Abstract
Coronary microvascular dysfunction (CMD) also known as syndrome X, is characterized by typical anginal symptoms, evidence of myocardial ischemia on non-invasive testing and normal to minimal coronary disease on coronary angiography. It has a female preponderance and has been detected in up to 50% of women presenting with chest pain symptoms. Definitive diagnosis of CMD is critical as recent evidence suggests that women with this condition are at increased risk of cardiovascular events in the future. Invasive coronary reactivity testing on coronary angiography is considered to be the ‘gold standard’ for diagnosis of CMD. Non-invasive imaging techniques such as PET and cardiac magnetic resonance hold promise for detection of CMD in the future.
Keywords: cardiac magnetic resonance, coronary microvascular dysfunction, diagnosis, myocardial perfusion reserve
Coronary artery disease (CAD) is the leading cause of mortality and morbidity in women and is characterized by atypical symptoms, differing patterns of coronary atherosclerosis and extensive comorbidities when compared with men. As many as 50% of women, presenting with symptoms of angina, have normal or minimal CAD on coronary angiography [1]. Such patients are frequently given the assurance that they have no CAD. However data from the Women’s Ischemia Syndrome Evaluation (WISE) study suggests that these patients are at higher risk of repeat hospital admissions, increased rates of progression to obstructive CAD and greater overall cardiovascular mortality and morbidity when compared to the general population [2]. While the study conclusions are not definitive, prompt diagnosis of this condition may help better our understanding of this condition.
Women who meet the above criteria of anginal symptoms and normal to minimal CAD have been designated as having ‘cardiac syndrome X’, ‘coronary microvascular dysfunction’ (CMD) or ‘microvascular angina’ [3]. Studies involving patients with CMD have revealed a number of underlying pathophysiological mechanisms including endothelial dysfunction, reduced coronary flow reserve (CFR) and autonomic imbalance [4]. Given the poor prognosis associated with CMD, varying diagnostic techniques have been used to confirm the diagnosis of CMD. While invasive coronary reactivity testing performed during coronary angiography has been widely considered to be the ‘gold standard’ for the diagnosis of CMD, newer non-invasive techniques have emerged and show promise for the detection of CMD [5].
In this review we examine the various invasive and non-invasive techniques available for the detection and quantification of CMD.
Definition
The currently accepted definition of CMD includes a reduced CFR of <2.5 in response to adenosine which is the lower limit of normal flow reserve in coronary arteries without obstructive CAD [5]. CFR can be measured invasively by quantitative coronary angiography and intracoronary Doppler flow wire, but non-invasive techniques such as PET have shown a reduced CFR of <2.5 in a subgroup of women from the WISE study who exhibited chest pain and non-obstructive CAD [6].
Pathophysiology
Several pathophysiological mechanisms have been proposed for CMD. Studies have described abnormalities in the micro-circulation including smooth muscle hypertrophy in subjects with CMD [7]. Burke et al. found that women demonstrated a higher frequency of coronary plaque erosion and microembolization that could result in microvascular dysfunction [8]. Autonomic imbalance has also been suggested as a possible underlying mechanism. Lanza et al. found that 75% of patients with CMD exhibited enhanced cardiac adrenergic nerve function and postulated that increased adrenergic drive led to increased microvascular tone and sensitivity to vasoconstrictor stimuli [9]. Gulli et al. similarly reported that reduced parasympathetic tone was present in two-thirds of the patients with CMD [10]. An imbalance between the endothelial derived nitric oxide, a vasodilator and endothelin-1, a vasoconstrictor, has been suggested as a possible cause of CMD [11]. Reduced levels of nitric oxide and increased levels of endothelin-1 may contribute to the altered microvascular tone in these subjects.
CFR is the increase in blood flow in response to metabolic or pharmacological interventions such as dipyridamole or adenosine. Reduced CFR is commonly noted in studies of patients with CMD [12–15]. Reduced CFR in response to intracoronary adenosine injection has also been detected in up to 47% of women with angina and minimal to non-obstructive CAD on cardiac catheterization, suggesting an endothelium-independent mechanism for CMD [16].
Endothelial dysfunction has been also postulated as one of the several possible causes for CMD. Recent studies showed that altered endothelial progenitor cells which are part of the response mechanism to vascular injury may contribute to endothelial dysfunction in CMD [17]. Abnormal sensitivity to pain, pain perception and cardiac sensitivity has been studied as part of the pathophysiology of CMD. Abnormal pain perception has been demonstrated using abnormal cerebral activity detected by cerebral single photon emission computed tomography (SPECT) imaging that occurred simultaneously with chest pain and ischemic ECG changes induced following dobutamine infusion in patients with CMD [18]. Finally factors such as hyperglycemia [19], inflammation [20] and vascular smooth muscle abnormalities [21] have also been implicated in the pathogenesis of CMD.
Epidemiology
CMD is seen in both men and women, although studies have shown a preponderance of female subjects with this condition [22]. Mertz et al. showed that up to 50% of women undergoing coronary angiography for anginal symptoms have normal coronaries [23]. Women with CMD are usually in the perimenopausal or menopausal stages of life, with onset of symptoms between 40 and 50 years [12].
Diagnosis
The diagnosis of CMD requires the exclusion of cardiac and noncardiac conditions that could be alternative explanations for chest pain. Diagnosis such as diabetes mellitus, coronary artery spasm, left ventricular hypertrophy and cardiomyopathy preclude the diagnosis of CMD. Diagnosis of CMD involves the demonstration of microvascular dysfunction either invasively or through non-invasive methods. Currently, the diagnosis of CMD requires the exclusion of obstructive CAD by coronary angiography, followed by evaluation of microvascular coronary function by Doppler guide wire in the cardiac catheterization laboratory for endothelial function testing in response to intracoronary acetylcholine, and CFR testing in response to adenosine by coronary reactivity testing [24]. Current non-invasive methods of diagnosis include contrast echocardiography, cardiac magnetic resonance imaging (CMR), PET and SPECT (Table 1).
Table 1.
Overview of various diagnostic techniques in coronary microvascular dysfunction.
| Technique | Mechanism | Stressor Agent | Measurement | Diagnostic techniques |
|---|---|---|---|---|
|
Invasive
| ||||
| Coronary angiography | Endothelium-dependant vasodilation | Acetycholine | CFR ≤50% or increases in coronary diameter ≤20% after maximum dose of acetylcholine | Intracoronary Doppler flow wire and quantitative coronary angiography |
| Endothelium-independent vasodilation | Adenosine | CFR < 2.5 or CFVR < 2.24 | Intracoronary Doppler flow wire and quantitative coronary angiography | |
|
| ||||
| Inflammatory markers and vascular tone modifiers | N/A | CRP, homocysteine, endothelin-1 and nitric oxide levels | Standard testing through blood draws | |
|
| ||||
| Non-invasive | ||||
|
| ||||
| Stress echocardiography | Endothelium-independent vasodilation | Adenosine | CFR < 2.0 | Doppler echocardiography |
|
| ||||
| Contrast echocardiography | Myocardial perfusion | Dipyridamole | Myocardial blood flow reserve < 2.0 | Ultrasound contrast agent (Definity) and intermittent/ultraharmonic imaging modality |
|
| ||||
| PET imaging (H215O and C15O, 13N-NH3, 82Rb, 18FDG) | Myocardial perfusion and cellular metabolism | Dipyridamole or adenosine | CFR < 2.5 | Quantitative and qualitative myocardial perfusion |
|
| ||||
| MR spectroscopy | Cellular metabolism (measurement of myocardial high energy phosphates) | Isometric handgrip exercise | Drop in phosphocreatine: ATP ratio during handgrip > 2 SD below mean in controls. | Magnetic resonance spectroscopy |
|
| ||||
| Stress CMR | Myocardial perfusion | Adenosine | CFR cut off variable in different studies. | Quantitative and qualitative myocardial perfusion |
|
| ||||
| Peripheral endothelial testing | Reactive hyperemia Digital reactive hyperemia | Cuff inflation for 5 min Cuff inflation for 5 min | Brachial artery flow mediated dilation Reactive hyperemia index | Brachial artery ultrasonography Peripheral arterial tonometry using Endo-PAT device |
|
| ||||
| Autonomic function testing | Sympathetic/parasympathetic activity | N/A | Heart rate variability | 24-h ambulatory ECG monitors |
18FDG: 18F fluoro-deoxyglucose; CFR: Coronary flow reserve; CFVR: Coronary flow velocity reserve; CRP: C-reactive protein; N/A: Not applicable; SD: Standard deviation.
Invasive testing
Evaluation of the coronary microcirculation involves assessment of coronary blood flow and measurement of CFR. The physiological response of the coronary system to increased myocardial demand involves increased coronary blood flow due to vasodilation of the epicardial and smaller coronary resistance vessels which is mediated by both endothelium-dependent and non-endothelium-dependent mechanisms [5]. Invasive testing of CMD involves the use of quantitative coronary angiography to evaluate changes in coronary vessel wall diameter in response to vasodilators such as adenosine, nitroglycerine and acetylcholine. Measurement of coronary flow velocity and CFR is performed using an intracoronary Doppler wire.
Assessment of endothelial dependent microvascular function
Intracoronary acetylcholine testing is widely used for the evaluation of coronary endothelial function. Injection of intracoronary acetylcholine along with coronary angiography is performed to measure endothelium-dependant vasodilatation in both epicardial and smaller resistance vessels [25]. Normal coronary endothelial function is characterized by coronary vasodilatation and a three-fourfold increase in coronary blood flow in response to acetylcholine [26]. A reduction in the coronary blood flow, no change or attenuation in the coronary blood flow in response to intracoronary acetylcholine suggests coronary endothelial dysfunction. Acetylcholine induces coronary microvascular dilation by the release of nitric oxide in controls with atypical chest pain [13]. Mohri et al. showed that nitric oxide-dependent dilation of the coronary microvessels was impaired in patients with angina and normal coronary arteries [27]. These authors suggested that the impaired nitric oxide-dependent vasodilation may increase small-vessel tone and predispose to hyperconstriction in response to acetylcholine. Ong et al. examined 39 women with CMD with the response to intracoronary acetylcholine during coronary angiography [28]. A significant proportion demonstrated microvascular vasoconstriction in response to increasing doses of intracoronary acetylcholine, similar to the response seen in subjects with coronary artery stenosis.
Endothelium-mediated coronary vasomotor function can also be determined by the cold pressor test which involves immersion of a patient’s hand in ice water for 90 sec [29]. Increased sympathetic stimulation leads to elevation of epinephrine and norepinephrine and elevated mean arterial pressure with coronary vasodilation in normal subjects while coronary vasoconstriction occurs in subjects with stenotic segments. The vasoconstrictive response to acetylcholine and CPR may be also related in part to increased smooth muscle reactivity. Zeiher et al., showed that patients with mild CAD, who had a blunted increase in coronary blood flow on the cold pressor test also showed a similar reduced response to coronary blood flow following intracoronary acetylcholine administration suggesting that the cold pressor test might be useful to assess endothelial dependent coronary vasodilatation [30].
Assessment of endothelial independent microvascular function
CMD can be also diagnosed in women without obstructive CAD by detecting microvascular dysfunction by an attenuated increase or decrease in coronary flow in response to hyperemic stimuli resulting in a reduced CFR. The current definition of microvascular dysfunction for CMD requires a <2.5-fold increase in coronary volumetric blood flow in response to maximal hyperemic stimuli such as adenosine [31]. CFR is most commonly measured invasively by quantitative coronary angiography and intracoronary Doppler ultrasonography (Figure 1). In the WISE study, CFR and coronary flow velocity reserve were measured in 48 women with anginal symptoms and normal coronaries. Coronary velocity and diameter were measured at baseline and after a hand-injected intracoronary bolus of adenosine. Coronary velocity reserve, which correlated well with CFR, was determined by the ratio of average peak coronary velocity after adenosine to baseline velocity. They found that a coronary velocity reserve threshold of 2.24 provided optimum sensitivity (90%) and specificity (89%) for the diagnosis of microvascular dysfunction based on a CFR of <2.5 [32]. The WISE investigators examined the prevalence of coronary microvascular disease in 159 women with chest pain and non-obstructive disease. They found that coronary micro-vascular dysfunction was present in one-half of the subjects studied on the basis of a reduced coronary flow velocity reserve [16]. Wessel et al. found that traditional risk factors for atherosclerosis, other than increasing age, did not reliably predict microvascular dysfunction in women [33]. Coronary reactivity testing was crucial to establish the diagnosis of CMD in these subjects.
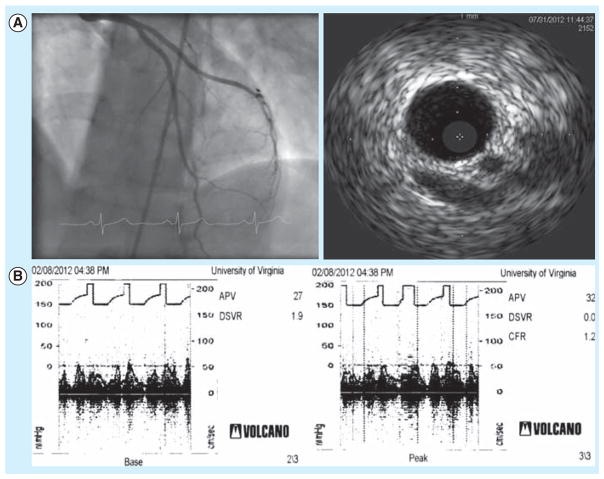
Figure 1. 49-year-old woman with chest pain.
(A) Cardiac catheterization (left) showed minimal disease in left anterior descending artery. Intravascular ultrasound (right) showed 15% plaque burden in the left anterior descencing artery. (B) Coronary flow reserve (CFR) testing for patient in (A) as performed with intracoronary Doppler blood flow velocity waveforms before adenosine (left), and after adenosine infusion (right). CFR is the ratio of average peak velocities before and after adenosine. The measured CFR was 1.2 and suggestive of coronary microvascular dysfunction in this patient.
Intracoronary nitroglycerin injection is also used to detect non-endothelial dependent macrovascular function as part of coronary reactivity testing. Since the coronary microcirculation lacks the enzyme needed to convert nitroglycerin to its active form, nitric oxide, nitroglycerin produces a dose-related dilation of coronary vessels >200 μm in diameter and hence has no effect on smaller coronary vessels [26]. Normal nitroglycerin response is defined as a diameter increase >20% [34]. Subjects with CMD do not show a difference in the increase in coronary blood flow response to nitro-glycerin as compared to normal controls [13].
Endothelin/inflammatory biomarkers
Imbalance between the endothelial vasodilator nitric oxide and vasoconstrictor endothelin-1 (ET-1) has been implicated in the pathogenesis of CMD. Lanza et al. detected a significant increase in ET-1 levels in subjects with CMD at baseline and post-atrial pacing vs control subjects [35]. Similar findings were seen in other studies with higher levels of ET-1 detected in subjects with CMD as compared to controls [11,36]. Blunted levels of nitric oxide were also seen along with elevated ET-1 levels in subjects with CMD as compared with controls [36]. Higher ET-1 levels in patients with syndrome X might cause an increase in coronary microvascular tone at rest by a direct vasoconstrictor effect and also by sensitization of small coronary arteries to catecholamines. ET-1 might also affect the abnormal coronary flow response to acetylcholine in CMD patients. This was evidenced by studies that showed an increase in ET-1 levels post-acetylcholine administration in patients with coronary endothelial dysfunction [37].
Inflammation is another possible underlying mechanism for CMD. Tousoulis et al. measured plasma levels of soluble vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) in women with CMD and found elevated levels of both as compared to controls [38]. However, others did not find any difference in the levels of inflammatory biomarkers such as C-reactive protein (CRP), IL-6, IL-18, TNF-α, TGF-β1 and ICAM-1 in subjects with CMD as compared to controls [39]. Elevated homocysteine levels linked with the C677T mutation in the methylenetetrahydrofolate reductase gene were noted in women with CMD over controls in one study, suggesting a role of homocysteine metabolism in endothelial cell dysfunction [40]. A recent study evaluated 21 subjects with CMD and detected increased levels of CRP in those subjects with CMD and reduced CFR as compared to controls [41]. While data exists to suggest that inflammation may play a role in the pathogenesis of CMD, larger studies are needed to validate this observation.
Non-invasive testing
Non-invasive evaluation of coronary circulation includes contrast echocardiography, PET, CMR and SPECT.
Echocardiography
Initial evaluation of patients with syndrome X or CMD included exercise stress testing. Abnormal response to exercise stress testing was part of the original diagnostic criteria for CMD. However, Cannon et al. showed that exercise testing can fail to detect patients with CMD [42]. Panza et al. studied 70 patients (44 women) with chest pain and normal angiograms who underwent exercise treadmill testing, radionuclide angiography at rest and during exercise, thallium stress testing and transesophageal dobutamine stress echocardiography. They noted that there was no concordance between the tests regarding the presence of ischemia and wall motion abnormalities. Despite the presence of chest pain and ECG abnormalities, no wall motion abnormalities were detected, highlighting the difficulty in diagnosing CMD [43]. Similar findings were reported in a study examining 33 patients (14 women) with chest pain and normal coronaries with perfusion defects on thallium SPECT undergoing dobutamine stress echocardiography (DSE) [44]. None of the patients developed regional wall abnormalities on DSE despite the high prevalence of perfusion defects on SPECT, the presence of chest pain and ST segment depression. The authors concluded that DSE might be insensitive to ischemia caused by microvascular dysfunction.
Vinereanu et al. used adenosine stress echocardiography in nine patients (eight women) with CMD [45]. They noted that all patients had global and regional diastolic dysfunction following adenosine infusion but suggested that their pilot study needed confirmation in a larger series of patients. Measurement of coronary flow velocity reserve (CFVR) using transthoracic echocardiography with adenosine or dipyridamole infusion has been validated in small studies against coronary angiography [46] or PET [47]. Sade et al. measured CFVR using transthoracic echo in 68 women with chest pain and normal angiograms and found impaired CFVR (<2.0 by definition) in 28 women [48]. They found that impaired CFVR correlated closely with epicardial fat thickness also measured by echocardiography. Another study measured coronary microvascular vasodilatation in response to adenosine and to CPT in 71 patients with CMD (48 women) using transthoracic echocardiography [49]. They also noted diminished responses to adenosine and CPT in CMD patients as compared to controls. Myocardial contrast echocardiography has also been used to detect perfusion defects and evaluate the CFR in patients with CMD. Galiuto et al. evaluated the use of both transthoracic and myocardial echocardiography in measuring CFR and myocardial blood flow respectively, following adenosine infusion in 17 subjects with CMD (11 women) and 17 controls [50]. CFR as measured in the LAD was lower in CMD patients as compared to controls and myocardial blood flow ratio using myocardial contract echocardiography was significantly lower in subjects with CMD than in controls. Rinkevich et al. also measured myocardial blood flow reserve using myocardial contrast echocardiography in 18 women with CMD as compared to age matched controls and found impaired myocardial blood flow reserve in subjects with CMD [51]. While studies measuring myocardial blood flow reserve or CFVR using echocardiography show promising results, these are single-center, small sample studies that require validation in larger populations.
SPECT imaging
SPECT imaging measures the relative distribution of myocardial blood flow at rest and stress. In one of the earliest studies to evaluate CMD, Fragasso et al. studied myocardial perfusion in 25 subjects (18 women) with CMD using stress-redistribution thallium-201 SPECT as compared to age matched controls. They found perfusion abnormalities in 97% of patients, with reverse redistribution in a significant proportion of patients which they suggested was due to inhomogeneous perfusion [52]. Another study examining myocardial function and perfusion using technetium-99m methoxy-isobutyl-isonitrile (99mTc-MIBI) gated-SPECT (exercise-rest protocol using an exercise bicycle) in 59 post-menopausal women with angina and normal coronaries found perfusion defects in approximately 35% of patients [53]. Such patients were more likely to also have reduced post-stress left ventricular ejection fraction (LVEF) and endothelial dysfunction as measured by brachial artery ultrasound. However the use of SPECT imaging to detect abnormal perfusion in CMD patients, especially women, is limited for the following reasons: false positives due to breast tissue and obesity, missing smaller areas of perfusion defects in comparatively smaller hearts due to limitations in spatial resolution with SPECT and a failure to detect global reduction in myocardial perfusion as SPECT perfusion deficits are identified by regional differences in blood flow [54].
PET imaging
PET measures absolute myocardial blood flow in units of ml blood/min/g myocardium and can quantify perfusion, thus detecting variations at the level of the coronary microcirculation in patients without obstructive CAD (Figure 2). Geltman et al. studied 17 patients (four women) with anginal symptoms and normal coronaries and 16 normal controls using PET imaging with oxygen-15-labeled water (H215O) and oxygen-15-labeled carbon monoxide (C15O) before and after intravenous dipyridamole infusion [55]. Regional myocardial perfusion and perfusion reserve was calculated in all subjects. Eight of the 17 patients had an impaired myocardial perfusion reserve of <2.5. In the patients with impaired perfusion reserve, perfusion at rest was significantly higher than that of normal subjects and maximal flow and perfusion reserve were significantly reduced. WISE study investigators studied 34 women with chest pain and no significant CAD and nine female control subjects who underwent 13N-NH3 PET to measure adenosine-induced changes in myocardial perfusion [6]. They noted that 25 patients (74%) had impaired CFR in at least one coronary artery territory. Microvascular dysfunction in these subjects was heterogeneous with discordance of microvascular function among different coronary artery territories. They felt that assessment of the microcirculation in all three coronary artery territories was essential in women with angina and normal coronaries to detect CMD.
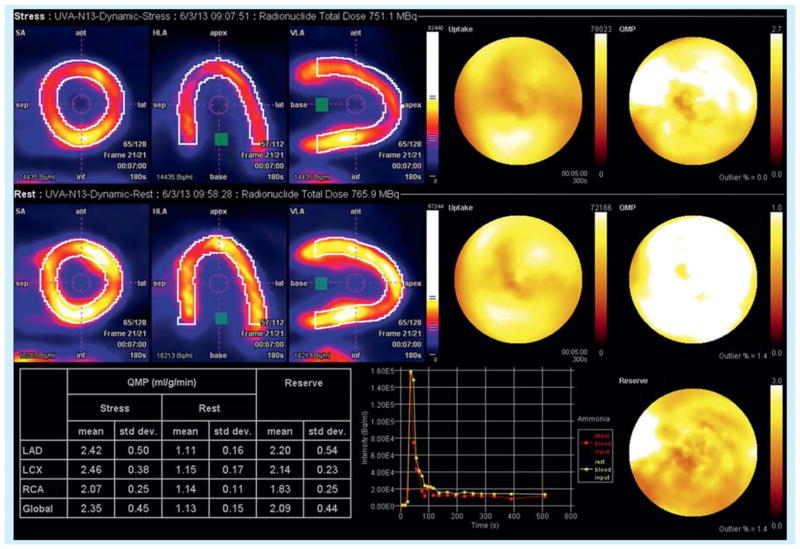
Figure 2. 56-year-old woman with recurrent angina, positive stress test and multiple cardiac catheterizations showing minimal coronary artery disease.
13N-NH3 PET perfusion imaging shows impaired increase in global perfusion post-adenosine administration. Measured coronary flow reserve is 2.09.
Further insight into the pathophysiology of CMD was provided by Satake et al., who noted increased myocardial 18F fluoro-deoxyglucose (FDG) uptake in 24 subjects (17 women) with CMD undergoing FDG PET as compared to 11 controls [56]. Endomyocardial biopsy in these subjects revealed significant increase in smooth muscle cells and thickening of the vascular wall, even in capillary vessels and the small vessel lumen was markedly narrowed. None of these findings were noted in the biopsy specimens of control subjects confirming the nature of small vessel disease in patients with CMD. Furthermore, investigators have shown that measuring rest/stress myocardial perfusion with 82Rb PET aided in the detection of microvascular dysfunction in 1034 subjects [57]. They noted that measuring heterogeneity in resting myocardial perfusion detected microvascular dysfunction in those subjects. While measurement of CFR with PET is an established method for detection of CMD and has been used to monitor response to various therapies [58], its limited availability has hampered its usage in this setting.
Cardiac magnetic resonance
The earliest studies used 31P-NMR spectroscopy, a technique which measures myocellular creatine phosphate (PCr), ATP, inorganic phosphate (Pi) and pH. The PCr:ATP ratio is a sensitive and specific marker for ischemia. Investigators enrolled 35 women with chest pain and normal coronaries and 12 age-matched, weight-matched controls to undergo 31P-NMR spectroscopy [59]. Myocardial high-energy phosphates were measured at 1.5 Tesla before, during, and after isometric handgrip exercise and the change in the ratio of phosphocreatine to ATP during exercise was measured. Seven (20%) of the 35 women had decreases in the PCr: ATP ratio during handgrip that were more than 2 SD below the mean value in the control subjects, demonstrating evidence of an abnormal metabolic response to handgrip exercise in these women.
CMR has been recently used to quantify myocardial perfusion and detect reduced myocardial perfusion in patients with CMD (Figure 3). Figure 3A demonstrates an epicardial to endocardial gradient and on the right a perfusion map generated using a high resolution spiral pulse sequence [60]. Use of CMR myocardial perfusion to study CMD was first employed by Panting et al., who determined myocardial-perfusion by CMR imaging in 20 patients with CMD and ten matched controls, both at rest and during an infusion of adenosine [61]. Myocardial perfusion index was measured in all subjects. The investigators noted that in controls subjects the myocardial perfusion index increased in both the subendocardium and subepicardial layers with adenosine administration. In patients with CMD, the myocardial perfusion index did not change significantly post adenosine administration in the subendocardium as compared with controls, but increased in the subepicardium similar to controls. They noted that subjects with CMD had subendocardial hypoperfusion in response to stress as compared to control subjects. Another study compared the perfusion defects seen in CMD subjects following stress CMR with dobutamine with the measured CFVR in the respective coronary artery territory after adenosine administration using transthoracic echocardiography [62]. The investigators observed a significant correlation between the dobutamine-induced myocardial perfusion defects on CMR and reduced CFVR in the LAD coronary artery territory in CMD subjects thus demonstrating that microcirculatory dysfunction had resulted in perfusion defects in those patients.
Figure 3. 57-year-old woman with persistent chest pain and non-obstructive coronary artery disease on cardiac catheterization. Stress cardiac magnetic resonance was performed.
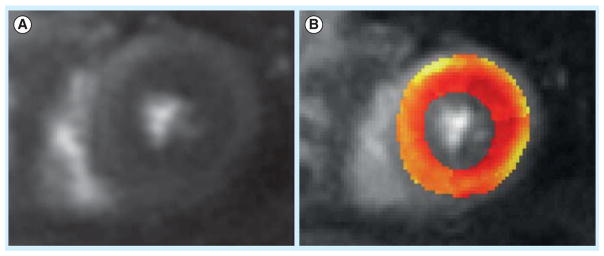
(A) Mid-ventricular short axis view containing both papillary muscles. (B) Perfusion image of the same short axis with a superimposed perfusion map with color coded areas. Red signifies decreasing blood flow all the way to yellow, which shows maximum perfusion. This patient has reduced subendocardial and a normal increase in epicardial perfusion following adenosine infusion. Calculated coronary flow reserve in this case was 1.42 and suggestive of coronary microvascular dysfunction (color figure can be found online at: www.expert-reviews.com/full/doi/10.1586/14779072.2013.833854).
Velmefoort et al. studied the use of perfusion by CMR imaging in 20 subjects (15 women) with anginal symptoms and chest pain [63]. They found no evidence of subendocardial hypoperfusion but uniform increase in myocardial perfusion index in both myocardial layers in all subjects. They suggested that the relatively small sample size and presence of frequent subendocardial artifacts could have adversely affected their results. Similar findings were reported by Karamitsos et al., who assessed quantitative perfusion and regional myocardial oxygenation in 18 subjects (15 women) with CMD as compared to 14 controls [64]. They found no differences in myocardial perfusion or oxygenation between the two groups. Increased sensitivity to chest pain following adenosine administration was the only differentiating characteristic of the CMD group. Quantitative perfusion by CMR with newer sequences that have improved spatial and temporal resolution may be able to better evaluate CMD in patients, but currently invasive coronary reactivity remains the gold standard.
Peripheral endothelial testing
Since coronary endothelial dysfunction has been observed in patients with CMD, non-invasive measures of endothelial function have also been utilized in the diagnosis of this condition. Lekakis et al. found that flow-mediated dilatation of the brachial artery measured by Doppler ultrasonography was comparable in subjects with CMD and those with coronary stenosis, but was significantly lower in CMD patients when compared with age-matched controls [65]. The study provided further evidence that endothelial dysfunction in patients with microvascular angina is not confined to the coronary micro-circulation but also extends to large peripheral conduit arteries. Pulse wave velocity (PWV), a measure of arterial stiffness, was found to be increased in subjects with CMD as compared to controls and was associated with a decrease in endothelium-dependent vasodilatation in CMD subjects when compared to controls [66]. Increased PWV in CMD subjects compared to controls has also been described in other studies [67].
Impaired skin microvascular function as measured by laser Doppler imaging has been observed in women with CMD when compared to controls [68]. Finally Matsuzawa et al. evaluated endothelial function by peripheral arterial tonometry in 158 post-menopausal women with obstructive CAD, non-obstructive CAD and controls [69]. Peripheral arterial tonometry is measured by the Endo-PAT 2000 device which is a non-invasive, automatic and quantitative clinical test for digital measurement of hyperemic response. The device measures hyperemia in the digits of the upper arm post-cuff occlusion of the upper arm for 5 min. They found that the reactive hyperemia index was attenuated markedly in both obstructive and non-obstructive CAD patients as compared to controls.
Reactive hyperemia index by peripheral tonometry is another non-invasive method to detect the presence of obstructive and non-obstructive CAD though it did not differentiate between the two groups in this study. Methods of peripheral endothelial function such as pulse wave velocity and peripheral arterial tonometry are newer methods that play a largely supportive role in detecting CMD. However larger studies are needed to confirm the usefulness of these parameters in making a diagnosis of CMD.
Prognosis
While some studies show that the presence of CMD is associated with a benign prognosis [70], other studies suggest otherwise [71]. Schächinger et al. studied 42 women who had a vasoconstrictor response to intracoronary acetylcholine suggestive of CMD and found that 30% of these patients developed CAD over a 10-year follow-up period [72]. Suwaidi et al. followed patients with CMD based on vasoconstrictor response to intracoronary acetylcholine and found that patients with a severe vasoconstrictor response were at highest risk of cardiovascular events over a period of 28 months [73]. Vasoconstrictor response to intracoronary acetylcholine has been independently linked to increased cardiovascular events in recent studies [74]. WISE investigators followed women with CMD based on reduced CFR in response to intracoronary adenosine and found that reduced CFR (<2.3) was independently linked with adverse cardiovascular events in those patients [14]. However the WISE study was limited by several factors including relative lack of endothelium-independent microvascular testing, fewer major adverse events and enrollment of relatively fewer women. The overall prognosis of MCD is unclear though recent studies have suggested an increased risk of cardiovascular events in this patient population.
Summary
In our view at the present time a diagnosis of CMD can be made if there are typical symptoms of chest pain, with or without presence of ischemia on non-invasive testing, normal to minimal coronary disease on coronary angiography and any one of the following: a) abnormal reactive response to intracoronary acetylcholine testing; b) reduced CFR in response to intracoronary adenosine; or c) a reduced CFR in response to adenosine on PET scanning. Newer techniques such as CMR perfusion have shown promise in the detection of CMD but require larger studies for validation of its use in this population.
Conclusions
CMD is a common condition seen in as many as 50% of women with anginal symptoms and normal coronaries on coronary angiography. Various pathophysiological mechanisms have been proposed for this condition. Currently, the gold standard for the diagnosis of CMD requires the exclusion of obstructive CAD by coronary angiography, followed by evaluation of microvascular coronary function by Doppler guide wire in the cardiac catheterization laboratory for endothelial function testing in response to intracoronary acetylcholine and CFR testing in response to adenosine by coronary reactivity testing. Non-invasive imaging methods such as PET imaging and CMR hold promise for detection of CMD. Since recent studies suggest that CMD is associated with an increased risk of cardiovascular events, diagnosis of this condition especially in women may offer an opportunity for early therapeutic interventions, leading to a reduction in cardiovascular events in the future.
Expert commentary
CMD is a cause of significant morbidity especially in women presenting to the emergency department with chest pain. Hence, prompt diagnosis of this condition is essential in order to better manage such patients. Invasive coronary reactivity testing during coronary angiography with intracoronary acetylcholine and adenosine remains the current standard for diagnosis. However quantitative perfusion assessment by PET is an effective alternative in making the diagnosis. The development of sequences with newer and improved temporal and spatial resolution makes stress CMR also an attractive noninvasive imaging option to efficiently detect CMD.
Five-year view
Further understanding of the pathophysiology of CMD and its prognosis is likely over the next few years given the various techniques available to study this condition. Larger studies will be performed to estimate the prevalence of this condition in the general population. Safe and effective diagnosis of CMD will likely be made by non-invasive techniques such as stress CMR. These diagnostic techniques will also be used to assess response to therapy for CMD.
Key issues.
Coronary microvascular dysfunction (CMD), also known as syndrome X, is characterized by typical anginal symptoms, evidence of myocardial ischemia on non-invasive testing and normal to minimal coronary disease on coronary angiography.
It is seen commonly in women, with studies showing that up to 50% of women presenting with anginal symptoms have CMD.
Several pathophysiological mechanisms have been proposed to explain the microvascular dysfunction that occurs in the coronary vascular system.
Diagnosis of CMD involves demonstration of microvascular dysfunction either invasively or noninvasively.
Invasive testing of CMD is the current gold standard for diagnosis and involves the use of quantitative coronary angiography to evaluate changes in coronary vessel wall diameter in response to vasodilators such as adenosine and acetylcholine.
The current definition for CMD requires <2.5-fold increase in coronary volumetric blood flow on intracoronary angiography in response to adenosine.
PET imaging is an established method to detect CMD and a coronary flow reserve of <2.5 has been shown in studies to be diagnostic of CMD.
Stress CMR is also a newer imaging method that holds considerable promise for the effective diagnosis of CMD.
Current evidence suggests that the diagnosis of CMD is not benign and is associated with worsening cardiovascular risk. Larger studies are needed to further validate this finding.
Footnotes
Financial & competing interests disclosure
C Kramer receives research equipment support from Siemens Healthcare and is a consultant for Synarc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Phan A, Shufelt C, Merz CN. Persistent chest pain and no obstructive coronary artery disease. JAMA. 2009;301(14):1468–1474. doi: 10.1001/jama.2009.425. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RO, 3rd, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61(15):1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 4•.Jones E, Eteiba W, Merz NB. Cardiac syndrome X and microvascular coronary dysfunction. Trends Cardiovasc Med. 2012;22(6):161–168. doi: 10.1016/j.tcm.2012.07.014. Study exploring the mechanistic pathways underlying MHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36(8):291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marroquin OC, Holubkov R, Edmundowicz D, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J. 2003;145(4):628–635. doi: 10.1067/mhj.2003.95. [DOI] [PubMed] [Google Scholar]
- 7.Opherk D, Zebe H, Weihe E, et al. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981;63(4):817–825. doi: 10.1161/01.cir.63.4.817. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Farb A, Malcom GT, et al. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97(21):2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 9.Lanza GA, Giordano A, Pristipino C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I] metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96(3):821–826. doi: 10.1161/01.cir.96.3.821. [DOI] [PubMed] [Google Scholar]
- 10.Gulli G, Cemin R, Pancera P, et al. Evidence of parasympathetic impairment in some patients with cardiac syndrome X. Cardiovasc Res. 2001;52(2):208–216. doi: 10.1016/s0008-6363(01)00369-8. [DOI] [PubMed] [Google Scholar]
- 11.Cox ID, Botker HE, Bagger JP, et al. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol. 1999;34(2):455–460. doi: 10.1016/s0735-1097(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 12.Cannon RO. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54(10):877–885. doi: 10.1016/j.jacc.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egashira K, Inou T, Hirooka Y, et al. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med. 1993;328(23):1659–1664. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 14•.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. doi: 10.1016/j.jacc.2010.01.054. Study showing the adverse prognosis associated with MHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeiher AM, Drexler H, Wollschläger H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84(5):1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 16•.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. Study demonstrating the high prevalence of MHD in women. [DOI] [PubMed] [Google Scholar]
- 17.Huang PH, Chen YH, Chen YL, et al. Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart. 2007;93(9):1064–1070. doi: 10.1136/hrt.2006.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen SD, Paulesu E, Wise RJ, Camici PG. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart. 2002;87(6):513–519. doi: 10.1136/heart.87.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadhav S, Ferrell W, Greer IA, et al. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48(5):956–963. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 20.Cosin-Sales J, Pizzi C, Brown S, Kaski JC. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J Am Coll Cardiol. 2003;41(9):1468–1474. doi: 10.1016/s0735-1097(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 21.Pepine CJ, Kerensky RA, Lambert CR, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47(3 Suppl):S30–S35. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 23.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 24.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121(21):2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 25.Campisi R. Noninvasive assessment of coronary microvascular function in women at risk for ischemic heart disease. Int J Clin Pract. 2008;62(2):300–307. doi: 10.1111/j.1742-1241.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 26.Hasdai D, Cannan CR, Mathew V, Holmes DR, Jr, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53(3):203–208. doi: 10.1016/0167-5273(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 27.Mohri M, Koyanagi M, Egashira K, et al. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351(9110):1165–1169. doi: 10.1016/S0140-6736(97)07329-7. [DOI] [PubMed] [Google Scholar]
- 28.Ong P, Athanasiadis A, Mahrholdt H, et al. Increased coronary vasoconstrictor response to acetylcholine in women with chest pain and normal coronary arteriograms (cardiac syndrome X) Clin Res Cardiol. 2012;101(8):673–681. doi: 10.1007/s00392-012-0442-4. [DOI] [PubMed] [Google Scholar]
- 29.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77(1):43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 30.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84(5):1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 31.Klocke FJ. Measurements of coronary flow reserve: defining pathophysiology versus making decisions about patient care. Circulation. 1987;76(6):1183–1189. doi: 10.1161/01.cir.76.6.1183. [DOI] [PubMed] [Google Scholar]
- 32•.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33(6):1469–1475. doi: 10.1016/s0735-1097(99)00072-8. WISE study evaluating the normal cut-offs for CFR and CFVR testing in women. [DOI] [PubMed] [Google Scholar]
- 33.Wessel TR, Arant CB, McGorray SP, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Clin Cardiol. 2007;30(2):69–74. doi: 10.1002/clc.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5(6):646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanza GA, Lüscher TF, Pasceri V, et al. Effects of atrial pacing on arterial and coronary sinus endothelin-1 levels in syndrome X. Am J Cardiol. 1999;84(10):1187–1191. doi: 10.1016/s0002-9149(99)00532-9. [DOI] [PubMed] [Google Scholar]
- 36.Piatti P, Fragasso G, Monti LD, et al. Endothelial and metabolic characteristics of patients with angina and angiographically normal coronary arteries: comparison with subjects with insulin resistance syndrome and normal controls. J Am Coll Cardiol. 1999;34(5):1452–1460. doi: 10.1016/s0735-1097(99)00379-4. [DOI] [PubMed] [Google Scholar]
- 37.Lerman A, Holmes DR, Bell MR, Garratt KN, Nishimura RA, Burnett JC. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92(9):2426–2431. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 38.Tousoulis D, Davies GJ, Asimakopoulos G, et al. Vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 serum level in patients with chest pain and normal coronary arteries (syndrome X) Clin Cardiol. 2001;24(4):301–304. doi: 10.1002/clc.4960240409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kip KE, Marroquin OC, Shaw LJ, et al. Global inflammation predicts cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Am Heart J. 2005;150(5):900–906. doi: 10.1016/j.ahj.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Alroy S, Preis M, Barzilai M, et al. Endothelial cell dysfunction in women with cardiac syndrome X and MTHFR C677T mutation. Isr Med Assoc J. 2007;9(4):321–325. [PubMed] [Google Scholar]
- 41.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome x patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6(6):660–667. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Cannon RO, 3rd, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85(3):883–892. doi: 10.1161/01.cir.85.3.883. [DOI] [PubMed] [Google Scholar]
- 43.Panza JA, Laurienzo JM, Curiel RV, et al. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29(2):293–301. doi: 10.1016/s0735-1097(96)00481-0. [DOI] [PubMed] [Google Scholar]
- 44.Zouridakis EG, Cox ID, Garcia-Moll X, et al. Negative stress echocardiographic responses in normotensive and hypertensive patients with angina pectoris, positive exercise stress testing, and normal coronary arteriograms. Heart. 2000;83(2):141–146. doi: 10.1136/heart.83.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinereanu D, Fraser AG, Robinson M, Lee A, Tweddel A. Adenosine provokes diastolic dysfunction in microvascular angina. Postgrad Med J. 2002;78(915):40–42. doi: 10.1136/pmj.78.915.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32(5):1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- 47.Saraste M, Koskenvuo J, Knuuti J, et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol. 2001;21(1):114–122. doi: 10.1046/j.1365-2281.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 48.Sade LE, Eroglu S, Bozbas H, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204(2):580–585. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 49.Sestito A, Lanza GA, Di Monaco A, et al. Relation between cardiovascular risk factors and coronary microvascular dysfunction in cardiac syndrome X. J Cardiovasc Med. 2011;12(5):322–327. doi: 10.2459/JCM.0b013e3283406479. [DOI] [PubMed] [Google Scholar]
- 50.Galiuto L, Sestito A, Barchetta S, et al. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99(10):1378–83. doi: 10.1016/j.amjcard.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 51.Rinkevich D, Belcik T, Gupta NC, et al. Coronary autoregulation is abnormal in syndrome X: insights using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2013;26(3):290–296. doi: 10.1016/j.echo.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fragasso G, Rossetti E, Dosio F, et al. High prevalence of the thallium-201 reverse redistribution phenomenon in patients with syndrome X. Eur Heart J. 1996;17(10):1482–1487. doi: 10.1093/oxfordjournals.eurheartj.a014710. [DOI] [PubMed] [Google Scholar]
- 53.Peix A, Garcia EJ, Valiente J, et al. Ischemia in women with angina and normal coronary angiograms. Coron Artery Dis. 2007;18(5):361–366. doi: 10.1097/MCA.0b013e3281689a3f. [DOI] [PubMed] [Google Scholar]
- 54.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl):S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 55•.Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol. 1990;16(3):586–595. doi: 10.1016/0735-1097(90)90347-r. Study that first evaluated the use of PET imaging to detect MHD in women. [DOI] [PubMed] [Google Scholar]
- 56.Osamichi S, Kouji K, Yoshimaro I, et al. Myocardial glucose metabolism assessed by positron emission tomography and the histopathologic findings of microvessels in syndrome X. Circ J. 2004;68(3):220–226. doi: 10.1253/circj.68.220. [DOI] [PubMed] [Google Scholar]
- 57.Johnson NP, Gould KL. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med. 2005;46(9):1427–1437. [PubMed] [Google Scholar]
- 58.Campisi R, Nathan L, Pampaloni MH, et al. Noninvasive assessment of coronary microcirculatory function in postmenopausal women and effects of short-term and long-term estrogen administration. Circulation. 2002;105(4):425–430. doi: 10.1161/hc0402.102860. [DOI] [PubMed] [Google Scholar]
- 59.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342(12):829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 60.Salerno M, Sica C, Kramer CM, Meyer CH. Improved first-pass spiral myocardial perfusion imaging with variable density trajectories. Magn Reson Med. 2012 doi: 10.1002/mrm.24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948–1953. doi: 10.1056/NEJMoa012369. First study to evaluate the use of stress CMR to detect abnormal myocardial perfusion in women with MHD. [DOI] [PubMed] [Google Scholar]
- 62.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51(4):466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 63.Vermeltfoort IA, Bondarenko O, Raijmakers PG, et al. Is subendocardial ischemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007;28(13):1554–1558. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 64•.Karamitsos TD, Arnold JR, Pegg TJ, et al. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3-T cardiovascular magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5(2):194–200. doi: 10.1161/CIRCIMAGING.111.969667. Study that first used a fully quantitative method of CMR imaging to detect MHD in women. [DOI] [PubMed] [Google Scholar]
- 65.Lekakis JP, Papamichael CM, Vemmos CN, Voutsas AA, Stamatelopoulos SF, Moulopoulos SD. Peripheral vascular endothelial dysfunction in patients with angina pectoris and normal coronary arteriograms. J Am Coll Cardiol. 1998;31(3):541–546. doi: 10.1016/s0735-1097(97)00542-1. [DOI] [PubMed] [Google Scholar]
- 66.Kidawa M, Krzeminska-Pakula M, Peruga JZ, Kasprzak JD. Arterial dysfunction in syndrome X: results of arterial reactivity and pulse wave propagation tests. Heart. 2003;89(4):422–426. doi: 10.1136/heart.89.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arroyo-Espliguero R, Mollichelli N, Avanzas P, et al. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur Heart J. 2003;24(22):2006–2011. doi: 10.1016/j.ehj.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 68.Jadhav ST, Ferrell WR, Petrie JR, et al. Microvascular function, metabolic syndrome, and novel risk factor status in women with cardiac syndrome X. Am J Cardiol. 2006;97(12):1727–1731. doi: 10.1016/j.amjcard.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 69.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55(16):1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki H, Matsubara H, Koba S, et al. Clinical characteristics and follow-up in patients with microvascular angina. Circ J. 2002;66(7):691–695. doi: 10.1253/circj.66.691. [DOI] [PubMed] [Google Scholar]
- 71.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998;148(10):958–966. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 72.Schlächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 73.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 74••.Von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. Important WISE study showing the adverse prognosis associated with MHD. [DOI] [PubMed] [Google Scholar]