Summary
Co-evolution of beneficial microorganisms with the mammalian intestine fundamentally shapes mammalian physiology. Herein we report that the intestinal microbe Bacteroides fragilis modifies the homeostasis of host invariant natural killer T (iNKT) cells by supplementing the host’s endogenous lipid antigen milieu with unique inhibitory sphingolipids. The process occurs early in life and effectively impedes iNKT cell proliferation during neonatal development. Consequently, total colonic iNKT cell numbers are restricted into adulthood and hosts are protected against experimental iNKT cell–mediated, oxazolone-induced colitis. In studies with neonatal mice lacking access to bacterial sphingolipids, we found that treatment with B. fragilis glycosphingolipids—exemplified by an isolated peak (M.W.=717.6) called GSL-Bf717—reduces colonic iNKT cell numbers and confers protection against oxazolone-induced colitis in adulthood. Our results suggest that the distinctive inhibitory capacity of GSL-Bf717 and similar molecules may prove useful in the treatment of autoimmune and allergic disorders in which iNKT cell activation is destructive.
Introduction
As humans, we require symbiotic microbes to establish and maintain health. Microbes equipped with beneficial properties are preferentially granted membership in our intestinal community. Understanding the specific molecules and immune mechanisms used by microbes to elicit their beneficial phenotype is a key step towards informed use of the microbiota to help resolve many health issues (Bäckhed et al., 2005; Chow et al., 2010; Honda and Littman, 2012). Currently, these molecules and mechanisms remain largely unknown. One exception to this dearth of knowledge on the contribution of specific microbial products to the host immune system is the body of literature on polysaccharide A (PSA) (Mazmanian et al., 2005; Mazmanian et al., 2008; Round et al., 2011) produced by the common intestinal symbiont Bacteroides fragilis.
Symbiotic bacteria possess enigmatic phenotypes that are unusual in environmental bacterial species and pathogens. For example, bacterial sphingolipids are produced predominantly by species in the phylum Bacteroidetes (Ingar and Erik, 2001; Kato et al., 1995), many of which are associated almost exclusively with mammalian hosts. The only identified function of bacterial sphingolipids is the activation of invariant natural killer T (iNKT) cells by glycosphingolipids produced by certain soil-dwelling Sphingomonas species in the phylum Proteobacteria—one of only a few known sphingolipid producers outside the Bacteroidetes (Kinjo et al., 2005; Mattner et al., 2005). iNKT cells recognize non-polymorphic major histocompatibility complex class I–like, CD1d protein–presented lipid antigens, of which the best studied are glycosphingolipids (Cohen et al., 2009). With their remarkable ability to quickly release high levels of cytokines upon activation (Kronenberg, 2005; Matsuda et al., 2008), iNKT cells are critical players in innate and adaptive immunity. Previously, our group demonstrated that specific pathogen–free (SPF) mice had lower iNKT cell numbers in the colonic lamina propria (LP) than did germ-free (GF) mice; accordingly, SPF mice were protected from experimental iNKT cell–mediated, oxazolone-induced colitis, whereas GF mice were not (Olszak et al., 2012). These results suggested that sphingolipids produced by symbiotic bacteria might play an important role in host colonic iNKT cell homeostasis and in the oxazolone colitis susceptibility phenotype.
Results
B. fragilis sphingolipids modulate host colonic iNKT cell homeostasis and protect the host from a colitis challenge
In the model organism B. fragilis NCTC 9343, the enzyme encoded by gene BF2461 has a high degree of homology (E values ≤−44 by standard BLASTP search) (Altschul, 2005) with the eukaryotic enzyme serine palmitoyltransferase (SPT). SPT, the first committed enzyme in sphingolipid biosynthesis, produces 3-ketosphinganine from palmitoyl-CoA and serine (Lowther et al., 2012). We knocked out gene BF2461 from wild-type B. fragilis NCTC 9343 (BFWT) to create a mutant strain BFΔSPT, and we complemented this mutant with a full copy of BF2461 in trans (C-delta). We found the BFWT and BFΔSPT in vitro growth kinetics were generally comparable although BFΔSPT had a slightly longer doubling time (64±0 min vs. 74±1 min, Fig. S1A). Using thin-layer chromatography, we compared lipid extracts from BFWT and BFΔSPT strains and identified several spots that were present in the former but lacking in the latter. We further treated the two samples with mild alkaline hydrolysis to differentiate sphingolipids from phospholipids, the latter being the most common components of bacterial lipid membranes. The spots that were unique to the BFWT strain were indeed sphingolipids, as determined by their resistance to hydrolysis; in comparison, the spots that were present in both strains were hydrolyzed after treatment, a result suggesting that these spots were phospholipids. C-delta conferred the wild-type profile of sphingolipid generation (Fig. S1B).
After mono-colonizing GF mice with either BFWT bacteria (termed BFWT mice) or BFΔSPT bacteria (termed BFΔSPT mice), we monitored absolute and relative numbers of iNKT cells in their pups’ colonic LP from birth to 9 weeks of age as well as in age-matched GF and SPF mice (Figs. 1A–1C). We found that iNKT cells were absent from the colon in all mice at birth but then were present in numbers that gradually increased until reaching steady state at the age of 6 weeks. However, the relative (to CD3+ T cells) and absolute numbers of iNKT cells in GF and BFΔSPT mice were significantly higher than those in SPF and BFWT mice, despite lower cell numbers in BFΔSPT mice than in GF mice. We also found that colonic LP CD3+ T cell numbers were similar in GF, BFWT and BFΔSPT mice (Fig. S1C). These results suggest that bacterial sphingolipids from a single microbe, B. fragilis, negatively regulate iNKT cell numbers in the colon, although we do not know whether colonic iNKT cells from the BFWT and BFΔSPT mice are functionally similar, e.g., the same capacity for cytokine production upon activation. In addition, C-delta-mono-associated mice had colonic iNKT cell numbers similar to those in BFWT mice (Fig. S1D).
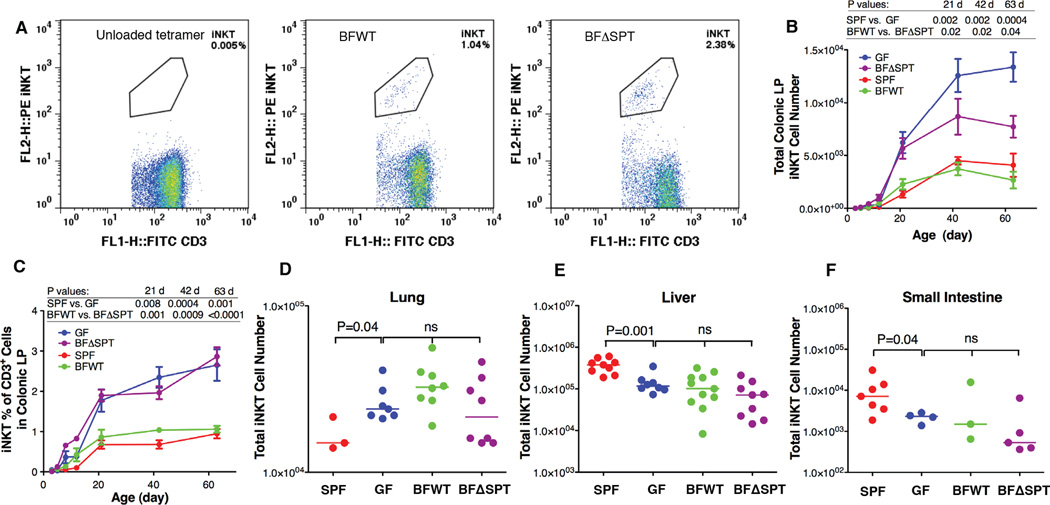
Figure 1.
B. fragilis sphingolipids modulate homeostasis of colonic LP iNKT cells. Representative FACS plots of iNKT cell gating are shown in (A). Total numbers of colonic LP iNKT cells (B) and their percentages in CD3+ populations (C) were higher in GF and BFΔSPT than in SPF and BFWT mice. B. fragilis mono-colonized mice had iNKT cell counts similar to those of GF mice in lung (D), liver (E) and small intestine (F). Data in panels (B) and (C) (days 21,42 and 63) were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM (standard error of the mean); n≥3 for each group. Data in panels (D)–(F) were analyzed by the Mann Whitney test. See also Fig. S1.
In previous comparisons of iNKT cell numbers in SPF and GF mice, we found that SPF animals had higher numbers in the thymus, spleen, and liver but lower counts in the colon and lung (Olszak et al., 2012). In the current studies, we found that, despite iNKT cell number differences in the colon, BFWT mice did not differ from BFΔSPT mice, or GF mice, in terms of iNKT cell numbers in the lung, liver, small intestine, thymus, spleen, or Peyer’s patches (Figs. 1D–1F and Figs. S1E–S1G). These results indicate that B. fragilis sphingolipids exert effects on iNKT cells only in the colon, where this bacterium is most abundant. A possible reason for the local effect of the B. fragilis glycosphingolipids in these mice is that their quantity, stability, and/or potency are not high enough to have an effect outside the large intestine for iNKT cell regulation.
To investigate whether the difference in colonic iNKT cells between BFWT and BFΔSPT mice has biological significance, we subjected these mice to an oxazolone colitis challenge, in which intestinal inflammation characteristic of human ulcerative colitis is induced and is dependent on iNKT cell–produced interleukin 13 (IL-13) (Boirivant et al., 1998; Fuss et al., 2004; Heller et al., 2002). As expected, BFΔSPT mice had more severe weight loss (Fig. 2A), higher cumulative histopathology scores (Fig. 2B), and higher levels of IL-13 and interleukin 4 (IL-4) release than BFWT mice, although the difference was not significant in interleukin 1β (IL-1β) production (Figs. 2C–2E). Furthermore, we confirmed that the BFΔSPT mouse phenotype was CD1d-dependent: blocking of CD1d with a monoclonal antibody (19G11) during the neonatal period reduced iNKT cell numbers and prevented the colitis phenotype when these mice were challenged in adulthood (Fig. S2A–C). It is possible that different functional characteristics, e.g., cytokine production during colitis stimulation, of the iNKT cells from these two mono-associated mice may also partially contribute to the colitis results. Nonetheless, our results showed a direct link between symbiotic bacterial sphingolipids and both host iNKT cell homeostasis and disease susceptibility.
Figure 2.
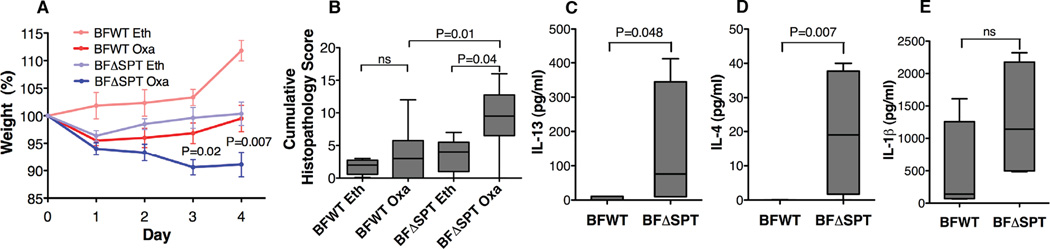
B. fragilis sphingolipids modulate host oxazolone-induced iNKT cell–mediated colitis phenotype. Upon oxazolone colitis challenge, BFΔSPT mice had more severe weight loss (A; n≥6; P values compare BFWT Oxa and BFΔSPT Oxa at days 3 and 4, respectively) and higher cumulative histopathology scores (B) than did BFWT mice. In tissue explant cultures, levels of IL-13 (C) and IL-4 (D) production in colonic tissue after oxazolone challenge were higher in BFΔSPT mice than in BFWT mice although IL-1β production was not (E). Data in (A) were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM; Data in (B)–(E) were analyzed by the Mann Whitney test; n≥3 for each group; representative of 3 experiments. See also Fig. S2.
B. fragilis sphingolipids inhibit host colonic iNKT cell proliferation during neonatal development
We next investigated a number of possible causes for our findings that mice mono-colonized with BFWT and BFΔSPT had different iNKT cell homeostasis in the colonic LP. We studied the relative numbers of BFWT and BFΔSPT bacteria contained within the colons, including microbes that were either in the lumen or tissue-associated (Fig. S3A and B) and we did not find appreciable differences in bacterial numbers between the two strains in any condition tested. We next studied the ability of B. fragilis to normalize the elevated colonic levels of CXCL16 observed in GF mice (Olszak et al., 2012) and found that the two types of mono-colonized mice had similar cxcl16 mRNA levels in the colon tissues, comparable to that of the GF mice (Fig. S3C). Thirdly, we analyzed the activation (Wingender et al., 2012) (Figs. S3D) and apoptosis (Table S1) of colonic iNKT cells in BFWT and BFΔSPT mice and found little difference. Lastly we investigated whether PSA expression was quantitatively different in BFWT and BFΔSPT strains, which could lead to differential activation of PSA-mediated pathways (Fig. S3E). We found that PSA production levels were statistically identical in the two strains. Thus we discovered no evidence to support any of the above mechanisms accounting for the differences in iNKT cell homeostasis in mice colonized with the BFWT vs. BFΔSPT bacteria.
One other possibility we considered was that some bacterial sphingolipids, including those of B. fragilis, inhibit the expansion of the iNKT cell population in the colon. To test this hypothesis, we measured the expression of Ki-67 (a nuclear protein marker for cellular proliferation) in iNKT cells within the colonic LP of mice from birth to 9 weeks of age. We found significantly higher mean fluorescence intensity (MFI) expression for this protein in both GF and BFΔSPT mice than in either SPF or BFWT mice during the neonatal period, particularly between days 5 and 12; at 8 days of age, the percentage of Ki-67+ iNKT cells was also higher in GF and BFΔSPT mice. Proliferation was reduced to similar low levels in all mice after 21 days (Figs. S3F, 3A and S3G). To verify this observation, we used an alternative approach—the bromodeoxyuridine (BrdU) method—to measure DNA replication in colonic LP iNKT cells in 8-day-old mice. We confirmed that GF and BFΔSPT mice had higher levels of DNA replication in these cells than did SPF and BFWT mice, respectively (Fig. S3H and 3B). These studies showed that symbiotic bacterial sphingolipids can modulate the homeostasis of colonic iNKT cells by inhibiting cell proliferation—and thus their accumulation—during neonatal development.
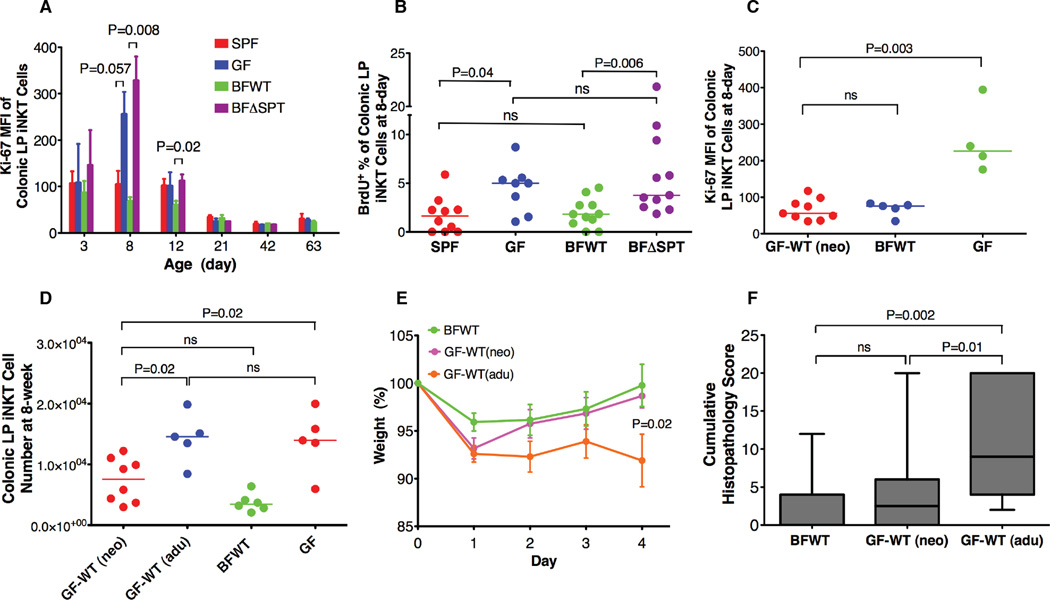
Figure 3.
B. fragilis sphingolipids inhibit colonic LP iNKT cell proliferation during neonatal development and restrict the accumulation of these cells in adult mice. Proliferation rates (A and B; n≥3) were higher in GF and BFΔSPT mice than in SPF and BFWT mice at early ages. Colonic LP iNKT cell counts were not restored in GF-WT(adu) mice, but GF-WT(neo) mice had levels of colonic LP iNKT cell proliferation and total cell numbers similar to those in BFWT mice (C and D). After oxazolone challenge, only GF-WT(adu) mice had severe weight loss and high cumulative histopathology scores (E and F; n≥10; P value in panel E compares BFWT and BF-WT(adu) mice at day 4). Data in (A) (days 8 and 12) and (E) were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM. MFI, mean fluorescence intensity. Data in (B)–(D) and (F) were analyzed by the Mann Whitney test. See also Fig. S3.
On the basis of these findings, we hypothesized that only when mice are exposed to symbiotic sphingolipids very early in life are their iNKT cell numbers restricted in adulthood. We designed two co-housing experiments to test this hypothesis. In the first, GF dams were mated with BFWT monocolonized mice. Ki-67 expression in offspring pups’ colonic LP iNKT cells was measured at 8 days of age (Fig. 3C), and iNKT cell numbers were measured at 8 weeks (Fig. 3D). As expected, proliferation levels and total cell numbers in pups born to dams receiving BFWT bacteria [GF-WT(neo)] were similar to those in BFWT mice. In the second experiment, we co-housed GF pups at 10–14 days of age with BFWT mice [GF-WT(adu)], just after the cell proliferation window had closed. As expected, although GF-WT(adu) mice harbored numbers of BFWT bacteria equivalent to those in GF-WT(neo) mice, the GF-WT(adu) animals had much higher colonic LP iNKT cell numbers at 8 weeks of age. When we challenged these mice with oxazolone, GF-WT(neo) mice responded similarly to BFWT mice, with a significant reduction in the severity of the colitis phenotype and intestinal inflammation (as assessed by weight loss and colitis scores) from values obtained in GF-WT(adu) mice (Figs. 3E and 3F). These studies established a critical time window for exposure to sphingolipid-producing symbionts to maintain host iNKT cell homeostasis and influence disease susceptibility. Interestingly, when we co-housed GF pregnant dams with BFWT mice three days before delivery, the pups’colonic iNKT cell numbers were not normalized to the BFWT level but more resembled the numbers found in GF mice, although at time of delivery the mother was heavily colonized with BFWT bacteria (data not shown). This finding indicates that either bacterial sphingolipids are important to iNKT cell development at the prenatal stage or that as yet unidentified factors also control iNKT cell homeostasis.
Purified B. fragilis glycosphingolipids inhibit iNKT cell activation in vitro and in vivo
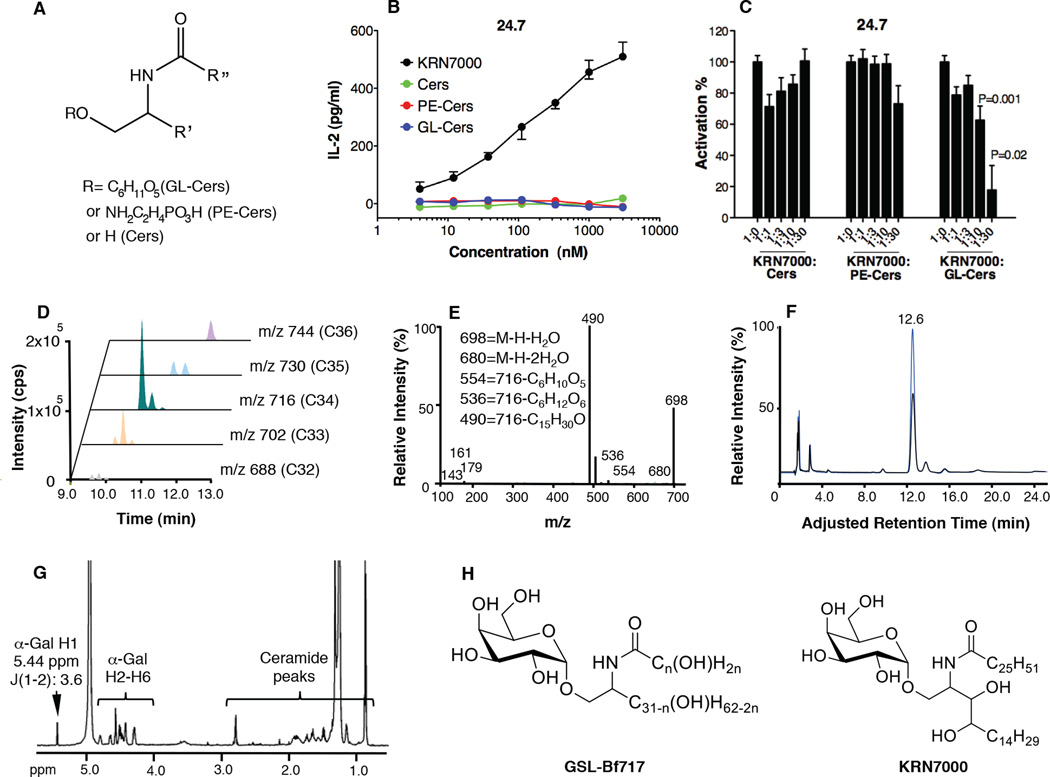
In order to identify bioactive sphingolipids of B. fragilis, we performed comparative lipidomic profiling of BFWT and BFΔSPT bacteria by HPLC–tandem mass spectrometry. In BFWT lipid extracts, we identified three types of sphingolipids with characteristic MS/MS fragmentation patterns: ceramides (Cers), glycosylceramides (GL-Cers, signature fragments of 161,179), and phosphoethanolamine-ceramides (PE-Cers, signature fragment of 140) (Fig. 4A and data not shown); none of these sphingolipids was detected in BFΔSPT lipid extracts. To determine whether any of these sphingolipids activated iNKT cells, we conducted co-culture assays. Bone marrow dendritic cells (BMDCs) were pulsed with each sphingolipid type and then incubated with iNKT cell hybridoma 24.7. None of the lipids in the tested conditions activated iNKT cells to produce appreciable interleukin 2 (IL-2), although the prototypical ligand KRN7000 caused robust activation (Fig. 4B). We next tested whether these lipids were inhibitory to iNKT cells. We pulsed BMDCs with each sphingolipid type in the presence of KRN7000 and then incubated the cells with hybridoma 24.7, measuring the production of IL-2 as an indicator of iNKT cell activation; 100% activation was defined by the level of IL-2 production in the KRN7000 alone condition. In this assay, GL-Cers, but not the other two lipid types, significantly reduced iNKT cell activation by KRN7000 (Fig. 4C). It is very likely that the difference in activity among the three classes of lipid molecules lies in the different head groups. Most probably the hexose head group allows molecules in the GL-Cers fraction to interact more stably with CD1d and/or iNKT cells compared to phosphoethanolamine and hydrogen head groups in PE-Cers and Cers fractions, respectively.
Figure 4.
Chemical analysis of B. fragilis glycosphingolipid peak GSL-Bf717. Three distinct sphingolipid clusters were identified in B. fragilis (A). None of these clusters activated iNKT cells (B). GL-Cers was inhibitory to iNKT cell activation by KRN7000 in vitro (C). Data in panel (B) are representative of 2 experiments, each with triplicates, and presented as median ± range. Data in (C) complied three experiments (n≥6) and were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM. P values compare KRN7000 + GSL-Bf717 (at ratios of 1:10 and 1:30) with KRN7000 alone (ratio 1:0). (D) GL-Cers had 5 chain-length variants, C32–C36, and the C34 with M.W.=717.6 (m/z 716) was predominant. A peak from this variant, GSL-Bf717, was characterized by MS/MS (E), HPAEC (F) and 1H-NMR (G) and was found to have an α-galactosyl linkage. In (F), the hydrolyzed hexose head group co-eluted with the spiked galactose standard (blue) at 12.6 min. In (H), the proposed molecular configuration of GSL-Bf717 is shown for comparison with that of the prototypical agonist KRN7000.
To identify molecules inhibitory to iNKT cell activation, we further analyzed the GL-Cers cluster. We found that GL-Cers is composed of multiple molecular species, ranging from m/z 688 to m/z 744 (Fig. 4D). The observation that all species had characteristic fragments at m/z 143, 161, and 179, which were from the hexose head group, implied the source of the structural variability resided in the chain length of ceramide structures from C32 to C36 (Fig. 4E and data not shown). Tandem mass spectrometric analysis of a prominent m/z 716 peak (C34: M.W.=717.6) generated multiple daughter ions—assigned as m/z 698 ([M-H-H2O]), 680 ([M-H-2H2O]), 554 ([M-H-hexose+H2O]), 536 ([M-H-hexose]), and 490 ([M-H-C15H29O])—in addition to the above-mentioned hexose-derived fragments. Among these daughter ions, m/z 490 (loss of the hydroxyl aliphatic chain) assigned that sphingosine and fatty acid chain has one hydroxyl group each (Fig. 4E). The m/z 716 peak was purified by HPLC and further analyzed. By high-pH anion-exchange chromatography (HPAEC), we identified the structure of the monosaccharide head group as galactose (Fig. 4F). In addition, we used 1H-NMR (Fig. 4G) and 1H-1H-NMR (COSY, data not shown) to verify the identity of the hexose and characterize its glycosidic linkage. NMR analysis revealed a galactose residue linked α-glycosidically to the sphingoid backbone. This α-galactosylceramide peak is referred to as GSL-Bf717; a proposed molecular configuration is shown in Fig. 4H. In addition, we confirmed that GSL-Bf717 was detectable only in fecal samples from BFWT mice but not in samples from BFΔSPT mice. The estimated yield of GSL-Bf717 was ~1 ng per gram of fecal pellet. Although it shares key features with known iNKT cell agonist KRN7000, GSL-Bf717 has unique structural characteristics, such as shorter chain lengths and different hydroxyl compositions in both chains. Ceramides produced by B. fragilis are known to have branched acyl chains in iso- or anteiso- positions (Miyagawa et al., 1979), which may present yet another distinction between GSL-Bf717 and KRN7000. As a result of these dissimilarities, GSL-Bf717 may possibly compete with KRN7000 for the limited space in CD1d grooves but may be positioned differently so that the α-galactosyl head group does not interact with the iNKT cell receptor in the same way as KRN7000. It is unclear whether GSL-Bf717 and similar molecules are presented as antagonistic ligands or simply occupy the CD1d binding space due to high affinity. In either case the end consequence is that these bacterial glycosphingolipids and KRN7000 have opposing immunological effects; GL-Cers restrains iNKT cell activation and KRN7000 activates iNKT cells.
To confirm that GSL-Bf717 has inhibitory activity, we performed several co-culture assays. In co-cultures of BMDCs and iNKT cell hybridoma 24.7, GSL-Bf717 did not activate iNKT cells (Fig. 5A). Moreover, GSL-Bf717 did not activate non-invariant NKT cell line 14S6 (data not shown). We next conducted a CD1d-loading experiment using phycoerythrin-stained CD1d empty tetramers and the iNKT cell hybridoma 24.7. When the empty CD1d tetramers are loaded with lipid antigens, they can bind to the iNKT cell receptor and the complex can be detected by flow cytometry, as shown in Figs. S4A–S4D for tetramers either pre-loaded at the NIH facility (Fig. S4B) or lab-loaded (Fig. S4D) with PBS-57 (a lipid variant of KRN7000 that also stimulates iNKT cells). Previously empty tetramers added with either one of the three identified lipid fractions (Fig. 4A) or GSL-Bf717 did not bind to iNKT cells (Figs. S4E–S4H). In addition, GSL-Bf717-added, previously empty CD1d tetramers did not effectively stain liver lymphocytes in contrast to the control tetramers loaded with PBS-57 (Figs. S4I–S4K). These CD1d-loading experiments provide supporting evidence that the purified bacterial lipids do not activate 24.7 iNKT hybridoma cells in the tested conditions. The likely mechanisms could be either that the lipids do not sit or do not sit correctly in the CD1d binding grooves and/or that the iNKT TCRs do not recognize these CD1d-lipid complexes.
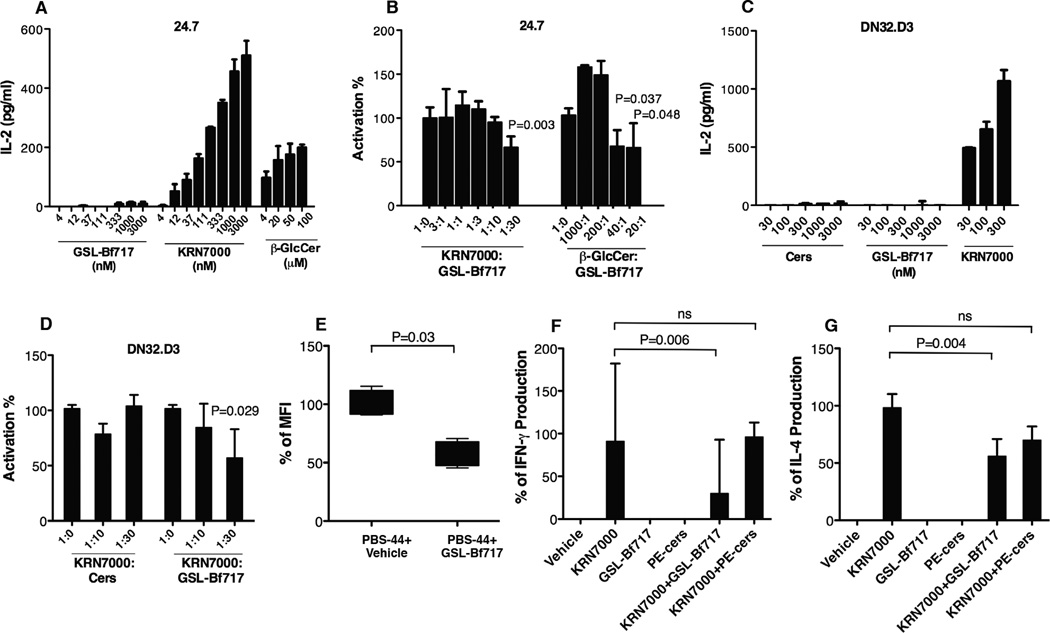
Figure 5.
B. fragilis GSL-Bf717 inhibits iNKT cell activation. GSL-Bf717 did not activate iNKT cells (A and C; data are representative of 2 independent experiments with each in triplicates) but did inhibit iNKT cell activation by agonist KRN7000 in both hybridomas 24.7 and DN32.D3 (B and D, data are representative of 2 independent experiments with each in triplicates; P value compares KRN7000 + GSL-Bf717 at a 1:30 ratio with KRN7000 alone, which is at ratio 1:0). GSL-Bf717 also inhibited activation by agonist β-GlcCer (right columns in B; P values compare β-GlcCer + GSL-Bf717 at ratios 40:1 and 20:1 with β-GlcCer alone, which is at ratio 1:0). The loading efficiency of PBS-44 to empty CD1d tetramer (phycoerythrin-labeled) was decreased significantly in presence of GSL-Bf717 as evidenced by the lowered MFI of CD1d tetramer-stained iNKT cells (E); n=4. GSL-Bf717 reduced serum levels of IFN-γ (F) and IL-4 (G) released by KRN7000-stimulated iNKT cells in vivo. 100% production was defined as the level of cytokines produced in KRN7000 only-treated mice; n≥4. Data in (A)–(F) are presented as median ± range. Data in (B), (D)–(F) were analyzed by the Mann Whitney test. Data in (G) were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM. See also Fig. S4.
When in competition with KRN7000 at a ratio of 1:30 in the co-culture assay using BMDCs and 24.7 cells, GSL-Bf717 in excess moderately (i.e., by 40%) inhibited KRN7000-induced activation of iNKT cells (Fig. 5B, left group of columns). This limited suppressive activity of GSL-Bf717 suggested that it likely acts as a competitive inhibitor to agonists in binding to CD1d, instead of as a true antagonist. However, when we tested another iNKT cell agonist, β-glucosylceramide (β-GlcCer, d18:1/24:1(15Z)), we found that GSL-Bf717 was more effective in inhibiting its function. β-GlcCer is an endogenous agonist that has much weaker activity than KRN7000 (Brennan et al., 2011). Indeed, in our assay, to induce comparable IL-2 production in the 24.7 hybridoma, it took micromolar concentrations of β-GlcCer as compared to nanomolar concentrations of KRN7000 (Fig. 5A). Remarkably, GSL-Bf717 not only could inhibit β-GlcCer function in a dose-dependent fashion, but also could achieve about 40% inhibition with as little as 2.5% of the β-GlcCer concentration (Fig. 5B, right group of columns, 40:1). We next used the hybridoma DN32.D3 to test the inhibitory activity of GSL-Bf717 on KRN7000 function. We found that GSL-Bf717 was not able to activate this iNKT cell line when using BMDC as the CD1d-expressing antigen presenting cell, but could inhibit iNKT cell activation by KRN7000 to a similar extent as in 24.7 (Fig. 5C and 5D). As seen with hybridoma 24.7, Cers could not inhibit DN32.D3 iNKT hybridoma cell activation by KRN7000. Comparison of Figs. 4C and 5B shows that the GL-Cers fraction was a more potent inhibitor of iNKT cell activation than was GSL-Bf717. It is highly likely that many B. fragilis glycosphingolipid species can additively or synergistically inhibit iNKT cells just as GSL-Bf717 does. Nonetheless, GSL-Bf717 is representative of these glycosphingolipids and can be used to further explore the inhibitory activity of GL-Cers.
To understand at the molecular level the inhibitory activity of GSL-Bf717, we performed a CD1d tetramer competitive loading experiment. We incubated PBS-44 (another variant of KRN7000 that is also agonistic to iNKT cells and can be efficiently loaded to empty CD1d tetramers) with phycoerythrin-labeled unloaded CD1d tetramers in the presence and absence of GSL-Bf717 in 1:1:3 ratio (tetramers: PBS-44: GSL-Bf717). We then used the CD1d-lipid complex to stain an excess number of iNKT hybridoma 24.7 cells and measured MFI. We found that in comparison to the PBS-44+vehicle control, addition of GSL-Bf717 to PBS-44 was able to significantly reduce the MFI of the stained iNKT cells to about 57% of the vehicle control, reflecting the decreased staining efficiency of the tetramers, as well as the poor loading capacity of PBS-44 to CD1d in presence of GSL-Bf717 (Fig. 5E). This experiment strongly suggested that the inhibitory function of GSL-Bf717 was likely mediated at the molecular level during CD1d-lipid interactions where GSL-Bf717 could either occupy the CD1d grooves and prevent PBS-44 loading, and/or that GSL-Bf717-loaded CD1d complex was not recognized properly by the iNKT cells (thus decreased MFI).
To investigate whether GSL-Bf717 inhibits iNKT cell agonist function in vivo, we intraperitoneally injected KRN7000 (100 ng) and GSL-Bf717 (1000 ng) into mice and measured serum interferon-γ (IFN-γ) and IL-4 production 4 hours later. When administered with GSL-Bf717, the ability of KRN7000 to produce both cytokines in serum was significantly reduced compared to that of KRN7000 alone; in contrast, PE-Cers at the same dose co-administered with KRN7000 did not reduce production of either cytokine (Figs. 5F and 5G). In this experiment, the production of these cytokines through KRN7000 stimulation was mediated by iNKT cells and dependent on CD1d expression: no IFN-γ or IL-4 was detected in CD1d-knockout mice in any group (data not shown). These results demonstrated that GSL-Bf717 inhibits agonist-induced iNKT cell activation in vivo.
GSL-Bf717 treatment of BFΔSPT mice restores colonic iNKT cell homeostasis and protects mice from colitis
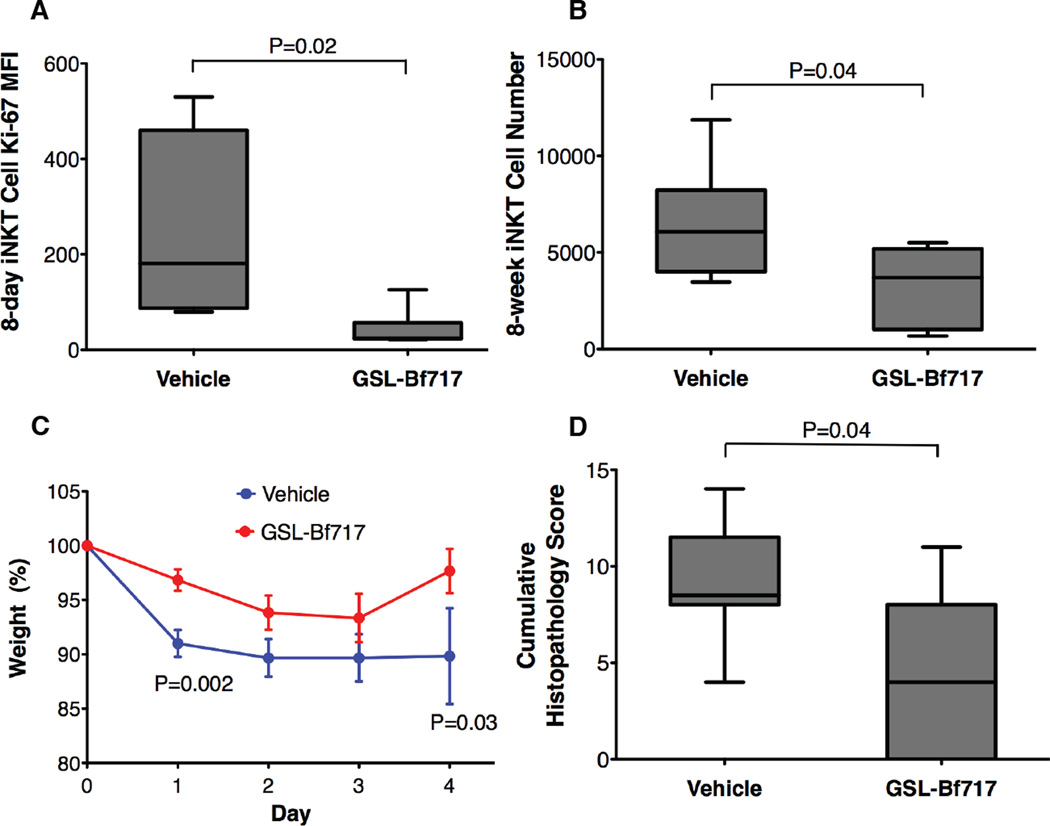
Endogenous lipids provide important antigens that drive in vivo activation of iNKT cells (Brennan et al., 2011; Rossjohn et al., 2012). Our studies with KRN7000 suggested that GSL-Bf717 might inhibit in vivo activation and expansion of iNKT cells by endogenous lipids during the neonatal period. We therefore administered GSL-Bf717 (100 ng/dose) five times by gavage to newborn BFΔSPT mice at 2–7 days of age and then measured the colonic iNKT cell Ki-67 levels at 8 days and total colonic iNKT cell numbers at 8 weeks. Compared with vehicle-treated mice, GSL-Bf717-treated BFΔSPT mice had significantly lower levels of Ki-67 expression at a very young age (Fig. 6A) as well as lower cell numbers in adulthood (Fig. 6B). When challenged with oxazolone, GSL-Bf717-treated mice lost less weight and had lower cumulative histopathology scores than did vehicle-treated mice (Figs. 6C and 6D). GSL-Bf717 is certainly not the only lipid that can mediate the above phenotypes, it may not even be the most potent one, yet it provides proof-of-principal evidence that bacterial glycosphingolipids can be orally administrated to susceptible hosts at a young age to perhaps permanently limit colonic iNKT cell numbers and reduce host colitis susceptibility into adulthood.
Figure 6.
GSL-Bf717 treatment of neonatal BFΔSPT mice restores colonic iNKT cell homeostasis and protects mice from colitis in adulthood. GSL-Bf717-treated mice had lower Ki-67 expression at 8-day (A; n≥4) and lower total counts of colonic LP iNKT cells at 8 weeks (B; n≥6). These mice were protected against the oxazolone challenge, with less weight loss (C; P values compare the two groups at days 1 and 4) and lower cumulative histopathology scores (D). Data are representative of 2 independent experiments; n=6 for each group. Data in (A), (B) and (D) were analyzed by the Mann Whitney test. Data in (C) were confirmed to have normal distribution by the KS normality test with α=0.05, analyzed by the Student’s t test and are presented as mean ± SEM.
Discussion
Although in utero development takes place in a sterile environment, mammals commence a lifelong relationship with microbes at birth and may be exposed to microbial products through the placenta during gestation. Symbionts thus have the opportunity to affect host physiology from an early age, when many host functions are actively evolving. This work offers mechanistic data that support the growing body of evidence indicating the importance of early-life microbial exposure in regulating adult immune homeostasis and disease susceptibility (Isolauri, 2009; Olszak et al., 2012; Strachan, 1989). Our results reveal an unexpected mechanism for the attainment of host immune balance: symbionts negatively regulate a crucial immune cell type, the iNKT cell, and prevent its excessive activation during a disease challenge. This modulation is fundamentally based on the ability of symbionts to modify the host lipid environment by means of bacterial glycosphingolipids. Indeed, the SPT homolog and potentially the ability to synthesize sphingolipids are well conserved in intestinal Bacteroides species (An et al., 2011; Kato et al., 1995) and the availability of these sphingolipids in the intestines is likely not an issue. Because of structural similarities with iNKT cell agonists, these non-activating sphingolipids effectively blend into the host lipid antigen pool and turn the hyper-proliferating environment during neonatal development into a restricted one. Consequently, iNKT cell activation and expansion caused by indigenous self and microbial stimuli are reduced. Importantly, in SPF and BFWT mice, despite lower numbers of colonic iNKT cells in the presence of these bacterial lipids, these cells still undergo some proliferation during the neonatal period (Fig.3A and S3G) suggesting that the host’s endogenous agonists are still recruited to expand the cell population in these mice. Wieland Brown et al. have very recently reported that some B. fragilis sphingolipids can provide weak agonistic activity to iNKT cells (Wieland Brown et al., 2013). Since we have found that B. fragilis produces a variety of sphingolipid species, it is not surprising that some of the lipid structures are stimulatory while others (as we are reporting) are inhibitory. It is very important to take note of the fact we have shown in this paper that the total net effect of these lipids, as demonstrated by comparison of the iNKT cell numbers in colons of BFWT and BFΔSPT mice, are inhibitory to iNKT cells in the colonic LP. GSL-Bf717 serves as a paradigm for these iNKT cell-restrictive lipids.
In our previous study comparing SPF and GF mice iNKT cells in the lung and colon, we identified that increased CXCL16 chemokine release from these tissues was a mechanism responsible in GF mice for the heightened iNKT cell accumulation in these tissues. In the present study, we found that the colonic cxcl16 mRNA expression in BFWT or BFΔSPT mice were at similarly high levels to those found in GF mice. Therefore, colonization by the single organism B. fragilis does not down-modulate CXCL16 expression in colon tissues and does not modify trafficking of these cells to the colon. However, the bacterial sphingolipids in BFWT mice can restrict local proliferation of colonic iNKT cells once the cells arrive. Ultimately, colonic iNKT cells achieve homeostasis with smaller total numbers in BFWT mice compared to those in BFΔSPT mice or GF mice, which do not possess the bacterial sphingolipids-mediated inhibition of proliferation. It is remarkable that mono-colonization by a single sphingolipid-producing bacterial species, which does not have the CXCL16 modulation capacity, can have impacts on host iNKT cell homeostasis and colitis susceptibility comparable to those attained by the whole microbiota, which implements the additional chemokine pathway. Taken together, our reported data on different CXCL16 expression in SPF vs GF mice and different iNKT cell proliferation in BFWT vs BFΔSPT mice, suggest that the microbiome uses multiple, perhaps organism-specific mechanisms, for keeping the numbers of iNKT cells in check.
The inhibitory bacterial glycosphingolipids further blur the conventional distinction between self and non-self in terms of immune recognition. Evidence in this study suggests that the host is strongly dependent on bacterial glycosphingolipid molecules for iNKT cell homeostasis during development. Strikingly, our data show that an absence of these decisive molecules in young GF and BFΔSPT mice has a lasting impact on the animals’ iNKT cell homeostasis and causes an irreversible increase in their susceptibility to colitis. Our results highlight the importance of sphingolipid-producing symbionts as a vital component of the colonic microflora in early life, despite the fact that they are probably not the dominant members of intestinal community at this age (Koenig et al., 2011; Yatsunenko et al., 2012). It is also possible that the inhibitory bacterial sphingolipids are passed to the pups from dams through milk. Nevertheless, although the enzymatic pathway required for production of these glycosphingolipids is not encoded in the eukaryotic genome, we propose these molecules—exemplified by GSL-Bf717—as prototypes for self-inhibitors of iNKT cells, which is currently a scarcely explored yet important field in iNKT cell biology.
Our results also propose a novel paradigm for host–symbiont interactions. In host–pathogen interactions, specific virulence factors directly target host cells and pathways with high efficacy and result in specific immunologic response profiles. Herein we report that, in contrast to pathogens, symbionts rather indirectly and subtly change the chemical environment mediating host functions. Instead of active stimulation, which has a palpable impact on host tissues, symbiosis factors—in this study glycosphingolipids—act silently, passively and with relatively lower efficiency (since one molecule can occupy only one CD1d groove). The overall mode of action by symbiosis factors is to regulate rather than to stimulate. Ineptitude in stimulating the host immune system results in their evasion of host immune surveillance and continual association with the host. The timing of symbiotic molecules’ activity depends upon not microbes, but host physiological factors, such as neonatal development and possibly immune stimulation. The lower efficiency of the symbiotic microbial molecules is compensated for by the bulk presence of these factors and the huge numbers of microbes producing them (Ley et al., 2006; Zitomersky et al., 2011). We propose that these attributes are essential for establishing a symbiotic relationship between microbes and immune systems. It is tempting to speculate that other, still unidentified symbiosis factors may function in a similar way to control host immune homeostasis. In addition, although both are α-glycosphingolipids, GSL-Bf717 is the diametrical opposite—in terms of function—of the sphingolipids produced by pathobiont Sphingomonas species. This observation likely reflects the fine structural differences between these molecules that result in dissimilar interactions with CD1d and the iNKT cell receptor. More importantly, the opposite phenotypes reflect their profoundly different relationships with the host and highlight a fundamental distinction between symbiosis and pathogenesis.
Along with our studies of PSA, our evidence on the function of GSL-Bf717 offers new insight into the functions of microbiota at the molecular level. PSA and GSL-Bf717 are synthesized from the same bacterium (B. fragilis) but function differently: PSA facilitates maturation of a balanced CD4+ T cell population, regardless of the host’s age, while GSL-Bf717 is crucial to iNKT cells in a specific time window of life. These examples, although thus far limited, strongly indicate that microbes deeply modulate host immune functions with diverse effects. In addition, with their unusual inhibitory properties, GSL-Bf717 or inhibitors yet to be discovered in the microbiota may offer promise as novel therapeutic agents that can target the deleterious impact of iNKT cells in many human disorders (Braun et al., 2010; Fuss et al., 2004; Matangkasombut et al., 2009; Peternel and Kastelan, 2009).
Experimental Procedures
Mice and cells
Swiss Webster mice maintained under SPF or GF conditions were purchased from Taconic USA. C57BL/6 wild-type mice were purchased from the Jackson Laboratory. CD1d-deficient mice (Smiley et al., 1997) were maintained in a SPF barrier facility at Harvard Medical School. GF and mono-associated mice were bred and maintained in vinyl isolators in the animal facility at Harvard Medical School. BMDCs were purified from mouse femurs and cultured for 8 days in C-RPMI-10 with GM-CSF (20 ng/ml; Biosource). All procedures were approved by the Harvard Medical Area Standing Committee on Animals.
Bacterial culture and mutant construction
B. fragilis strain NCTC 9343 was grown in flasks in an anaerobic chamber in rich medium (An et al., 2011). Mutant strain BFΔSPT was constructed with a pNJR6 suicide vector; the first 50 and last 94 nucleotides of gene BF2461 were retained, and the 1041 intervening nucleotides were deleted (Comstock et al., 1999). The complemented strain C-delta, maintained by erythromycin at 20 µg/ml, was constructed by expression of the full BF2461 gene on plasmid PFD340 and conjugation of the complementing plasmid to the BFΔSPT mutant.
Co-housing experiment
GF females or young (10- to 14-day-old) GF pups and their dams were co-housed in an isolator with BFWT mice (male for mating with GF females and female for the litters) for an extended period. Effective colonization by BFWT bacteria was verified by examination of stool samples from adult mice after 24 hours.
B. fragilis lipid extraction
Total bacterial sphingolipids were purified from overnight anaerobic cultures of B. fragilis (either BFWT or BFΔSPT) by a modified Bligh-Dyer method. Washed bacterial pellets were stirred overnight in chloroform-methanol-water (1:2:0.8). The insoluble fraction was precipitated; the liquid fraction was mixed with equal volumes of chloroform and water (final composition, 1:1:0.9) and centrifuged for phase separation. The lower organic phase was then collected, dried, and resuspended with the initial eluent. An open silica column was used to fractionate the purified lipids with stepwise increases in the polarity of the eluents (i.e., chloroform-methanol at ratios of 8:1 to 1:2). Each fraction was dried and resuspended in chloroform. For differentiation of sphingolipids from non-sphingolipids, total lipids were treated with 0.02 N NaOH for 30 min at 37°C before being subjected to thin-layer chromatography. Lipid stocks (10 mg/ml) were prepared in chloroform, and working solutions (1 mg/ml) were prepared in dimethyl sulfoxide.
Lipidomic profiling
To identify BFWT-specific sphingolipids, silica column–fractionated lipid fractions of the BFWT and BFΔSPT strains were analyzed with reverse-phase HPLC-MS/MS (Agilent C18 column connected with Thermo Scientific LTQ-XL; gradients of water to methanol, 10:90 to 0:100) to identify spots present in BFWT but missing in BFΔSPT. Identified lipid species of interest were purified by reverse-phase HPLC (Varian Prostar/Agilent C18 column) and subjected to structural analysis.
CD1d tetramer loading and staining assay
For single lipid loading, phycoerythrin-tagged unloaded-mCD1d tetramers (1 µM; NIH Tetramer Core Facility) were incubated overnight with 4 µM lipids in a 10-µl volume. After three washes with PBS, the complex was resuspended in 10 µl of water. The solution (5 µl) was used to stain iNKT cell hybridoma 24.7 or liver lymphocytes (1×105 cells), together with FITC-labeled anti-CD3 (or TCR-β) (1 µl) and 7-AAD (5 µl) in total of 50-µl volume for 30 min at 4°C. Cells were then analyzed by flow cytometry. For competitive loading, the 1 µM empty tetramers were incubated with 4 µM PBS-44, in presence or absence of 12 µM bacterial lipid GSL-Bf717 in a total volume of 6 µl PBS-0.1% Tween 20. After incubation for 6–8 hours at 37°C in dark, each incubation mixture was washed and 15% of the mixture was then used to stain iNKT cell hybridoma 24.7 as described above. PBS-57 and PBS-44 were provided by Dr. Gurdyal Besra, University of Birmingham, UK and Dr. Paul Savage, Brigham Young University, USA, respectively.
BMDC–T cell co-culture assay
BMDCs (100 µl; 5 × 105 cells/ml) were pulsed with lipids in a 96-well plate in triplicate for 4 hours at 37°C. The wells were washed three times before addition of NKT hybridoma cells of lines 24.7 (Behar et al., 1999), DN32.D3 (Lantz and Bendelac, 1994), and 14S6 (Behar et al., 1999) in 200 µl volume (2.5 × 105 cells/ml). After 24 h at 37°C, culture supernatants were subjected to IL-2 enzyme-linked immunosorbent assay (ELISA) (R&D). For competition assays, bacterial lipids and KRN7000 (100 nM, Avanti Polar lipids) or β-GlcCer (20 µM, C24:1 Glucosyl(β) Ceramide (d18:1/24:1(15Z)), Avanti Polar lipids) were pulsed simultaneously with BMDCs.
In vivo cytokine inhibition assay
C57BL/6 wild-type or CD1d-knockout mice (6 weeks old) received one 100-µl intraperitoneal injection of KRN7000 (1 µg/ml) or vehicle control (0.9% NaCl). In certain groups, this injection was followed by an intraperitoneal injection of 100-µl (10 µg/ml) bacterial lipid GSL-Bf717 or PE-Cers (estimated M.W.≈678). Each wild-type group included 4–8 mice; each CD1d-knockout group consisted of 2 mice. After 4 hours, serum samples were prepared and subjected to ELISA for measurements of IFN-γ and IL-4.
Lipid treatment
GSL-Bf717 was purified and dissolved in ethanol at a concentration of 200 µg/ml. Before treatment, the lipid sample was diluted in 0.9% NaCl to a concentration of 100 ng/30 µl. A 30-µl volume of the GSL-Bf717 solution or 0.9% NaCl was administered by oral gavage to newborn BFΔSPT mice (in an isolator) 5 times during the first week of life; polyethylene tubing with an inside diameter of 0.28 mm and an outside diameter of 0.61 mm (BD) was used for gavage. For oxazolone colitis challenge studies, 6- to 8-week-old female mice were used.
Statistical analysis
Two-tailed, non-paired Student’s t test or the two-tailed Mann Whitney test was used to determine P values as specified in each figure legend. One-way ANOVA was performed for multi-group comparison and reported in Table S2. Statistical significance was defined as P < 0.05. Detailed report for each test is listed in Table S2. Horizontal lines in dot plots represent median values, and each dot represents one mouse. Whiskers in Box plots are minima and maxima of the data.
Supplementary Material
Highlights.
Symbiotic bacterial glycosphingolipids modulate host iNKT cell homeostasis.
Early exposure to these lipids protects the host from later colitis challenges.
These lipids inhibit iNKT cell proliferation.
Treatment with microbial sphingolipids protects the host from colitis.
Acknowledgements
We thank Shakir Edwards for handling of the gnotobiotic mice, Dr. Roderick Bronson for histopathology sample evaluation, Dr. Bella Printseva and Wen Zheng for technical assistance and Julie B. McCoy for editing. We thank Dr. Yusuf Hannun at Stony Brook University for discussions and help with lipid analysis. Phycoerythrin-tagged PBS-57-loaded and -unloaded mCD1d tetramers were obtained from the NIH Tetramer Core Facility. Dr. Gurdyal Besra at the University of Birmingham, UK, kindly provided PBS-57. Dr. Paul Savage at Brigham Young University kindly provided PBS-44. This work was supported by NIH grants AI090102 (D.L.K.) and NIH DK44319, DK51362, DK53056, and DK88199 (R.S.B.); AI007061 (S.F.O.); a Crohn’s and Colitis Foundation of America Senior Research Award (D.L.K.); a Crohn’s and Colitis Foundation of America Postdoctoral Fellowship Award (D.A.); and the Harvard Digestive Diseases Center (DK034854).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul S, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK. Protein database searches using compositionally adjusted substitution matrices. FEBS Journal. 2005;272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Na C, Bielawski J, Hannun Y, Kasper D. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4666–4671. doi: 10.1073/pnas.1001501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Behar S, Podrebarac T, Roy C, Wang C, Brenner M. Diverse TCRs recognize murine CD1. Journal of Immunology. 1999;162:161–167. [PubMed] [Google Scholar]
- Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone Colitis: A Murine Model of T Helper Cell Type 2 Colitis Treatable with Antibodies to Interleukin 4. The Journal of Experimental Medicine. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Covarrubias R, Major A. Natural killer T cells and atherosclerosis: form and function meet pathogenesis. Journal of Innate Immunity. 2010;2:316–324. doi: 10.1159/000296915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P, Tatituri RV, Brigl M, Kim E, Tuli A, Sanderson J, Gadola S, Hsu F-F, Besra G, Brenner M. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nature Immunology. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J, Lee S, Shen Y, Khosravi A, Mazmanian S. Host-bacterial symbiosis in health and disease. Advances in Immunology. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N, Garg S, Brenner M. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Advances in Immunology. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- Comstock LE, Coyne MJ, Tzianabos AO, Pantosti A, Onderdonk AB, Kasper DL. Analysis of a capsular polysaccharide biosynthesis locus of Bacteroides fragilis. Infect Immun. 1999;67:3525–3532. doi: 10.1128/iai.67.7.3525-3532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss I, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg R, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. The Journal of Clinical Investigation. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Fuss I, Nieuwenhuis E, Blumberg R, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman D. The microbiome in infectious disease and inflammation. Annual Review of Immunology. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingar O, Erik J. Sphingolipids in Bacteria and Fungi. Anaerobe. 2001;7 [Google Scholar]
- Isolauri EK Marko, Rautava Samuli, Salminen Seppo, Laitinen Kirsi. Obesity - extending the hygiene hypothesis. Nestlé Nutrition Workshop Series Paediatric Programme. 2009;64:75–85. doi: 10.1159/000235784. [DOI] [PubMed] [Google Scholar]
- Kato M, Muto Y, Tanaka-Bandoh K, Watanabe K, Ueno K. Sphingolipid composition in Bacteroides species. Anaerobe. 1995;1:135–139. doi: 10.1006/anae.1995.1009. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing G-W, Poles M, Ho D, Tsuji M, Kawahara K, Wong C-H, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Koenig J, Spor A, Scalfone N, Fricker A, Stombaugh J, Knight R, Angenent L, Ley R. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology:progress and paradoxes. Annual Reviews Immunology. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8− T cells in mice and humans. The Journal of Experimental Medicine. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Lowther J, Naismith J, Dunn T, Campopiano D. Structural, mechanistic and regulatory studies of serine palmitoyltransferase. Biochemical Society Transactions. 2012;40:547–554. doi: 10.1042/BST20110769. [DOI] [PubMed] [Google Scholar]
- Matangkasombut P, Pichavant M, Dekruyff R, Umetsu D. Natural killer T cells and the regulation of asthma. Mucosal Immunology. 2009;2:383–392. doi: 10.1038/mi.2009.96. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Current Opinion in Immunology. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mazmanian S, Liu C, Tzianabos A, Kasper D. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian S, Round J, Kasper D. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Miyagawa E, Azuma R, Suto T, Yano I. Occurrence of free ceramides in Bacteroides fragilis NCTC 9343. J Biochem. 1979;86:311–320. doi: 10.1093/oxfordjournals.jbchem.a132528. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera M, Richter J, Franke A, Glickman J, Siebert R, Baron R, Kasper D, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peternel S, Kastelan M. Immunopathogenesis of psoriasis: focus on natural killer T cells. Journal of the European Academy of Dermatology and Venereology. 2009;23:1123–1127. doi: 10.1111/j.1468-3083.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Pellicci D, Patel O, Gapin L, Godfrey D. Recognition of CD1d-restricted antigens by natural killer T cells. Nature reviews Immunology. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J, Lee S, Li J, Tran G, Jabri B, Chatila T, Mazmanian S. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science (New York, NY) 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299 doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland Brown L, Penaranda C, Kashyap P, Williams B, Clardy J, Kronenberg M, Sonnenburg J, Comstock L, Bluestone J, Fischbach M. Production of α-Galactosylceramide by a Prominent Member of the Human Gut Microbiota. PLoS biology. 2013;11 doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, Braun J, Mazmanian S, Kronenberg M. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey F, Manary M, Trehan I, Dominguez-Bello M, Contreras M, Magris M, Hidalgo G, Baldassano R, Anokhin A, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomersky N, Coyne M, Comstock L. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infection and Immunity. 2011;79:2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.