Abstract
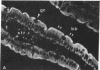
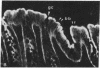
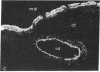
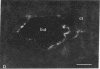
We have investigated the presence of villin (a Ca2+-regulated actin binding protein) in various tissues (normal or malignant) and in established cell lines by using sensitive immunochemical techniques on cell extracts and immunofluorescence analysis on frozen sections. Our results show that villin is a marker that can be used to distinguish normal differentiated epithelial cells from the simple epithelia lining the gastrointestinal tract and renal tubules. Villin is found in the absorptive cells of the small and large intestines, in the duct cells of pancreas and biliary system, and in the cells of kidney proximal tubules. Furthermore, undifferentiated normal and tumoral cells of intestinal origin in vivo and in cell culture express villin. Therefore, expression of villin is seen in cells that do not necessarily display the morphological features characteristic of their terminally differentiated state, such as the microvilli-lined brush border. We suggest the possible clinical implications of using villin as a marker in the diagnosis of metastatic adenocarcinomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher A., Osborn M., Wehland J., Weber K. Villin associates with specific microfilamentous structures as seen by immunofluorescence microscopy on tissue sections and cells microinjected with villin. Exp Cell Res. 1981 Sep;135(1):213–219. doi: 10.1016/0014-4827(81)90313-x. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci U S A. 1979 May;76(5):2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Immunolocalization of the 110,000 molecular weight cytoskeletal protein of intestinal microvilli. J Mol Biol. 1981 Oct 15;152(1):49–66. doi: 10.1016/0022-2836(81)90095-4. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Mannherz H. G. Distribution of actin and the actin-associated proteins myosin, tropomyosin, alpha-actinin, vinculin, and villin in rat and bovine exocrine glands. Eur J Cell Biol. 1983 May;30(2):167–176. [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Isolation and characterization of mammalian villin and fimbrin, the two bundling proteins of the intestinal microvilli. Eur J Cell Biol. 1983 Sep;31(2):249–255. [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Freiburghaus A., Grenier J. F., Hadorn B. Biosynthesis of brush border glycoproteins by human small intestinal mucosa in organ culture. Biochim Biophys Acta. 1977 Jun 16;467(3):327–339. doi: 10.1016/0005-2736(77)90310-8. [DOI] [PubMed] [Google Scholar]
- Herzlinger D. A., Easton T. G., Ojakian G. K. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J Cell Biol. 1982 May;93(2):269–277. doi: 10.1083/jcb.93.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981 Dec 25;256(24):12836–12839. [PubMed] [Google Scholar]
- Ishimura K., Fujita H., Ban T., Matsuda H., Sobue K., Kakiuchi S. Immunocytochemical demonstration of caldesmon (a calmodulin-binding, F-actin-interacting protein) in smooth muscle fibers and absorptive epithelial cells in the small intestine of the rat. Cell Tissue Res. 1984;235(1):207–209. doi: 10.1007/BF00213742. [DOI] [PubMed] [Google Scholar]
- Lacroix B., Kedinger M., Simon-Assmann P., Haffen K. Early organogenesis of human small intestine: scanning electron microscopy and brush border enzymology. Gut. 1984 Sep;25(9):925–930. doi: 10.1136/gut.25.9.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Paulin D., Babinet C., Weber K., Osborn M. Antibodies as probes of cellular differentiation and cytoskeletal organization in the mouse blastocyst. Exp Cell Res. 1980 Dec;130(2):297–304. doi: 10.1016/0014-4827(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Paulin D., Jakob H., Jacob F., Weber K., Osborn M. In vitro differentiation of mouse teratocarcinoma cells monitored by intermediate filament expression. Differentiation. 1982;22(2):90–99. doi: 10.1111/j.1432-0436.1982.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Rabito C. A., Ausiello D. A. Na+-dependent sugar transport in a cultured epithelial cell line from pig kidney. J Membr Biol. 1980;54(1):31–38. doi: 10.1007/BF01875374. [DOI] [PubMed] [Google Scholar]
- Rabito C. A., Kreisberg J. I., Wight D. Alkaline phosphatase and gamma-glutamyl transpeptidase as polarization markers during the organization of LLC-PK1 cells into an epithelial membrane. J Biol Chem. 1984 Jan 10;259(1):574–582. [PubMed] [Google Scholar]
- Raul F., Simon P., Kedinger M., Haffen K. Intestinal enzymes activities in isolated villus and crypt cells during postnatal development of the rat. Cell Tissue Res. 1977 Jan 12;176(2):167–178. doi: 10.1007/BF00229460. [DOI] [PubMed] [Google Scholar]
- Reggio H., Coudrier E., Louvard D. Surface and cytoplasmic domains in polarized epithelial cells. Prog Clin Biol Res. 1982;91:89–105. [PubMed] [Google Scholar]
- Reggio H., Webster P., Louvard D. Use of immunocytochemical techniques in studying the biogenesis of cell surfaces in polarized epithelia. Methods Enzymol. 1983;98:379–395. doi: 10.1016/0076-6879(83)98166-1. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H., Reddy B. S., Wynder E. L. Colon cancer: its epidemiology and experimental production. Cancer. 1977 Nov;40(5 Suppl):2414–2420. doi: 10.1002/1097-0142(197711)40:5+<2414::aid-cncr2820400904>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Triadou N., Kedinger M., Augeron C., Robine-Léon S., Pinto M., Rousset M., Haffen K. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983 Oct 15;32(4):407–412. doi: 10.1002/ijc.2910320403. [DOI] [PubMed] [Google Scholar]