Abstract
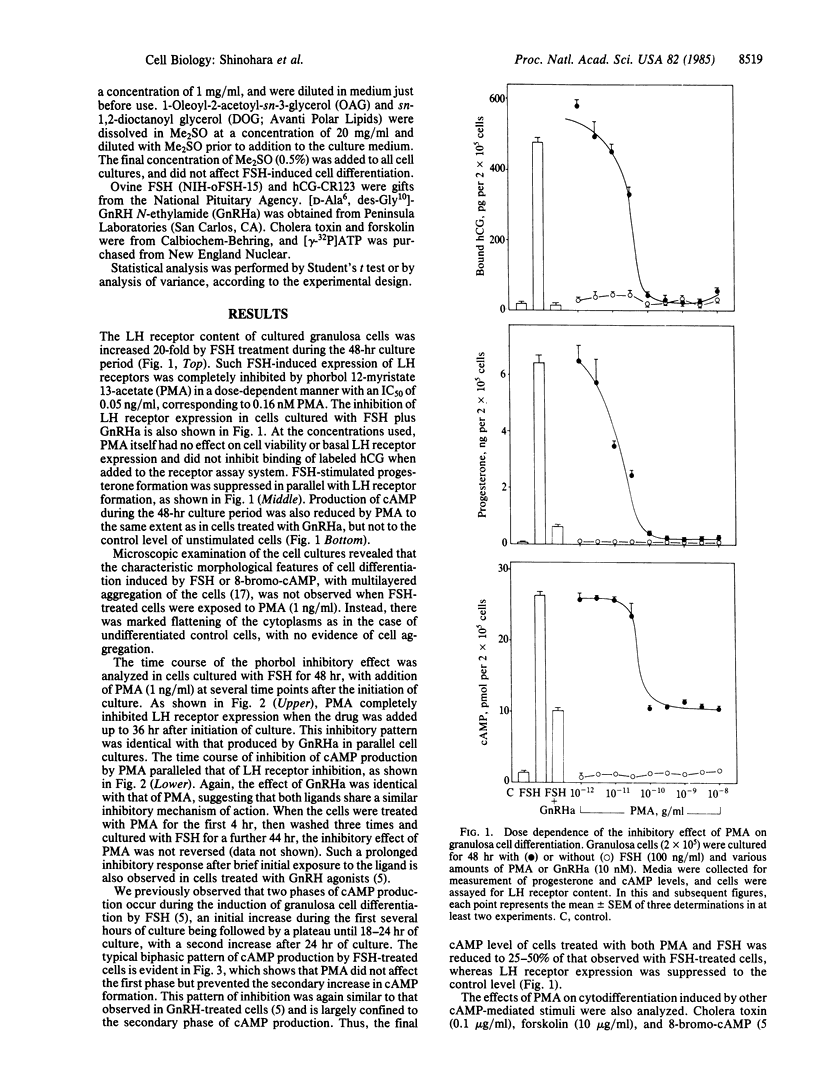
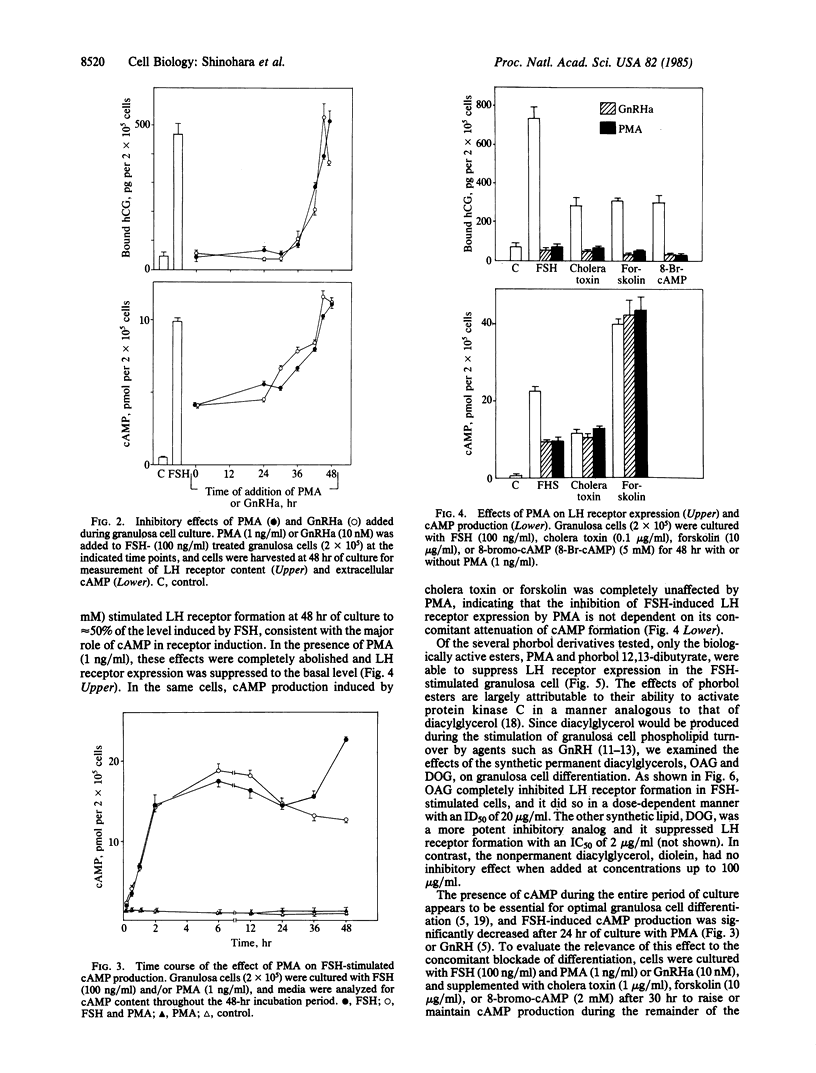
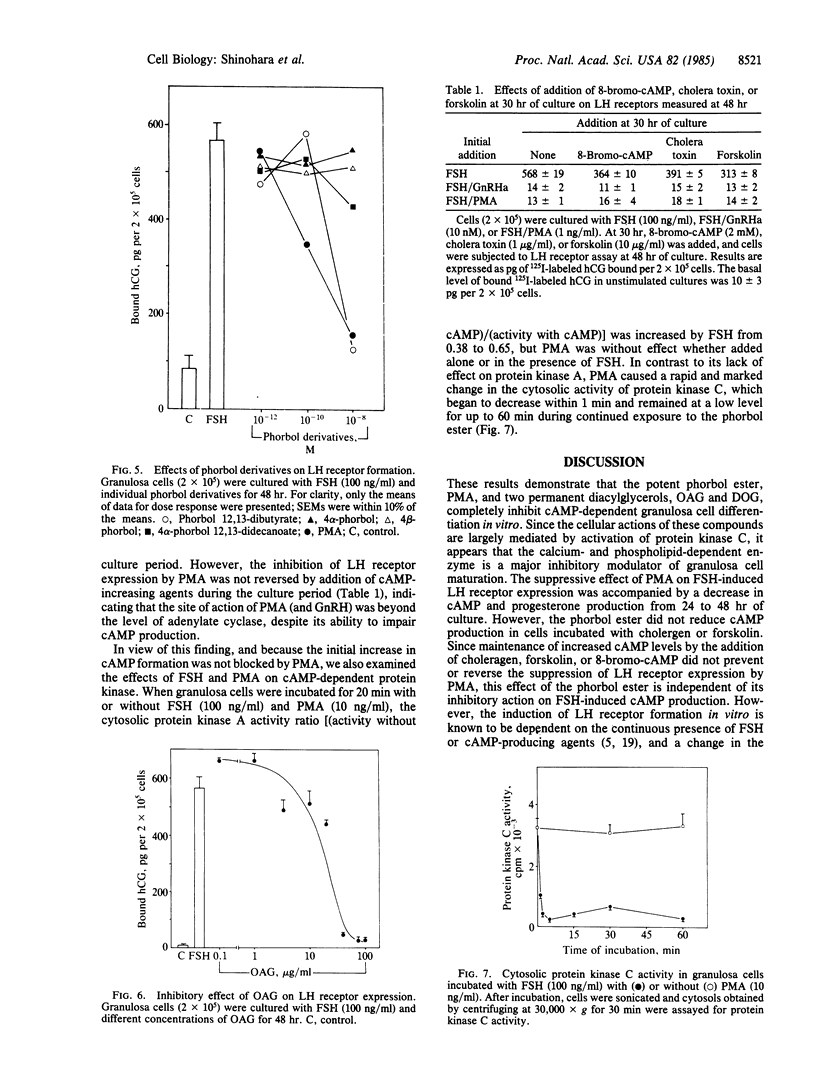
The induction of granulosa cell differentiation by follicle-stimulating hormone (FSH) is characterized by cellular aggregation, expression of luteinizing hormone (LH) receptors, and biosynthesis of steroidogenic enzymes. These actions of FSH are mediated by activation of adenylate cyclase and cAMP-dependent protein kinase and can be mimicked by choleragen, forskolin, and cAMP analogs. Gonadotropin releasing hormone (GnRH) agonists inhibit these maturation responses in a calcium-dependent manner and promote phosphoinositide turnover. The phorbol ester phorbol 12-myristate 13-acetate (PMA) also prevented FSH-induced cell aggregation and suppressed cAMP formation, LH receptor expression, and progesterone production, with an ID50 of 0.2 nM. In FSH-treated cells, PMA did not reduce the initial increase in cAMP formation during the first 24 hr of culture but prevented its secondary increase from 24 to 48 hr. PMA also inhibited LH receptor induction by cholera toxin, forskolin, and 8-bromo-cAMP, but it did not impair cAMP responses to the former two agents, indicating that the site of action of the phorbol ester is distal to adenylate cyclase. The early stimulation of cAMP-dependent protein kinase activity by FSH was also unaffected by PMA, consistent with its lack of effect on the initial cAMP response to FSH. However, PMA caused a marked decrease in cytosolic protein kinase C activity within 1 min of its addition to the cells. The permeant diacylglycerols, 1-oleoyl-2-acetoyl-sn-glycerol and sn-1,2-dioctanoyl glycerol, also inhibited LH receptor formation, while the nonpermeant diacylglycerol, diolein, was inactive. These results indicate that in situ activation of protein kinase C by PMA or permeant diacylglycerols inhibits cAMP-dependent granulosa cell differentiation, and suggest that the inhibitory actions of GnRH agonists on granulosa cell maturation are also mediated by protein kinase C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Resnick C. E. Forskolin-induced differentiation of cultured rat granulosa cells: new evidence for an intermediary role of adenosine 3',5'-monophosphate in the mechanism of action of follicle-stimulating hormone. Endocrinology. 1984 Jul;115(1):183–190. doi: 10.1210/endo-115-1-183. [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Svoboda M. E., Van Wyk J. J. A novel role for somatomedin-C in the cytodifferentiation of the ovarian granulosa cell. Endocrinology. 1984 Sep;115(3):1227–1229. doi: 10.1210/endo-115-3-1227. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Knecht M., Catt K. J. Hormonal regulation of cytodifferentiation and intercellular communication in cultured granulosa cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3000–3004. doi: 10.1073/pnas.78.5.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. D., Buxton I. L., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Putative effect of C kinase on alpha s-GTP-catalytic subunit interaction. J Biol Chem. 1985 Mar 10;260(5):2625–2628. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Darbon J. M., Knecht M., Ranta T., Dufau M. L., Catt K. J. Hormonal regulation of cyclic AMP-dependent protein kinase in cultured ovarian granulosa cells. Effects of follicle-stimulating hormone and gonadotropin-releasing hormone. J Biol Chem. 1984 Dec 10;259(23):14778–14782. [PubMed] [Google Scholar]
- Davis J. S., Farese R. V., Clark M. R. Gonadotropin-releasing hormone (GnRH) stimulates phosphatidylinositol metabolism in rat granulosa cells: mechanism of action of GnRH. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2049–2053. doi: 10.1073/pnas.80.7.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham C. R., Zhu H., Masters B. S., Simpson E. R., Mendelson C. R. Regulation of aromatase activity of rat granulosa cells: induction of synthesis of NADPH-cytochrome P-450 reductase by FSH and dibutyryl cyclic AMP. Mol Cell Endocrinol. 1985 May;40(2-3):211–219. doi: 10.1016/0303-7207(85)90177-7. [DOI] [PubMed] [Google Scholar]
- Hirota K., Hirota T., Aguilera G., Catt K. J. Hormone-induced redistribution of calcium-activated phospholipid-dependent protein kinase in pituitary gonadotrophs. J Biol Chem. 1985 Mar 25;260(6):3243–3246. [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Fibach E., Yamasaki H., Weinstein I. B. Tumor promoters inhibit morphological differentiation in cultured mouse neuroblastoma cells. Science. 1978 May 5;200(4341):556–559. doi: 10.1126/science.644318. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Clark M. R. Phorbol ester regulation of rat granulosa cell prostaglandin and progesterone accumulation. Endocrinology. 1985 Jun;116(6):2320–2326. doi: 10.1210/endo-116-6-2320. [DOI] [PubMed] [Google Scholar]
- Knecht M., Amsterdam A., Catt K. J. Inhibition of granulosa cell differentiation by gonadotropin-releasing hormone. Endocrinology. 1982 Mar;110(3):865–872. doi: 10.1210/endo-110-3-865. [DOI] [PubMed] [Google Scholar]
- Knecht M., Amsterdam A., Catt K. The regulatory role of cyclic AMP in hormone-induced of granulosa cell differentiation. J Biol Chem. 1981 Oct 25;256(20):10628–10633. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Leung P. C., Raymond V., Labrie F. Stimulation of phosphatidic acid and phosphatidylinositol labeling in luteal cells by luteinizing hormone releasing hormone. Endocrinology. 1983 Mar;112(3):1138–1140. doi: 10.1210/endo-112-3-1138. [DOI] [PubMed] [Google Scholar]
- Ludwig K. W., Niles R. M. Suppression of cyclic AMP-dependent protein kinase activity in murine melanoma cells by 12-0-tetradecanoylphorbol-13-acetate. Biochem Biophys Res Commun. 1980 Jul 16;95(1):296–303. doi: 10.1016/0006-291x(80)90738-x. [DOI] [PubMed] [Google Scholar]
- Naor Z., Yavin E. Gonadotropin-releasing hormone stimulates phospholipid labeling in cultured granulosa cells. Endocrinology. 1982 Nov;111(5):1615–1619. doi: 10.1210/endo-111-5-1615. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Takai Y., Kishimoto A., Kikkawa U., Kaibuchi K. Phospholipid turnover in hormone action. Recent Prog Horm Res. 1984;40:301–345. doi: 10.1016/b978-0-12-571140-1.50012-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Osborne R., Tashjian A. H., Jr Modulation of peptide binding to specific receptors on rat pituitary cells by tumor-promoting phorbol esters: decreased binding of thyrotropin-releasing hormone and somatostatin as well as epidermal growth factor. Cancer Res. 1982 Nov;42(11):4375–4381. [PubMed] [Google Scholar]
- Perchellet J. P., Boutwell R. K. Enhancement by 3-isobutyl-1-methylxanthine and cholera toxin of 12-O-tetradecanoylphorbol-13-acetate-stimulated cyclic nucleotide levels and ornithine decarboxylase activity in isolated epidermal cells. Cancer Res. 1980 Aug;40(8 Pt 1):2653–2660. [PubMed] [Google Scholar]
- Polan M. L., DeCherney A. H., Haseltine F. P., Mezer H. C., Behrman H. R. Adenosine amplifies follicle-stimulating hormone action in granulosa cells and luteinizing hormone action in luteal cells of rat and human ovaries. J Clin Endocrinol Metab. 1983 Feb;56(2):288–294. doi: 10.1210/jcem-56-2-288. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaloff D. L., Limbird L. E. Luteinizing hormone receptor appearance in cultured porcine granulosa cells requires continual presence of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5631–5635. doi: 10.1073/pnas.80.18.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Tureck R. W., Mastroianni L., Jr, Blasco L., Strauss J. F., 3rd Inhibition of human granulosa cell progesterone secretion by a gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1982 May;54(5):1078–1080. doi: 10.1210/jcem-54-5-1078. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Kolp L. A. Mechanisms subserving insulin's differentiating actions on progestin biosynthesis by ovarian cells: studies with cultured swine granulosa cells. Endocrinology. 1985 Feb;116(2):651–659. doi: 10.1210/endo-116-2-651. [DOI] [PubMed] [Google Scholar]
- Welsh T. H., Jr, Jones P. B., Hsueh A. J. Phorbol ester inhibition of ovarian and testicular steroidogenesis in vitro. Cancer Res. 1984 Mar;44(3):885–892. [PubMed] [Google Scholar]


