Abstract
The fungal pathogens Fusarium solani and Fusarium oxysporum cause severe corneal disease in the United States and worldwide and were the causative organisms in a recent outbreak of contact lens-associated keratitis. To characterize innate immunity in Fusarium keratitis, we developed a murine model in which conidia are injected into the corneal stroma. Immunocompetent C57BL/6 mice rapidly developed severe corneal opacification associated with neutrophil infiltration and clearance of Fusarium hyphae. In contrast, neutrophil infiltration was delayed in MyD88−/− mice, resulting in uncontrolled growth of Fusarium hyphae in the corneal stroma and anterior chamber, and eventually resulting in corneal perforation. Corneal opacification scores in TLR2−/−, TLR4−/−, and TLR2/4−/− mice were similar to those of C57BL/6 mice; however, TLR4−/− and TLR2/4−/− mice had impaired antifungal responses. The phenotype of infected IL-1R1−/− mice was similar to that of MyD88−/− mice, with uncontrolled fungal growth resulting in corneal perforation. IL-1R1−/− mice also produced significantly less CXCL1/KC in the corneal stroma compared with C57BL/6 mice consistent with delayed neutrophil recruitment to the corneal stroma. Together, these findings indicate that IL-1R1 and MyD88 regulate CXC chemokine production and neutrophil recruitment to the cornea, and that TLR4 has an important role in controlling growth and replication of these pathogenic fungi.
Fusarium species achieved notoriety as fungal pathogens after a recent outbreak of corneal infection related to a contact lens care solution in which the Centers for Disease Control and Prevention reported 318 cases of Fusarium keratitis in the United States in 2005 and 2006 (1), and appears to be associated with biofilm formation (2). Fusarium was already well recognized as an important cause of microbial keratitis unrelated to contact lens wear, with high prevalence in warm, humid areas of the United States (3) and in southern and southeastern Asia, especially India and China (4 – 6).
Members of the F. solani and Fusarium oxysporum species complex are ubiquitous in the environment, where they survive as plant pathogens and saprophytes (7), and Fusarium species and other filamentous fungi are an increasing cause of invasive human mycoses in immune-compromised or neutropenic individuals, where they cause systemic fusariosis and endophthalmitis (8 –11). However, the most common manifestation of Fusarium infection is keratitis, which occurs in otherwise healthy individuals engaged in agricultural work (12, 13). For example, recent studies on the prevalence of noncontact lens-associated microbial keratitis in India and China showed that Fusarium is a major cause of disease and that agricultural work is an important risk factor (4, 14).
Despite the frequency, chronicity, and severity of the disease, very little is known about the host response to Fusarium. Epidemiological studies indicate that the pathogenesis of this disease involves traumatic injury incurred during farm work facilitating entry of Fusarium spores (conidia) into the corneal stroma. Once present at this site, germination, toxin secretion, and hyphal invasion of the tissues either initiates a host response that controls infection at the expense of causing extensive tissue damage, or the host is unable to control Fusarium growth, and keratitis results from unregulated microbial growth and resulting tissue destruction.
Animal models of Fusarium keratitis are characterized by a profound neutrophil infiltrate and systemic steroid treatment causes unregulated fungal growth and subsequent destruction of the cornea (15–19), indicating an essential role for the host response in controlling fungal growth and development of keratitis. In the current study, we examined the host innate immune response to Fusarium using a murine model of keratitis in which Fusarium conidia are injected directly into the corneal stroma.
Our findings clearly demonstrate that development of Fusarium keratitis involves: 1) an essential role for MyD88 in Fusarium keratitis, since neutrophil recruitment to MyD88−/− corneal stroma is delayed and ineffective, resulting in unimpaired fungal growth in the stroma and anterior chamber, and corneal perforation; 2) no role for TLR2, because TLR2−/− mice have an identical phenotype as C57BL/6 mice; 3) a role for TLR4 in fungal killing, because TLR4−/− and TLR24−/− mice have delayed fungal clearance; and 4) an essential role for IL-1R1, which also signals through MyD88, because IL-1R1−/− mice have the same phenotype as MyD88−/− mice. These observations demonstrate a definitive role for the IL-1R1/MyD88 pathway in regulating Fusarium growth and severity of corneal disease and suggests a putative role for TLR4 in neutrophil-mediated antifungal activity.
Materials and Methods
Fusarium strain and growth conditions
F. oxysporum species complex strain MRL8996 (NRRL 47514) was obtained by corneal scraping from a patient with a severe case of fungal keratitis at the Cleveland Clinic Foundation (Cleveland, OH) as described (2). Organisms were stored at −80°C at the Center for Medical Mycology, University Hospitals Case Medical Center (Cleveland, OH), and a 50-μl stock was used to inoculate 50 ml of Sabouraud dextrose broth (Difco). After 48 h of growth under shaking incubator conditions at 30°C, a second culture was then inoculated and incubated under identical conditions for 40 h. To collect conidia, hyphae were removed by passing the culture suspension through sterile PBS-soaked gauze positioned at the tip of a 30-ml syringe. Conidia were centrifuged at 3000 rpm for 5 min., washed three times with PBS, and adjusted to 5 × 106 conidia/ml. Endotoxin-free water (MilliQ; Millipore) was used in all stages of fungal growth and isolation.
Source of mice
C57BL/6 mice (6 –12 wk old) were purchased from The Jackson Laboratory. TLR2-deficient, TLR4-deficient, and MyD88-deficient mice were provided by Shizuo Akira (Research Institute for Microbial Disease, Osaka University, Osaka, Japan). IL-1R-deficient mice on a C57BL/6 background were obtained from S. Mohr (Department of Medicine, Case Western Reserve University, Cleveland, OH). All animals were bred under specific pathogen-free conditions and maintained according to institutional guidelines.
Development of a mouse model of Fusarium keratitis
Based on preliminary dose-response studies, we found that 1 × 104 inoculum/2 μl (5 × 106 conidia/ml) in sterile PBS was optimal to induce keratitis and to recover organisms from immunocompetent C57BL/6 mice. An intrastromal corneal injection was performed by first abrading the epithelial layer using a 30-gauge needle, then injecting a 1 × 104 conidia in 2 μl using a 33-gauge Hamilton syringe. Mice were examined daily under a stereomicroscope for corneal opacification, ulceration, and perforation, and evaluated in a masked fashion by an experienced technician using an established scoring system (20, 21). At set time points, animals were sacrificed and eyes were either placed in 10% formaldehyde and embedded in paraffin and sectioned at 5-μm intervals, excised and placed in 1 ml of sterile saline, and homogenized for quantitative culture.
In vivo confocal microscopy
In vivo analysis of the corneal infiltration was evaluated using the ConfoScan3 microscope system (Nidek Technologies) as described in our previous studies (22, 23). Transparent gel (Genteal; Novartis Ophthalmics) was used between the corneal surface and objective, and corneas were examined using a ×40 objective. The software (NAVIS; Lucent Technologies) was used to capture sequential images of the entire cornea.
Quantitative fungal growth
Whole eyes were homogenized under sterile conditions using the Mixer Mill MM300 (Retsch) at 33 Hz for 4 min. Serial log dilutions were performed and plated onto Sabouraud dextrose agar (Difco). Plates were incubated at 30°C for 40 h and the number of CFU was determined by direct counting.
Histology and immunohistochemistry
Periodic acid-Schiff-hematoxylin staining of paraffin sections was performed following standard techniques. Briefly, after deparaffinization, slides were placed in periodic acid solution (Sigma-Aldrich) for 10 min and placed in Schiff’s solution (Sigma-Aldrich) for 20 min. The slides were counterstained with hematoxylin, then dehydrated and mounted under Per-mount (Fisher Scientific).
H&E staining was performed using Gill’s hematoxylin for 3–5 min following deparaffinization. The slides were dipped into glacial acetic acid solution, placed in Shandon Bluing Solution (ThermoShandon) for 1 min and counterstained with alcoholic Eosin Y solution (ThermoShandon). The slides were then dehydrated and mounted under Permount medium (Vector Laboratories).
To detect neutrophils, 5-μm paraffin sections were deparaffinized in Citrisolv (Fisher Scientific) and hydrated by serially dipping into 100, 95, and 80% ethanol, distilled water, and then PBS. Slides were blocked with 1.5% normal rabbit serum in PBS for 20 min, then incubated with primary rat anti-mouse neutrophil (NIMP-R14; Abcam) Ab with 1% fetal cow serum for 2 h at room temperature. The slides were then washed in PBS plus 0.05% Tween 20 and incubated with goat anti-rat-biotin (Southern Bio-technology Associates) diluted 1/200 in PBS for 30 min. The slides were washed in PBS/Tween 20 and streptavidin/alkaline phosphatase (Bio-Genex) and incubated for 30 min. Vector Red Solution (Vector Laboratories) was prepared in Tris buffer adjusted to pH 8.3 and was added to the slides and incubated in the dark for 10 min. The slides were counterstained in Gill’s hematoxylin (Vector Laboratories), then treated with 2% glacial acetic acid and 0.5% lithium carbonate. The slides were then dehydrated and placed again in Citrisolv, then mounted with Permount medium and allowed to dry overnight.
Cytokine assays
Infected corneas were dissected and stored overnight in 5% Natamycin Solution (Alcon). (Sterility was ensured by inoculating samples onto Sabouraud dextrose agar.) Corneal samples were then homogenized using the Retsch MM 300 ball miller at 33 Hz for 4 min. Half-well cytokine assays were performed using Duoset ELISA kits (R&D Systems) according to the manufacturer’s directions.
Statistics
Statistical analysis was performed using GraphPad Prism software. To determine statistical significance, we used nonparametric one-way ANOVA with post hoc Tukey’s multiple comparison. Differences were considered significant at p < 0.05.
Results
MyD88−/− mice develop delayed, but nonresolving corneal opacification
MyD88 has an important role in regulating the host response to systemic and pulmonary Aspergillus fumigatus infections and to systemic infection with Cryptococcus neoformans and Candida albicans (24 –26). To determine the role of MyD88 in Fusarium keratitis, corneas of C57BL/6 and MyD88−/− mice were inoculated with 1 × 104 conidia in 2 μl, the severity of keratitis was examined by slit-lamp microscopy, and clinical scores were evaluated in a masked fashion as described previously (21).
As shown in Fig. 1, C57BL/6 mice developed severe corneal opacification within 24 h, which remained high for 7 days and gradually resolved over 14 days. In marked contrast, MyD88−/− mice had moderate corneal opacification after 24 h compared with C57BL/6 mice (Fig. 1). However, by 48 h, MyD88−/− corneas showed increased corneal opacification scores and in contrast to C57BL/6 mice, corneal disease in MyD88−/− progressed rapidly to perforation after 72 h. Clinical stromal disease scores for day 1 are shown in Fig. 2. Together, these findings demonstrate that MyD88 regulates the outcome of Fusarium keratitis.
FIGURE 1.
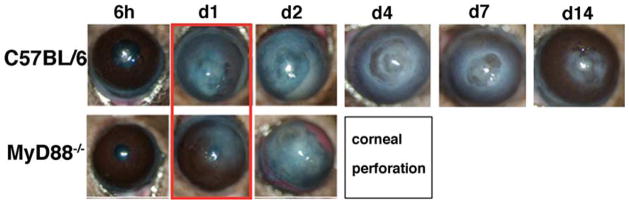
Fusarium- induced corneal opacification in MyD88−/− mice. Corneal opacification was examined using slit-lamp biomicroscopy in C57BL/6 and MyD88−/− mice after intrastromal injection of 1 × 104 conidia. Representative corneas at each time point are shown, with the major difference highlighted on day 1. Data are representative of four experiments with five mice per group.
FIGURE 2.
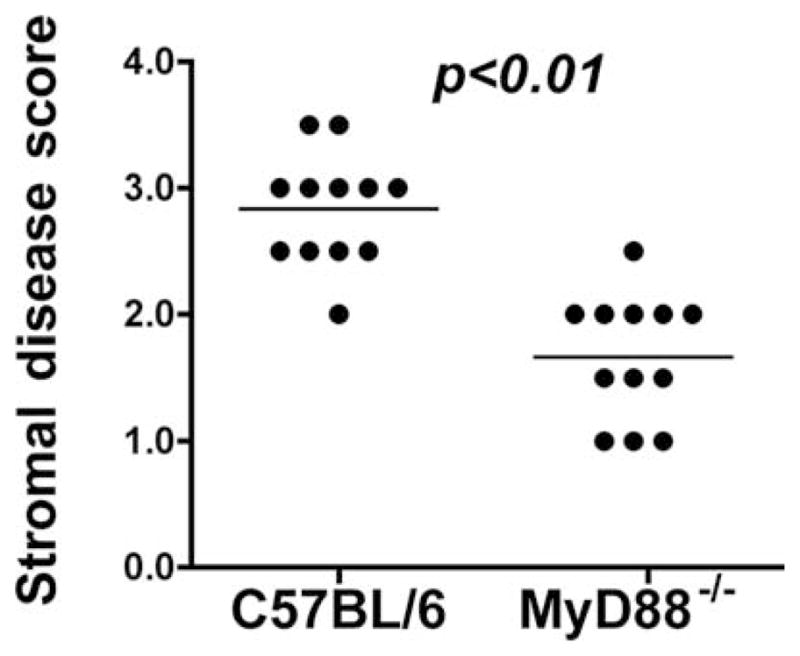
Quantification of corneal opacification. Stromal disease scores on day 1 were based on previously described criteria (21): 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; and +3, +4 = dense or extremely dense opacity, covering the entire anterior segment. Data points indicate individual corneas and are representative of three repeat experiments.
Impaired neutrophil infiltration and fungal clearance in MyD88−/− corneas
To examine the early response of C57BL/6 and MyD88−/− mice to Fusarium challenge, before detectable corneal opacification, corneas were examined by in vivo confocal microscopy, which provides a series of images throughout the cornea and can clearly identify cellular infiltrates in the corneal stroma (22, 23). In vivo confocal microscopy images of the central corneal stroma 6 h after injection of conidia revealed extensive growth of hyphal elements in the central corneal stroma in C57BL/6 and in MyD88−/− mice (Fig. 3, a and c), indicating that conidia had germinated in both strains of mice by this time point.
FIGURE 3.
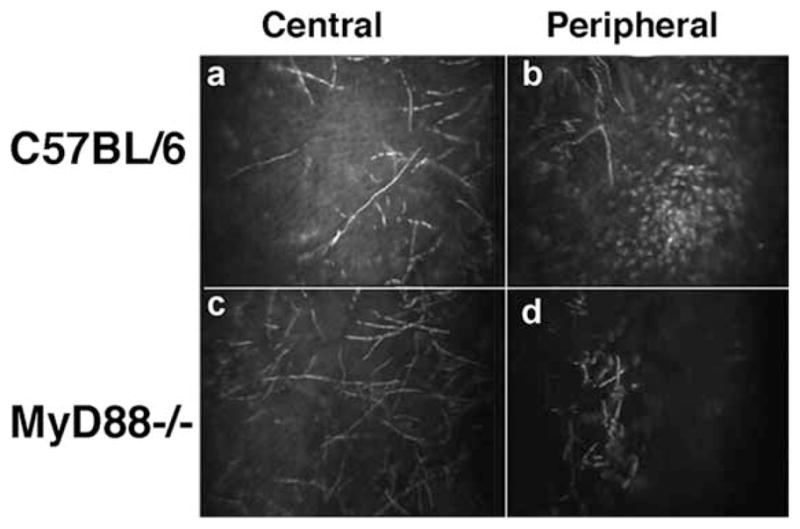
In vivo confocal microscopy of C57BL/6 and MyD88−/− corneas. C57BL/6 and MyD88−/− mice were injected intrastromally with 1 × 104 conidia from a clinical isolate of F. oxysporum. After 6 h, mice were examined by in vivo confocal microscopy (Confoscan). Representative images from the central and peripheral corneal stroma are shown. Note the presence of hyphae in the central corneal stroma of C57BL/6 and MyD88−/− mice (a and c); however, a cellular infiltrate is present in the peripheral cornea of C57BL/6, but not MyD88−/− mice (b and d).
Because the mammalian cornea is normally avascular, cellular infiltration occurs from peripheral limbal vessels. As shown in Fig. 3, b and d, cellular infiltration in the peripheral corneal stroma was clearly detected in C57BL/6 mice, but was absent in MyD88−/− mice. This observation demonstrates a critical role for MyD88 in cellular infiltration to the corneal stroma.
To characterize the cellular infiltrate, Fusarium-infected C57BL/6 and MyD88−/− corneas were examined by histology and immunohistochemical analyses 24 and 48 h after intrastromal injection of conidia. Fig. 4 shows corneal sections stained with periodic acid-Schiff for the presence of fungi and counterstained with hematoxylin. Neutrophils were identified by immunostaining using mAb NIMPR-14 (22).
FIGURE 4.
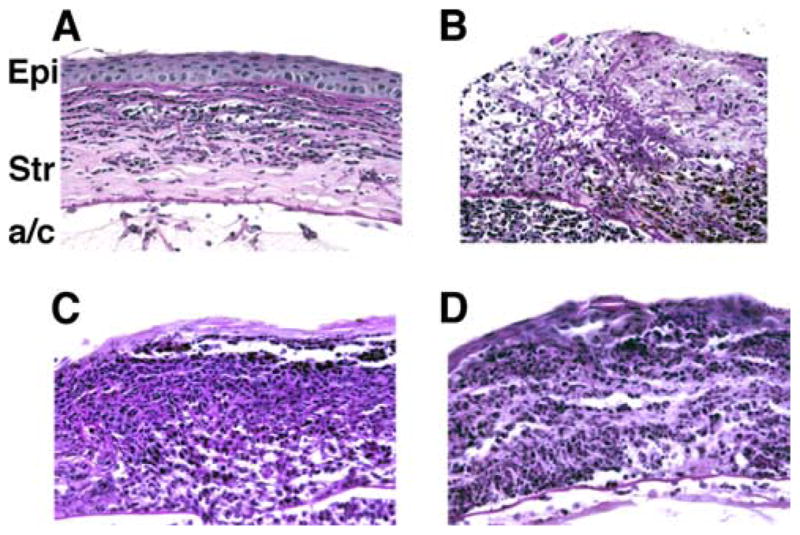
Histological analysis of C57BL/6 and MyD88−/− corneas. Fusarium conidia (1 × 104) were inoculated into the corneal stroma of C57BL/6 and MyD88−/− mice. After 6, 24, and 48 h, mice were sacrificed and eyes were processed for histology and immunohistochemistry. A and B, Representative sections of MyD88−/− corneas showing Fusarium hyphae in the corneal stroma (Str) and anterior chamber (a/c) 24 h (A) and 48 h (B) after inoculation of conidia. C and D, C57BL/6 corneas 24 h (C) and 48 h (D) after inoculation. Note the intense cellular infiltrate in the corneal stroma and anterior chamber of C57BL/6 mice compared with MyD88−/− mice and, conversely, the presence of Fusarium hyphae of MyD88−/− but not C57BL/6 mice. Original magnification, ×400. Epi, Epithelium.
As shown in Fig. 4 (upper panels), hyphae were present throughout the MyD88−/− corneal stroma 24 h after intrastromal injection and in the anterior chamber, indicating that Fusarium hyphal elements had penetrated Descemet’s (basement) membrane. By 48 h, hyphae had replicated throughout the corneal stroma and anterior chamber. In contrast to MyD88−/− corneas, Fusarium hyphae were rarely detected in the C57BL/6-infected cornea (Fig. 4, lower panels); however, there was a pronounced cellular infiltrate in the stroma and anterior chamber within 24 h. Immunostaining with NIMPR-14 showed that the infiltrate at both sites was predominantly neutrophils (Fig. 5), thereby demonstrating that MyD88 regulates neutrophil infiltration to the corneal stroma in Fusarium keratitis.
FIGURE 5.
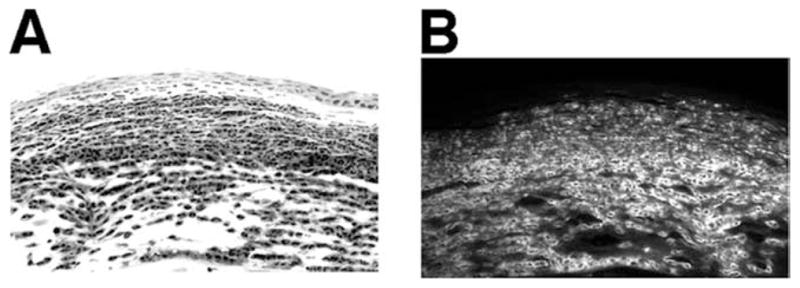
Neutrophils as a major component of the cellular infiltrate. C57BL/6 mice were inoculated intrastromally with Fusarium conidia as described in the legend to Fig. 3, eyes were dissected 24 h after inoculation, and 5-μm paraffin sections were immunostained with NIMPR-14, which specifically identifies murine neutrophils. Representative corneal sections are shown by light (A) and fluorescence (B) microscopy. Note that neutrophils are a major component of the cellular infiltrate. Sections are representative of four repeat experiments with five mice per group. Original magnification, ×400.
MyD88 regulates Fusarium replication in the cornea
To determine the role of MyD88 in fungal replication in the cornea and anterior chamber, 1 × 104 conidia were injected into the corneal stroma of C57BL/6 and MyD88−/− mice, and after 6, 24, and 48 h, eyes were dissected, homogenized, and plated for CFU determination. As shown in Fig. 6, although similar numbers of organisms were recovered from C57BL/6 and MyD88−/− mice at 6 h, after 24 and 48 h, the number of CFU diminished in C57BL/6 mice until no colonies were detected after 72 h. In contrast, the number of CFU isolated from MyD88−/− eyes increased over 48 h, which is consistent with the presence of hyphae in the cornea and anterior chamber. Together, these findings demonstrate an essential role for MyD88 in regulating fungal growth and replication, and support the notion that neutrophil infiltration is important in this process.
FIGURE 6.
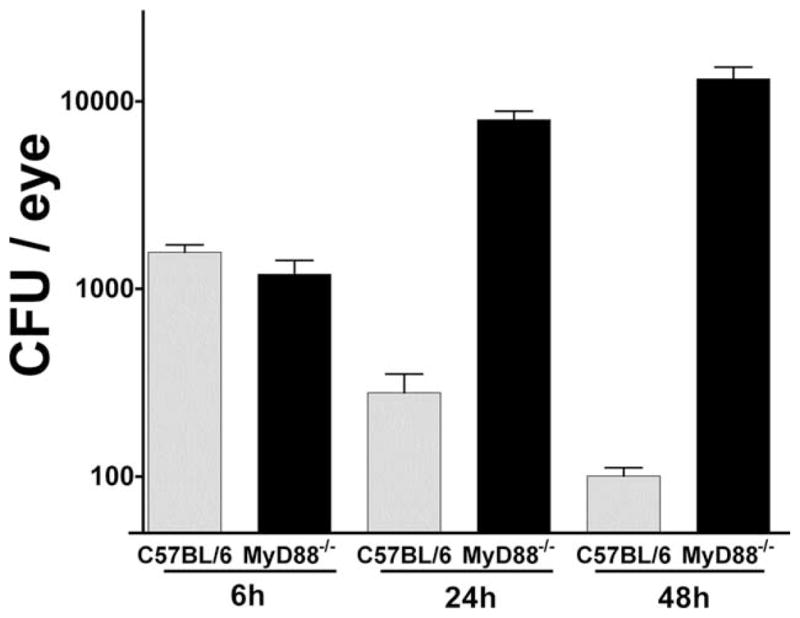
CFU from C57BL/6 and MyD88−/− eyes. Fusarium conidia (1 × 104) were inoculated into the corneal stoma of C57BL/6 and MyD88−/− mice. After 6, 24, and 48 h, mice were sacrificed, whole eyes (including cornea and anterior chamber) were homogenized, diluted, and the number of CFU was determined by direct counting. Note the reduction of Fusarium CFU from C57BL/6 mice over time, but increased CFU in MyD88−/− mice. Graphs are mean ± SEM for five mice per group. ANOVA comparing CFU from C57BL/6 and MyD88−/− eyes was p < 0.001 at 24 and 48 h. This experiment was repeated three times with similar results.
TLR4 mediates antifungal activity, but not neutrophil infiltration to the corneal stroma or development of keratitis
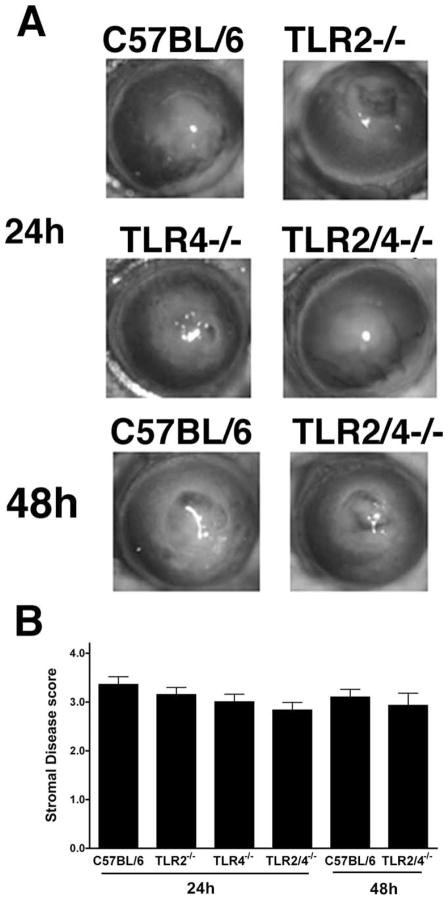
Since all TLRs except TLR3 signal through MyD88 (27) and TLR2 or TLR4 regulate the outcome of infections with A. fumigatus (28 –31) and C. albicans (32–34), we next examined whether the central role for MyD88 in Fusarium keratitis is based on activation of these pathogen recognition receptors. C57BL/6, TLR2−/−, TLR4−/−, and TLR2/4−/− mice were inoculated intrastromally with 1 × 104 conidia, and corneal opacification, neutrophil infiltration, and fungal killing was measured as described above.
Clinical evaluation of keratitis in TLR2−/−, TLR4−/−, and TLR2/4−/− mice compared with C57BL/6 mice showed no differences in corneal opacification among any of the strains after 24 or 48 h (Fig. 7). Consistent with this observation, the neutrophil infiltrate in TLR2−/−, TLR4−/−, and TLR2/4−/− mice was similar to that in C57BL/6 mice, indicating that there is no role for TLR2 or TLR4 in modulating cellular infiltration to the corneal stroma.
FIGURE 7.
Fusarium- induced corneal opacification in TLR2−/−, TLR4−/−, and TLR2/4−/− mice. Fusarium conidia (1 × 104) were inoculated into the corneal stoma of C57BL/6, TLR2−/−, TLR4−/−, and TLR2/ 4−/− mice. After 24 and 48 h, corneas were examined by slit-lamp biomicroscopy and scored as described above. A, Representative eyes of each group of mice. B, Clinical scores ± SEM for five mice per group. Note that there was no difference in corneal opacification among these mouse strains. This experiment was repeated three times with similar results.
Closer examination of histological sections revealed Fusarium hyphae in TLR4−/− and TLR2/4−/− corneas (Fig. 8, A and B), but not in TLR2−/− or C57BL/6 corneas (data not shown). To quantify the effect of TLR4 on Fusarium survival in the cornea and anterior chamber, eyes were homogenized and analyzed by CFU evaluation. As shown in Fig. 8C, ~500 CFU were recovered from TLR2−/− and C57BL per six eyes after 24 h, whereas ~2500 CFU were recovered from TLR4−/− and TLR2/4−/− eyes. No differences were detected between C57BL/6 mice and TLR2 mice or between TLR4−/− and TLR2/4−/− mice, indicating that TLR2 does not contribute to protection against Fusarium infections. After 48 h, CFU were significantly reduced in all strains and fungi were not detected after 72 h.
FIGURE 8.
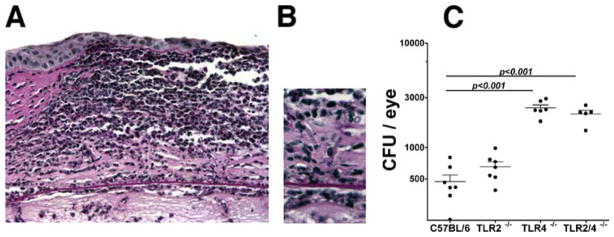
Fusarium survival in TLR4−/− and TLR2/4−/− mice compared with C57BL/6 and TLR2−/− mice. F. oxysporum conidia (1 × 104) were inoculated into the corneal stoma of C57BL/6, TLR2−/−, TLR4−/−, and TLR2/4−/− mice. After 24 h, 5-μm corneal sections were examined after periodic acid-Schiff/hematoxylin staining. A, Representative corneal section from a TLR2/4−/− mouse showing intense cellular infiltrate. B, Fusarium hyphae can be detected in the posterior corneal stroma. Original magnification, ×400. C, CFU in eyes dissected from C57BL/6, TLR2−/−, TLR4−/−, and TLR2/4−/− mice 24 h after inoculation. Note that TLR4−/− and TLR2/4−/− mice show significantly higher CFU compared with C57BL/6 and TLR2−/− mice. Data points represent individual eyes. The experiment was repeated three times with similar results.
Taken together, these findings indicate that: 1) TLR2 and TLR4 do not regulate cellular infiltration and development of corneal opacification; and 2) TLR4 has a significant role in antifungal activity, possibly when expressed on neutrophils.
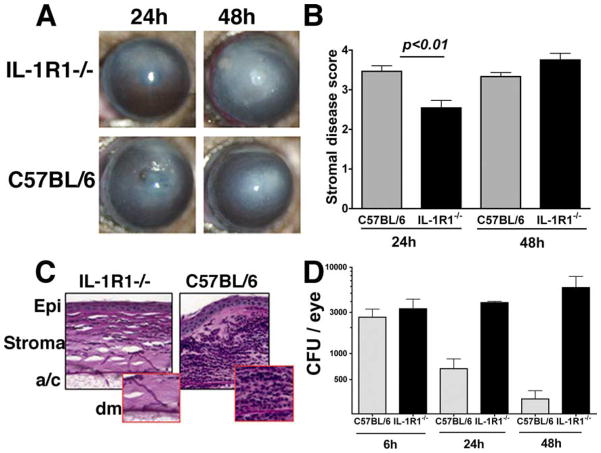
IL-1R1 is essential for fungal clearance, neutrophil infiltration to the corneal stroma, and development of keratitis
In addition to TLRs, IL-1R1 signals through the MyD88 common adaptor molecule (27); therefore, we next examined the role of this cytokine receptor in Fusarium keratitis. Corneas of C57BL/6 and IL-1R1−/− mice were inoculated intrastromally with Fusarium conidia as before and corneal opacification was assessed by slit-lamp examination. Fig. 9, A and B, shows that as with MyD88−/− mice, development of corneal opacification was impaired in severity in IL-1R1−/− mice compared with C57BL/6 mice after 24 h, increased at 48 h, and eventually perforated (data not shown). Histological analysis showed that IL-1R1−/− mice had few neutrophils, but numerous Fusarium hyphae were observed in the corneal stroma and anterior chamber compared with C57BL/6 mice (Fig. 8C). Consistent with these findings, CFU analysis showed that hyphae continued to replicate in IL-1R1−/− eyes in contrast to C57BL/6 mice where CFU were reduced by 90% after 72 h (Fig. 9D).
FIGURE 9.
Fusarium keratitis in IL-1R1−/− mice. Fusarium conidia (1 × 104) were inoculated into the corneal stoma of C57BL/6 and IL-1R1−/− mice. After 24 and 48 h, mice were examined by slit-lamp biomicroscopy and eyes were processed for histology or CFU as described above. A, Representative IL-1R1−/− and C57BL/6 corneas 24 h after intrastromal injection. B, Clinical scores at 24 h. C, Representative histological sections of IL-1R1−/− and C57BL/6 corneas. Original magnification, ×400. D, CFU in eyes from C57BL/6 and IL-1R1−/− mice recovered 24, 48, and 72 h after intrastromal injection. CFU are mean ± SEM of 5–10 mice/group. ANOVA comparing CFU from C57BL/6 and IL-1R1−/− eyes revealed p < 0.05 at 24 h and p < 0.01 at 48 h. The experiment was repeated three times with similar results. Epi, Corneal epithelium; a/c, anterior chamber; Dm, Descemet’s (basement) membrane.
Taken together, these findings demonstrate that IL-1R1−/− mice have a similar phenotype as MyD88−/− mice, in which corneal opacification in IL-1R1−/− mice reflects unregulated Fusarium replication rather than infiltrating neutrophils, and demonstrates that the outcome of Fusarium keratitis is mediated by an IL-1R1/ MyD88-dependent pathway.
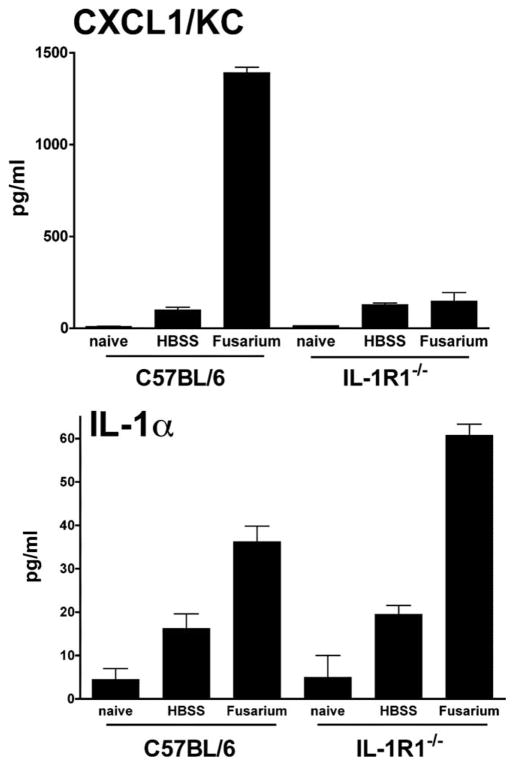
CXCL1/KC production in the cornea is dependent on IL-1R1
Since neutrophil recruitment to the corneal stroma is associated with clearance of the organisms, and our previous studies showed an essential role for CXCL1/KC in neutrophil recruitment to the corneal stroma (35), we next determined whether IL-1R1 mediates production of this chemokine in keratitis. C57BL/6 and IL-1R1−/− mice were inoculated intrastromally with 1 × 104 conidia as before, corneas were dissected after 4 h (before neutrophil infiltration), homogenized, and CXCL1 and IL-1α production was measured by ELISA.
As shown in Fig. 10, C57BL/6 mice produced high levels of CXCL1 in the cornea in response to Fusarium. In contrast, CXCL1 production in corneas of IL-1R1−/− mice was not elevated compared with trauma control, indicating that IL-1R1 has an essential role in CXC chemokine production. IL-1α production was not inhibited in IL-1R1−/− mice, indicating that these mice recognize and respond to Fusarium. IL-1β was not detected at this time point.
FIGURE 10.
CXCL1/KC and IL-1α production by C57BL/6 and IL-1R1−/− corneas. Fusarium conidia (1 × 104) were injected into the corneal stroma of C57BL/6 and IL-1R1−/− mice. After 4 h, corneas were dissected, homogenized, and insoluble material was removed by centrifugation. CXCL1/KC and IL-1α in supernatants was measured by ELISA. Data are mean ± SEM of five mice per group and are representative of two repeat experiments.
Discussion
The normal mammalian cornea maintains its transparent nature because of the barrier function of the external corneal epithelium, the highly organized, antiparallel arrangement of collagen fibrils, and the single layer of corneal endothelial cells lining the anterior chamber, which maintains the essential hydration level. Other factors also contribute to corneal transparency, including the translucent nature of the resident stromal keratocytes and the absence of blood vessels and resident inflammatory cells such as neutrophils. During microbial infection, the cornea becomes opaque after release of cytotoxic mediators from microbes and from activated neutrophils that infiltrate the corneal stroma.
Our previous studies showed that activation of TLR2 and TLR4 through MyD88 in the cornea rapidly induces production of proinflammatory and chemotactic cytokines, which lead to neutrophil recruitment to the corneal stroma and loss of corneal clarity (22, 23). Results from the current study show that although MyD88 is essential for neutrophil recruitment in response to Fusarium, TLR2 and TLR4 have no effect on this stage of the host response. Instead, we found a role for TLR4 in fungal killing, as the number of Fusarium colonies recovered from whole eyes (including cornea and anterior chamber) was significantly higher in TLR4−/− and TLR2/4−/− mice compared with C57BL/6 and TLR2−/− mice. The observation that TLR4 is important in fungal killing but not neutrophil recruitment suggests that it is TLR4 expression on neutrophils that mediates antifungal activity, possibly by production of reactive oxygen species (ROS).3 Although not shown in the current study, it is likely that MyD88 is also involved in TLR4 activation of neutrophils. TLR2 and TLR4 are important in the host response to other filamentous fungi, such as A. fumigatus (28, 31), and to the pathogenic yeasts C. albicans and Cryptococcus neoformans (32, 36). In addition, hyphal and macroconidia forms of the organisms stimulate different TLRs, including TLR4 activation of oxidative pathways (28, 34). Production of ROS by neutrophils is important in regulating fungal hyphae that cannot be ingested, since mice that are defective in ROS production are highly susceptible to infection with fungal pathogens such as C. albicans (37, 38), and this mechanism is likely to be involved in limiting Fusarium growth in the cornea and anterior chamber.
Given that neutrophil recruitment to the corneal stroma is MyD88 dependent, but independent of TLR2 and TLR4, we examined the role of IL-1R1, which also signals through MyD88. We found that IL-1R1 is required for production of CXCL1/KC and neutrophil recruitment, consistent with the role of IL-1 and CXC chemokines in LPS and Pseudomonas keratitis (36, 39). However, at least two observations indicate that other pathogen recognition molecules are involved in the pathogenesis of Fusarium keratitis: 1) IL-1R1 is required for CXC chemokine, but not IL-1α production, indicating that an unidentified receptor is needed for IL-1 production in the cornea; and 2) although TLR4 has an important role in fungal killing, the organisms are eventually cleared in TLR4−/− and TLR2/4−/− mice, indicating that other receptors are also involved. Future studies will examine the role of C-type lectins including Dectin-1, which is expressed on macrophages and neutrophils, recognizes β-(1,3)-glucan on the fungal cell wall, and has a cooperative role with TLRs (40 – 45). In addition, complement receptor 3 (CD11b/CD18) is expressed on neutrophils and is associated with ROS production (38). Macrophages and dendritic cells, but not neutrophils, are present in normal corneal stroma (46 – 49) and are likely to produce IL-1α and IL-1β in response to fungal cell wall components.
Taken together, we propose that the events leading to keratitis in immune-competent animals involves: 1) recognition of Fusarium by resident cells in the corneal stroma and production of IL-1α; 2) IL-1R1/MyD88-dependent CXC chemokine production and recruitment of neutrophils from peripheral vessels to the central cornea; 3) TLR4-dependent antifungal activity by neutrophils; 4) tissue damage and development of corneal opacification due to production of cytotoxic mediators by fungi or by products of neutrophil degranulation; and 5) eventual corneal scarring and resolution of inflammation. Future studies will examine the role of C-type lectins and additional host and fungal virulence factors that regulate the pathogenesis of this disease.
Acknowledgments
We express thanks to Eugenia Diaconu, Scott Howell, and Catherine Doller for excellent technical assistance. We also thank Dr. Shigeru Akira for TLR2, TLR4, and MyD88 gene knockout mice, Dr. Susanne Mohr for IL-1R gene knockout mice, and Drs. David Geiser and Kerry O’Donnell for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grants R01EY018612 (to E.P.), P30-EY11373 (to E.P.), R01DE017486 (to M.A.G.), and R01DE13932 (to M.A.G.) and by a Research to Prevent Blindness Foundation Senior Investigator Award (to E.P.). We also acknowledge support from the National Institutes of Health (R01 AI035097-10) and Bristol-Myers Squibb Freedom to Discover Award (to M.A.G.), American Heart Association Scientist Development Grant 0335313N (to P.K.M.), and Research to Prevent Blindness and Ohio Lions Eye Research Foundation unrestricted awards (to J.H.L.).
Abbreviation used in this paper: ROS, reactive oxygen species.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FM, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. J Am Med Assoc. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 2.Imamura Y, Chandra J, Mukherjee PK, Lattif AA, Szczotka-Flynn LB, Pearlman E, Lass JH, O’Donnell K, Ghannoum MA. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52:171–182. doi: 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101:1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 4.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61– 69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129–141. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhavan M, Ratnakar C, Veliath AJ, Kanungo R, Smile SR, Bhat S. Primary disseminated fusarial infection. Postgrad Med J. 1992;68:143–144. doi: 10.1136/pgmj.68.796.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayayo E, Guarro J, Pujol I. Endogenous endophthalmitis by Fusarium solani: an animal experimental model. Med Mycol. 1998;36:249–253. doi: 10.1080/02681219880000401. [DOI] [PubMed] [Google Scholar]
- 10.Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J Med Microbiol. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- 11.Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, Kontoyiannis DP. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis. 2006;42:1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 12.Thomas PA. Fungal infections of the cornea. Eye. 2003;17:852– 862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 13.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong WX, Sun SY, Zhao J, Shi WY, Xie LX. Retrospective study of suppurative keratitis in 1054 patients. Zhonghua yan ke za zhi. 2007;43:245–250. [PubMed] [Google Scholar]
- 15.Forster RK, Rebell G. Animal model of Fusarium solani keratitis. Am J Ophthalmol. 1975;79:510–515. doi: 10.1016/0002-9394(75)90629-7. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi Y, Kaufman HE, Kagawa S. Comparison of the pathogenicities of Fusarium solani and Candida albicans in the rabbit cornea. J Med Vet Mycol. 1986;24:369–376. [PubMed] [Google Scholar]
- 17.Kiryu H, Yoshida S, Suenaga Y, Asahi M. Invasion and survival of Fusarium solani in the dexamethasone-treated cornea of rabbits. J Med Vet Mycol. 1991;29:395– 406. [PubMed] [Google Scholar]
- 18.O’Day DM, Ray WA, Head WS, Robinson RD, Williams TE. Influence of corticosteroid on experimentally induced keratomycosis. Arch Ophthalmol. 1991;109:1601–1604. doi: 10.1001/archopht.1991.01080110139051. [DOI] [PubMed] [Google Scholar]
- 19.Wu TG, V, Keasler V, Mitchell BM, Wilhelmus KR. Immunosuppression affects the severity of experimental Fusarium solani keratitis. J Infect Dis. 2004;190:192–198. doi: 10.1086/421300. [DOI] [PubMed] [Google Scholar]
- 20.Hazlett LD. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 2002;21:383–390. doi: 10.1089/10445490260099665. [DOI] [PubMed] [Google Scholar]
- 21.Pearlman E, Lass JH, Bardenstein DS, Kopf M, Hazlett FE, Jr, Diaconu E, Kazura JW. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness) J Exp Med. 1995;182:931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of Toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthal Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 23.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 24.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, Pamer EG. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665– 675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Biondo C, Midiri A, Messina L, Tomasello F, Garufi G, Catania MR, Bombaci M, Beninati C, Teti G, Mancuso G. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur J Immunol. 2005;35:870– 878. doi: 10.1002/eji.200425799. [DOI] [PubMed] [Google Scholar]
- 26.Villamon E, Gozalbo D, Roig P, Murciano C, O’Connor JE, Fradelizi D, Gil ML. Myeloid differentiation factor 88 (MyD88) is required for murine resistance to Candida albicans and is critically involved in Candida-induced production of cytokines. Eur Cytokine Network. 2004;15:263–271. [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, Mosci P, Lipford GB, Pitzurra L, Romani L. TLRs govern neutrophil activity in aspergillosis. J Immunol. 2004;173:7406–7415. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 29.Braedel S, Radsak M, Einsele H, Latge JP, Michan A, Loeffler J, Haddad Z, Grigoleit U, Schild H, Hebart H. Aspergillus fumigatus antigens activate innate immune cells via Toll-like receptors 2 and 4. Br J Haematol. 2004;125:392–399. doi: 10.1111/j.1365-2141.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 30.Netea MG, Warris A, Van der Meer JW, Fenton MJ, Verver-Janssen TJ, Jacobs LE, Andresen T, Verweij PE, Kullberg BJ. Aspergillus fumigatus evades immune recognition during germination through loss of Toll-like receptor-4-mediated signal transduction. J Infect Dis. 2003;188:320–326. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 31.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5:561–570. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 32.Blasi E, Mucci A, Neglia R, Pezzini F, Colombari B, Radzioch D, Cossarizza A, Lugli E, Volpini G, Del Giudice G, Peppoloni S. Biological importance of the two Toll-like receptors, TLR2 and TLR4, in macrophage response to infection with Candida albicans. FEMS Immunol Med Microbiol. 2005;44:69–79. doi: 10.1016/j.femsim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 34.van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukocyte Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Miyagi K, Koguchi Y, Kinjo Y, Uezu K, Kinjo T, Akamine M, Fujita J, Kawamura I, Mitsuyama M, et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol. 2006;47:148–154. doi: 10.1111/j.1574-695X.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 37.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavigne LM, Albina JE, Reichner JS. β-Glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667– 8675. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- 39.Rudner XL, Kernacki KA, Barrett RP, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000;164:6576– 6582. doi: 10.4049/jimmunol.164.12.6576. [DOI] [PubMed] [Google Scholar]
- 40.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 41.Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 42.Reid DM, Montoya M, Taylor PR, Borrow P, Gordon S, Brown GD, Wong SY. Expression of the β-glucan receptor, Dectin-1, on murine leukocytes in situ correlates with its function in pathogen recognition and reveals potential roles in leukocyte interactions. J Leukocyte Biol. 2004;76:86–94. doi: 10.1189/jlb.0104031. [DOI] [PubMed] [Google Scholar]
- 43.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 44.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun. 2005;73:1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 47.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]