Abstract
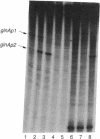
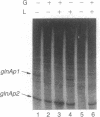
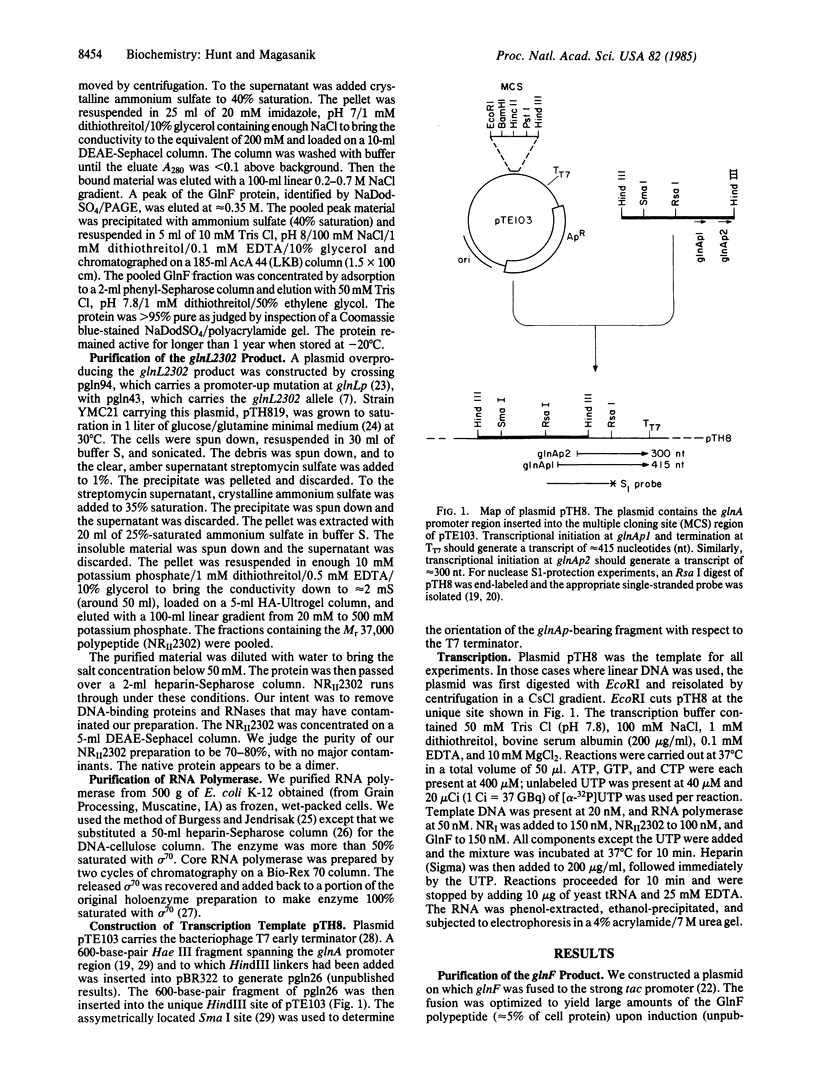
We have shown that the purified glnF (ntrA) product of Escherichia coli binds to core RNA polymerase. Together these proteins initiated transcription at the nitrogen-regulated promoter glnAp2 on a supercoiled template. The initiation of transcription at glnAp2 on a linear template required in addition NRI, the product of glnG (ntrC), and NRII2302, the product of a mutant allele of glnL (ntrB). These results identify the glnF product as a new sigma factor specifically required for the transcription of nitrogen-regulated and of nitrogen-fixation promoters. We propose rpoN as the proper designation for glnF, and sigma 60 for its product. Our results indicate that sigma 60 RNA polymerase recognizes the nitrogen-regulated/nitrogen-fixation promoter consensus sequence C-T-G-G-Y-A-Y-R-N4-T-T-G-C-A. Initiation of transcription in the intact cell appears to require in addition the active form of NRI, the product of glnG. Conversion of NRI to its active form is apparently brought about by NRII, the product of glnL, in response to nitrogen deprivation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausubel F. M. Regulation of nitrogen fixation genes. Cell. 1984 May;37(1):5–6. doi: 10.1016/0092-8674(84)90294-0. [DOI] [PubMed] [Google Scholar]
- Backman K. C., Chen Y. M., Ueno-Nishio S., Magasanik B. The product of glnL is not essential for regulation of bacterial nitrogen assimilation. J Bacteriol. 1983 Apr;154(1):516–519. doi: 10.1128/jb.154.1.516-519.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Castaño I., Bastarrachea F. glnF-lacZ fusions in Escherichia coli: studies on glnF expression and its chromosomal orientation. Mol Gen Genet. 1984;195(1-2):228–233. doi: 10.1007/BF00332751. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Backman K., Magasanik B. Characterization of a gene, glnL, the product of which is involved in the regulation of nitrogen utilization in Escherichia coli. J Bacteriol. 1982 Apr;150(1):214–220. doi: 10.1128/jb.150.1.214-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias A. A., Bastarrachea F. Nucleotide sequence of the glnA control region of Escherichia coli. Mol Gen Genet. 1983;190(1):171–175. doi: 10.1007/BF00330342. [DOI] [PubMed] [Google Scholar]
- Dixon R. Tandem promoters determine regulation of the Klebsiella pneumoniae glutamine synthetase (glnA) gene. Nucleic Acids Res. 1984 Oct 25;12(20):7811–7830. doi: 10.1093/nar/12.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Elliott T., Geiduschek E. P. Defining a bacteriophage T4 late promoter: absence of a "-35" region. Cell. 1984 Jan;36(1):211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G., Nikaido K. Nitrogen regulation in Salmonella typhimurium. Identification of an ntrC protein-binding site and definition of a consensus binding sequence. EMBO J. 1985 Feb;4(2):539–547. doi: 10.1002/j.1460-2075.1985.tb03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillardin C. M., Magasanik B. Involvement of the product of the glnF gene in the autogenous regulation of glutamine synthetase formation in Klebsiella aerogenes. J Bacteriol. 1978 Mar;133(3):1329–1338. doi: 10.1128/jb.133.3.1329-1338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E., Bancroft S., Rhee S. G., Kustu S. The product of a newly identified gene, gInF, is required for synthesis of glutamine synthetase in Salmonella. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1662–1666. doi: 10.1073/pnas.74.4.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J. Nitrogen control of the nif regulon in Klebsiella pneumoniae: involvement of the ntrA gene and analogies between ntrC and nifA. EMBO J. 1983;2(1):39–44. doi: 10.1002/j.1460-2075.1983.tb01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Regulation of nitrogen metabolism genes by nifA gene product in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):307–313. doi: 10.1038/301307a0. [DOI] [PubMed] [Google Scholar]
- Ow D. W., Xiong Y., Gu Q., Shen S. C. Mutational analysis of the Klebsiella pneumoniae nitrogenase promoter: sequences essential for positive control by nifA and ntrC (glnG) products. J Bacteriol. 1985 Mar;161(3):868–874. doi: 10.1128/jb.161.3.868-874.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Rothstein D. M., Magasanik B. Complex glnA-glnL-glnG operon of Escherichia coli. J Bacteriol. 1982 Apr;150(1):202–213. doi: 10.1128/jb.150.1.202-213.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Isolation of the nitrogen assimilation regulator NR(I), the product of the glnG gene of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5554–5558. doi: 10.1073/pnas.80.18.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Initiation of transcription by Escherichia coli RNA polymerase from supercoiled and non-supercoiled bacteriophage PM2 DNA. J Mol Biol. 1975 Feb 5;91(4):477–487. doi: 10.1016/0022-2836(75)90274-0. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Sternbach H., Engelhardt R., Lezius A. G. Rapid isolation of highly active RNA polymerase from Escherichia coli and its subunits by matrix-bound heparin. Eur J Biochem. 1975 Dec 1;60(1):51–55. doi: 10.1111/j.1432-1033.1975.tb20974.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Ow D. W., Ausubel F. M. Activation of Klebsiella pneumoniae and Rhizobium meliloti nitrogenase promoters by gln (ntr) regulatory proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4030–4034. doi: 10.1073/pnas.80.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- Ueno-Nishio S., Mango S., Reitzer L. J., Magasanik B. Identification and regulation of the glnL operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1984 Oct;160(1):379–384. doi: 10.1128/jb.160.1.379-384.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]