;37:558–567. doi: 10.3899/jrheum.090856. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. FDA warns of safety concern regarding rituxan in new patient population. [Accessed February 15, 2012]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108810.htm.
- 10.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 12.Cattaneo C, Spedini P, Casari S, Re A, Tucci A, Borlenghi E, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47:1013–1017. doi: 10.1080/10428190500473113. [DOI] [PubMed] [Google Scholar]
- 13.Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis. 2011;15:e2–e16. doi: 10.1016/j.ijid.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal L, Gafter-Gvili A, Leibovici L, Shpilberg O. Rituximab as maintenance therapy for patients with follicular lymphoma. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006552.pub2. CD006552. [DOI] [PubMed] [Google Scholar]
- 15.El-Hallak M, Binstadt BA, Leichtner AM, Bennett CM, Neufeld EJ, Fuhlbrigge RC, et al. Clinical effects and safety of rituximab for treatment of refractory pediatric autoimmune diseases. J Pediatr. 2007;150:376–382. doi: 10.1016/j.jpeds.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 16.Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CM. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–2642. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugle B, Solomon M, Harvey E, James A, Wadhwa A, Amin R, et al. Pneumocystis jiroveci pneumonia following rituximab treatment in Wegener’s granulomatosis. Arthritis Care Res. 2010;62:1661–1664. doi: 10.1002/acr.20279. [DOI] [PubMed] [Google Scholar]
- 19.Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29:814–824. doi: 10.1200/JCO.2010.28.9777. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]