Abstract
The mammalian cornea contains an extensive network of resident macrophages and dendritic cells. To determine the role of these cells in LPS-induced corneal inflammation, TLR4−/− mice were sublethally irradiated and reconstituted with bone marrow cells from either enhanced GFP (eGFP)+/C57BL/6 or eGFP+/TLR4−/− mice. The corneal epithelium was abraded, LPS was added topically, and cellular infiltration to the corneal stroma and development of corneal haze were examined after 24 h. TLR4−/− mice reconstituted with C57BL/6, but not TLR4−/− bone marrow cells donor cells were found to cause infiltration of eGFP+ cells to the cornea, including neutrophils, and also increased corneal haze compared with saline-treated corneas. In a second experimental approach, corneas of transgenic macrophage Fas induced apoptosis (Mafia) mice were stimulated with LPS. These mice express eGFP and a suicide gene under control of the c-fms promoter, and systemic treatment with the FK506 dimerizer (AP20187) causes Fas-mediated apoptosis of monocytic cells. AP20187-treated mice had significantly fewer eGFP+ cells in the cornea than untreated mice. After stimulation with LPS neutrophil recruitment and development of corneal haze were impaired in AP20187-treated mice compared with untreated controls. Furthermore, LPS induced CXCL1/KC and IL-1α production within 4 h in corneas of untreated Mafia mice, which is before cellular infiltration; however, cytokine production was impaired after AP20187 treatment. Together, results from both experimental approaches demonstrate an essential role for resident corneal monocytic lineage cells (macrophages and dendritic cells) in development of corneal inflammation.
The TLR family of pathogen recognition molecules plays a critical role in recognizing and responding to microbial pathogens, initiating antimicrobial responses that can also cause tissue damage (1, 2). In the eye, inflammatory responses that disrupt the refractive and transparent properties of ocular tissues along the visual axis, such as the cornea and lens, can have a devastating effect on visual function, which ultimately impacts on quality of life. The cornea functions not only as the principle refractory tissue of the eye, but also as a physical barrier to trauma and infection. When the cornea is subjected to trauma in the context of infection or exposure to microbial products, TLRs are activated and enable the host to recognize and respond to pathogenic microorganisms at the ocular surface (3, 4). Using animal models of LPS keratitis, we and others demonstrated that LPS induces pronounced neutrophil infiltration to the corneal stroma and loss of corneal clarity (1, 2, 5–14).
Although the mammalian cornea was for many years thought to be devoid of myeloid cells, recent studies demonstrate that the healthy mammalian corneal stroma and epithelium contain heterogeneous populations of macrophages and dendritic cells (DCs)4 (15–19). The murine corneal epithelium contains populations of MHC class II+ CD11c+ (similar to Langerhans cells in the skin) and MHC class II− CD11c+ DCs, while the corneal stroma also contains populations of DCs (19–22) and extensive CD11b+ tissue macrophages (15, 17, 18). Our previous studies showed that CX3CR1 (Fractalkine receptor) is important for homing of Langerhans cells to the corneal epithelium (17), and that DCs in the corneal stroma extend membrane nanotubes that are likely important for cell-cell communication, and which are increased after trauma and LPS stimulation (16). However, a definitive function for corneal DCs and tissue macrophages in innate immunity has not been demonstrated. Therefore, in the current study, we examined whether these cells contribute to the host response to bacterial products and induce an inflammatory response.
We used two complementary approaches to identify the potential role of macrophages and DCs in corneal inflammation: firstly, as enhanced GFP (eGFP)+ donor cells of myeloid lineage have been clearly shown in bone marrow chimeric mouse models to reconstitute the host cornea (8, 23), we used myeloid-derived donor eGFP+ TLR4+/+ cells to assess LPS responses in corneas of recipient TLR4−/− mice. In the second approach, we used a transgenic mouse model expressing an inducible suicide gene on a C-FMS promoter (c-fms), activation of which leads to fas-mediated killing of a significant portion of cells of the monocyte/macrophage lineage, including DCs (24). Our findings from both approaches support the conclusion that myeloid lineage cells in the cornea are the cells that primarily mediate LPS-induced corneal inflammation. These findings have implications for our understanding of corneal infections and corneal inflammation, which are important causes of blindness and visual impairment worldwide.
Materials and Methods
Animals
For bone marrow chimera experiments, 6- to 12-wk-old eGFP C57BL/6TgN (ACTbEGFP)10sb mice were used as donors, with C57BL/6 and TLR4−/− mice as recipients (The Jackson Laboratory). TLR4−/− mice, provided by Dr. S. Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan), were fully backcrossed to C57BL/6 mice. TLR4−/− mice were bred with eGFP C57BL/6TgN mice to produce eGFP TLR4−/− mice, which remained nonresponsive to LPS (data not shown) and could be tracked in vivo. All animals were housed in specific pathogen-free conditions in microisolator cages and were treated in accordance with the guidelines provided in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Generation of eGFP chimeric mice
Bone marrow cells from C57BL/6TgN (ACTbEGFP)10sb (eGFP) mice or eGFP mice crossed with TLR4−/− were isolated as described previously (8). Following euthanasia by CO2 asphyxiation, femurs and tibias were removed and the shafts centrifuged at 10,000 rpm for 30 s at 4°C. The pellet was resuspended in 1 ml of sterile RBC lysis buffer for 2–3 min, and cells were centrifuged at 1200 rpm for 5 min at room temperature then washed once using sterile DMEM. Recipient mice received 2 × 600 Gy doses of whole-body irradiation 3 h apart. Immediately following the second dose, mice were injected i.v. with 200 μl of DMEM containing 5 × 106 bone marrow cells. Our previous studies showed no difference in reconstitution between 2 and 8 wk after bone marrow transplant, (25); in the current study, mice were used 2–6 wk after bone marrow reconstitution.
Depletion of CSF-1 receptor expressing cells using Mafia mice
Burnett and colleagues generated transgenic mice designed for inducible macrophage and dendritic cell depletion by macrophage Fas-induced apoptosis (Mafia) (24, 26). The transgene is under control of the c-fms promoter that regulates expression of the CSF-1 receptor and is expressed on macrophages and DC. These mice express eGFP and a membrane-bound suicide protein comprising the human low-affinity nerve growth factor receptor, the FK506 binding protein and the cytoplasmic domain of Fas (24, 26). AP20187 is a covalently linked dimer (Ariad Pharmaceuticals) that cross links the FK506 binding protein region of the suicide protein and induces caspase 8-dependent apoptosis as described (24, 25). Mafia mice are on a C57BL/6 background and have a normal phenotype in the absence of the dimerizer (24, 25). Lyophilized AP20187 received from Ariad Pharmaceuticals was dissolved in 100% ethanol 13.75 mg/ml and stored at −20°C. To deplete macrophages and DCs, this stock solution was diluted to 0.55 mg/ml in 4% ethanol, 10% PEG-400, and 1.7% Tween 80 in sterile water, and Mafia mice were injected i.p. daily for 5 days before starting the experiment with 40 μg per injection (total = 200 μg).
Murine model of LPS-induced corneal inflammation
Mice were anesthetized by i.p. injection of 0.4 ml 2,2,2, tribomoethanol (1.2%) (Sigma-Aldrich) and the epithelium of the central cornea was demarcated with a 1 mm diameter circular trephine. Within this zone of central cornea, an epithelial debridement wound was generated using an Algerbrush II corneal rust ring remover with a 0.5 mm burr (Alger Equipment), as shown previously (11, 23). Immediately following epithelial debridement, either 20 μg of Ultra Pure, TLR4-specific Escherichia coli LPS (strain K12; Invivogen), 5 μg of the synthetic bacterial lipopeptide Pam3Cys-Ser-(Lys)4 (Pam3Cys; EMC Microcollections), or sterile saline was applied to the surface of each eye.
Epifluorescence microscopy
Mice were anesthetized by i.p. injection of 2,2,2, tribomoethanol, and positioned in a three-point stereotactic mouse restrainer. Cellular infiltration to the corneas was visualized using a high-resolution stereo fluorescence MZFLIII microscope (Leica Microsystems). In some experiments, corneas were dissected and examined using in inverted Leica DMI 6000B microscope, and images were captured using SpotCam software (RT Slider KE; Diagnostics Instruments).
In vivo confocal microscopy
To examine cellular infiltration, animals underwent euthanasia by CO2 asphyxiation, and were examined by in vivo confocal microscopy (Nidek Technologies America) within 2 min. Images were captured every 2 μm throughout the thickness of the cornea, and light intensity (reflectivity) values for each image were captured using accompanying software (NAVIS; Lucent Technologies), and exported as an Excel file. Reflectivity values for the corneal stroma were plotted in relation to distance from the corneal endothelium to basal epithelium to generate a curve, and the area under the curve was calculated using GraphPad Prism software. This measurement was termed stromal haze as described in our previous studies (5, 6). As measurements are completed within a few minutes after euthanasia, and control animals are treated identically, postmortem corneal edema was minimal and did not affect the measurements.
Immunohistochemical staining of frozen corneal sections
Eyes were enucleated and snap-frozen in embedding medium (Tissue-Tek) using dry ice. Five-micrometer cryosections were fixed in 4% paraformaldehyde (Sigma-Aldrich) for 30 min at RT, washed in PBS for 30 min then blocked with PBS plus 2% BSA. Sections were then incubated overnight with rat anti-mouse mAb NIMP-R14, which specifically stains neutrophils (Abcam) or with the pan macrophage marker F4/80 (BD Biosciences), which we and others show identifies macrophages in the corneal stroma (11, 15, 28).
Following 3 × 5 min washes with PBS, sections were incubated for 45 min with Strep Alexa Fluor 594-conjugated anti-rat IgG (1/200; Molecular Probes). Sections were washed three times and then mounted with Vecta-Shield mounting medium containing 4′, 6-diamidino-2-phenylindole (Vector Laboratories). Neutrophils were counted in each section (from limbus to limbus at ×400) by fluorescence microscopy (Olympus Optical).
Statistical analysis
Statistical analysis was performed for each experiment using an unpaired t test (Prism, GraphPad Software). A p value <0.05 was considered significant.
Results
TLR4-expressing bone marrow-derived donor cells confer LPS responsiveness to TLR4−/− chimeric mice in a model of corneal inflammation
Our previous studies demonstrated that corneal inflammation induced by bacterial products or killed bacteria is characterized by neutrophil infiltration to the corneal stroma, and with loss of normal corneal transparency (5–7), and that LPS-induced corneal inflammation is dependent on functional TLR4 (6, 14). As a first approach to examine the role of myeloid cells in LPS-induced corneal inflammation, we used bone marrow chimeras with TLR4−/−-recipient mice and donor cells from C57BL/6 or TLR4−/− mice expressing eGFP under a β-actin promoter.
Four weeks after myeloablation and bone marrow transplantation, donor eGFP+ cells were present in the normal cornea (Fig. 1, A and B), consistent with our previous observations on bone marrow cell turnover in the cornea (23). Trauma controls (corneal epithelial abrasion and saline only) exhibited a mild inflammatory cell infiltrate, as evident by eGFP+ cells (Fig. 1, C and D). In C57BL/6 eGFP → TLR4−/− mice, corneas that were abraded and stimulated with topical LPS showed a robust eGFP+ inflammatory cell infiltrate at both the limbus and in the central cornea (Fig. 1, E and F). To ensure that C57BL/6 eGFP → TLR4−/− chimeras were not impaired in their ability to respond to other stimuli, corneas were stimulated by topical application of the TLR2-specific ligand, Pam3Cys (Fig. 1, G and H). These corneas had a pronounced eGFP+ cell infiltrate in the limbal zone (Fig. 1G) and central cornea (Fig. 1H), indicating that these chimeras can respond normally to other TLR ligands.
FIGURE 1.
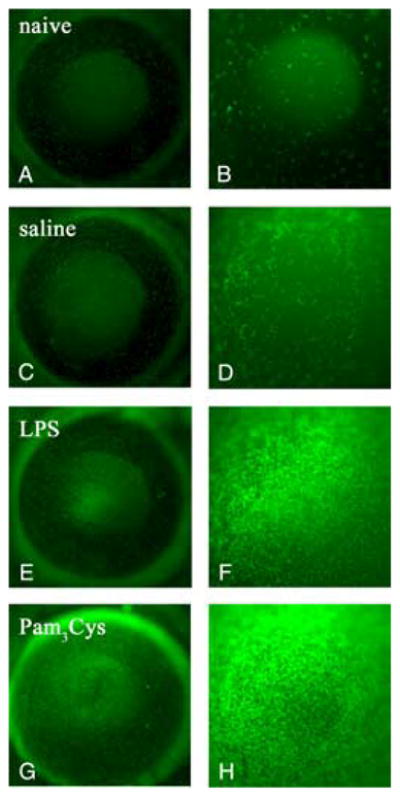
Cellular infiltration in TLR4−/− mice corneas reconstituted with eGFP+ C57BL/6 bone marrow cells. TLR4−/ − mice were irradiated and reconstituted with bone marrow from eGFP+ C57BL/6 mice. After 4 wk, corneas were abraded and exposed to saline, LPS, or Pam3Cys. After 24 h, in vivo epifluorescence microscopy images were captured at magnification ×32 (A, C, E, and G) or ×80 (B, D, F, and H). Representative images are shown from naive (A and B), saline treated (C and D), LPS-treated (E and F ), and Pam3Cys treated corneas (G and H). eGFP+ cells were present in the host corneas of naive mice (A, total cornea; B, central cornea) and a mild infiltrate of eGFP+ cells was observed in saline treated control mice, trauma induced response (C, whole cornea; D, central cornea). In LPS treated corneas, more eGFP+ cells were visible in both the limbal region (E) and in the central cornea (F ) compared with saline treated corneas. Pam3Cys treated corneas induced recruitment of eGFP+ cells in the limbal region (G) and in the central cornea (H). These experiments were repeated three times with four mice per group.
To determine the role of resident bone marrow-derived cells in neutrophil recruitment to the corneal stroma, corneas were abraded and treated with either saline, LPS, or Pam3Cys, and examined 24 h later at the optimal time point for neutrophil infiltration (5, 6). As shown in Fig. 2, A and B, trauma controls had fewer than fifty neutrophils/corneal section. Neutrophil recruitment to the corneal stroma of LPS-treated TLR4−/− → TLR4−/− chimeras was not elevated above that measured in saline controls. However, LPS stimulated of the cornea in C57BL/6 → TLR4−/− corneas had a significantly elevated neutrophil infiltrate compared with control mice, indicating that the donor TLR4+/+ cells that had partially repopulated the cornea can confer LPS-induced corneal inflammation. As an additional control, we found that there was no significant difference in neutrophil infiltration between C57BL/6 → TLR4−/− and C57BL/6 → C57BL/6 chimeras stimulated with the TLR2 ligand Pam3Cys (Fig. 2B).
FIGURE 2.
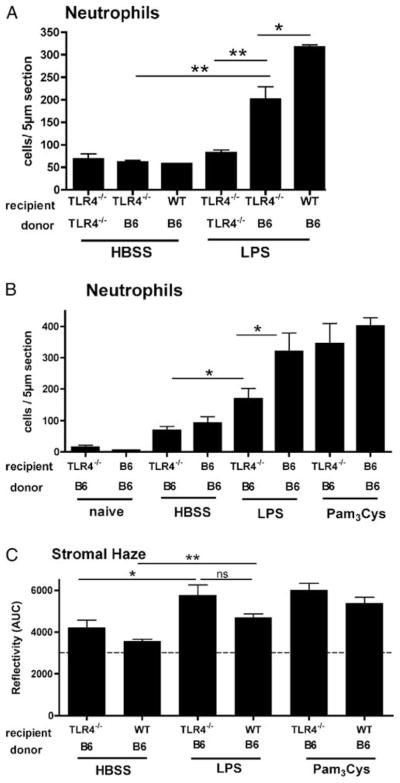
Neutrophil infiltration and development of stromal haze in TLR4−/− bone marrow chimeric. Recipient C57BL/6 and TLR4−/− mice were irradiated and reconstituted with bone marrow from eGFP+ C57BL/6 (B6) or eGFP+ TLR4−/− mice. After 2 (A) or 6 wk (B and C), corneas were abraded and stimulated with LPS, Pam3Cys, or Hanks’ buffered salt solution (HBSS), and neutrophils/5 μm section and stromal haze (reflectivity) were examined after 24 h as described above. A and B, Significant differences (*, p < 0.05; **, p < 0.01) were noted as follows: 1) LPS vs HBSS in B6 → TLR4−/− chimeras; 2) LPS-treated B6 → TLR4−/− vs TLR4−/ − → TLR4−/− chimeras, and 3) LPS-treated B6 → TLR4−/− vs B6 → B6 chimeras. No differences were detected in Pam3Cys-treated B6 → TLR4−/− vs TLR4−/− → TLR4−/− chimeras. C, Significant differences are indicated between LPS and HBSS-treated B6 → TLR4−/− and B6 → B6 chimeras.
To determine the role of bone marrow-derived cells on LPS induced corneal haze, we examined chimeras by in vivo confocal microscopy. C57BL/6 → TLR4−/− chimeric mice treated with LPS demonstrated greater corneal stromal haze compared with saline treated corneas ( p < 0.04; Fig. 2C), which further supporting a role for bone marrow derived cells in the host inflammatory response to LPS.
Macrophages and DCs in corneas of control and LPS-treated Mafia mice
As a second approach to examining the role of bone marrow derived cells in TLR4-induced corneal inflammation, we used transgenic mice that express the cytoplasmic domain of Fas with receptors for chemically inducible dimerization under control of the c-fms promoter. Cross-linking of the transgenic receptor induces apoptosis in cells expressing the macrophage Fas-induced apoptosis transgene. When these Mafia mice are treated systemically with the synthetic dimerizer (AP20187), macrophages and DC expressing this gene in most tissues undergo apoptosis (24). The plasmid used to generate these mice also expresses eGFP under control of the same promoter; therefore, macrophages and DCs from Mafia mice express eGFP. (Note that in contrast to bone marrow chimeric mice, neutrophils do not express eGFP in Mafia mice and are therefore not visualized by in vivo fluorescence microscopy).
The distribution and morphology of c-fms-expressing eGFP+ cells detected in the cornea of naive Mafia mice (Fig. 3, A and C) are consistent with our eGFP chimeras described above, and with our previous studies using CX3CR1 GFP transgenic mice (9, 10), demonstrating intraepithelial DCs or Langerhans cells in the corneal epithelium, and DCs and macrophages in the stroma. However, in corneas of mice treated with the AP20187 dimerizer, there was a loss of Langerhans-like cells in the epithelium, and an evident diminution of eGFP+ cells in the corneal stroma (Fig. 3, B and D). This finding is consistent with those of Burnett et al. (24), who showed in the initial description of these mice that low numbers of eGFP+ cells remained in the skin after dimerizer treatment and that the remaining eGFP+ cells exhibited abnormal morphology.
FIGURE 3.
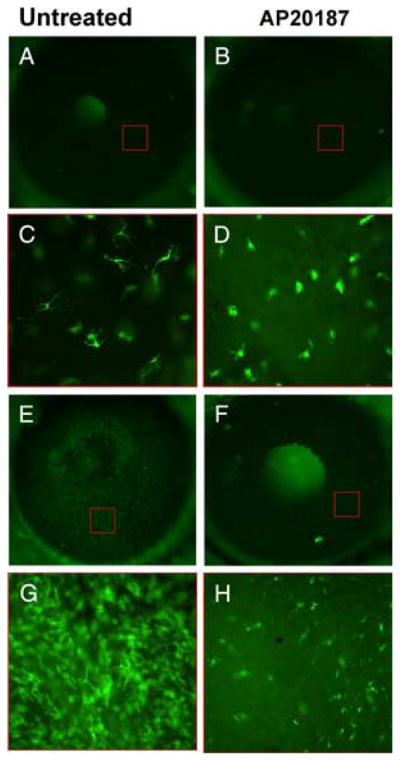
Depletion of c-fms– expressing eGFP+ cells (macrophage and DCs) in Mafia mice. Mafia mice were left untreated (A, C, E, and G) or were injected i.p. with the AP20187 dimerizer to deplete c-fms cells (B, D, F, and H). Corneas were viewed either by epifluorescence of the whole eye (A, B, E, and F), or at higher magnification after dissection of the cornea (C, D, G, and H). Before corneal stimulation (A–D) resident eGFP+ cells were detected in the corneas of untreated mice (A and C); however, there was an almost complete loss of typical dendriform intraepithelial Langerhans like cells in mice treated with the dimerizer (B and D), although eGFP+ cells were present in the corneal stroma. Corneas were abraded and stimulated with LPS as before, and examined after 24 h (E–H). In Mafia mice not injected with the dimerizer, there was an intense infiltrate of eGFP+ cells in all regions of the cornea (E and G), whereas there was an obvious reduction in the numbers of eGFP+ cells in dimerizer-treated mice (F and H). At the higher magnification, cells are evident in the corneal epithelium and stroma (G and H). Red boxes in A, B, E, and F indicate approximate regions where the higher magnification images were obtained. Images are representative of three repeat experiments with five mice per group. Original magnification A, B, E, and F, ×20; C and D, ×400. (The central bright area in panels A, B, and F is the mouse lens seen through the pupil.)
To determine the effect of LPS on the recruitment of inflammatory macrophages to the cornea of dimerizer treated and untreated Mafia mice, the corneas of both groups were examined by in vivo fluorescence microscopy. We found that corneas of LPS-challenged, untreated (no dimerizer) Mafia mice showed an intense eGFP+ cellular infiltrate 24 h after challenge (Fig. 3, E and G), whereas the corneas of dimerizer-treated Mafia mice contained very few eGFP+ cells in the corneal stroma (Fig. 3, F and H).
Ablation of resident corneal macrophages and DCs in Mafia mice abrogates LPS-induced neutrophil infiltration to the cornea and development of corneal haze
Previous studies showed that neutrophils comprise the major cell type infiltrating the corneal stroma following LPS stimulation (5, 6, 8, 12, 13). To determine the role of c-fms-expressing cells on LPS-induced corneal inflammation, Mafia mice were treated with AP20187 as described above, and the corneal epithelium was abraded and stimulated with LPS. After 24 h, eyes were enucleated, sectioned, and immunostained with NIMP-R14 Ab or with F4/80, which label cells of the macrophage/DC lineage (24). As with eGFP+ cells in Mafia mouse corneas shown above, F4/80+ cells increased in LPS-challenged corneas of untreated (no dimerizer) Mafia mice. Infiltration of F4/80+ cells was completely abrogated in AP20187 treated mice, confirming the in vivo epifluorescence microscopy findings shown above. LPS-treated corneas had elevated neutrophils in the corneal stroma compared with saline (trauma) controls (Fig. 4B). Furthermore, neutrophil infiltration was significantly reduced in AP20187-treated mice, which ablates only macrophages and DCs. Together, these findings indicate that c-fms-expressing cells have an essential role in mediating LPS-induced neutrophil infiltration to the cornea.
FIGURE 4.
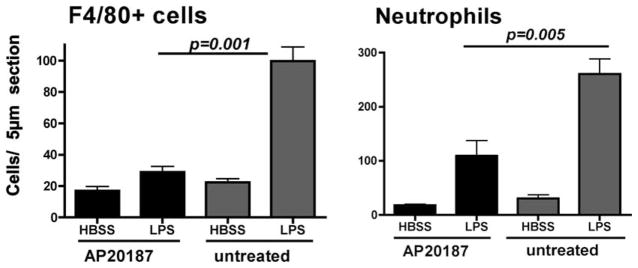
The effect of depletion of c-fms-expressing cells on cellular infiltration or recruitment to the corneal stroma. Mafia mice were treated as described in the legend to Fig. 3, and 24 h after stimulation with LPS, eyes were sectioned and immunostained for neutrophils (NIMP/R14+) and F4/80+ cells. Data represent four repeat experiments with three mice per group. Note significant difference between untreated and AP20187 dimerizer treated groups for neutrophils and macrophages.
To examine whether the absence of cellular infiltrate affects corneal refractile properties, we also examined these mice by in vivo confocal microscopy (Fig. 5). LPS-stimulated corneas of untreated Mafia mice displayed characteristic increases in corneal haze (Fig. 5, A and B), similar to those described in C57BL/6 mice (5, 6). In contrast, LPS-induced corneal haze in AP20187-treated Mafia mice was significantly reduced compared with untreated Mafia mice, thereby revealing a role for these cells in development of corneal inflammation.
FIGURE 5.
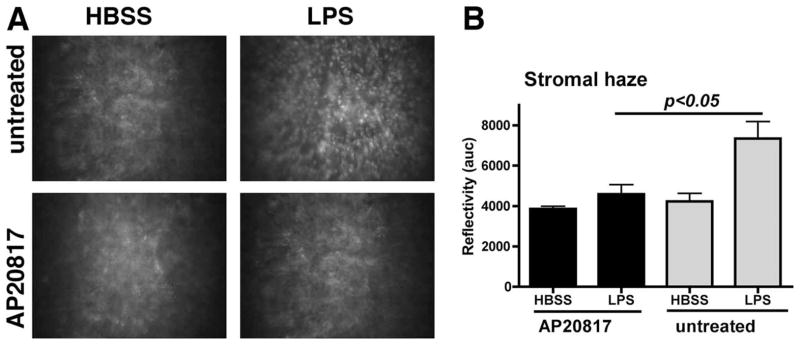
The effect of depletion of c-fms-expressing cells on corneal thickness and haze as visualized and measured by in vivo confocal microscopy. A, Representative images of cellular infiltrate in untreated Mafia mice compared with AP20187 treated Mafia mice. B, Quantitative differences in stromal haze (reflectivity). Similar results were found in three repeat experiments with five mice per group.
Recruitment of neutrophils to the corneal stroma after LPS stimulation requires production of CXC chemokines such as CXCL1/KC and involves proinflammatory cytokines such as IL-1α. To determine whether c-fms receptor-expressing cells are involved in early production of proinflammatory and chemotactic cytokines, corneas of AP20187 dimerizer-treated and untreated Mafia mice were abraded and stimulated with LPS as before. After 4 h (before neutrophil infiltration), corneas were homogenized, and cytokines were measured by ELISA as described previously (5, 6). We found that CXCL1/KC and IL-1α production were elevated in LPS-treated corneas compared with saline (HBSS) controls (Fig. 6); however, production of both cytokines was ablated in dimerizer-treated Mafia mice compared with untreated Mafia mice, indicating a role for resident macrophages and DCs in production of proinflammatory and chemotactic cytokines at this time point.
FIGURE 6.
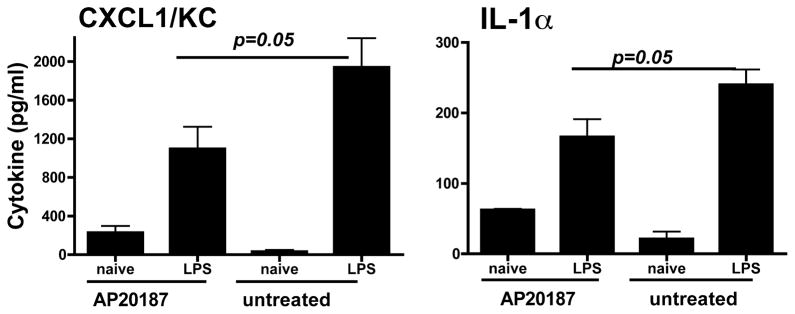
The effect of depletion of c-fms– expressing cells on cytokine production in the cornea Mafia mice were treated with AP20187 dimerizer as before, and corneas were abraded and stimulated with LPS. After 4 h, individual corneas were dissected, homogenized, and the cytokine concentration in each cornea was measured by ELISA. Data are mean ± SEM of four mice per group, and the experiment was repeated twice with similar results.
Discussion
Bone marrow-derived cells represent a first line of defense in responding to microbes and microbial products in the gut (28, 29), respiratory tract (30) and skin (31), where they recognize microbial products such as LPS and initiate an inflammatory response. Similarly, the use of bone marrow chimeric mice revealed a critical role for myeloid cells in LPS-induced airway inflammation (32).
We and others demonstrated that the corneal epithelium also contains cells with dendriform morphology and immunophenotype identical with skin Langerhans cells (17, 33, 34). Earlier reports also indicate that these Langerhans cells, which are more numerous in the peripheral cornea, have a role in corneal transplantation rejection and in the host response in Herpes simplex keratitis and in Pseudomonas aeruginosa keratitis (35–38).
Activation of the TLR family is important in regulating the response to infectious agents in the cornea, including Herpes simplex virus, as TLR9 chemokine and Type I IFN production in the cornea (39). Furthermore, lesion size is smaller in TLR2−/−, TLR9−/−, and MyD88−/− mice, although MyD88−/− mice die from lethal encephalitis (40). TLR2 and MyD88 also mediate corneal inflammation associated with ocular onchocerciasis (10), and TLR4 regulates the host response in Pseudomonas aeruginosa keratitis, with TLR4-deficient mice showing increased susceptibility (41). However, the role of corneal epithelial cells vs resident myeloid cells in TLR4 dependent corneal infection and inflammation has not been determined. Human corneal epithelial cells express TLR2–9, and produce cytokines and β-defensins in response to activation of TLR2, TLR3, and TLR5 (3, 4, 44 – 48); however, these cells do not respond to LPS unless coreceptors, especially MD-2, are added exogenously (14, 47–49).
In the current study, we found that TLR4 expressing bone marrow-derived cells confer LPS responsiveness in the cornea, implicating these cells in the initiation of the host response. This study did not determine the relative contribution of myeloid lineage cells compared with TLR4 expressing corneal epithelial cells in the presence of MD-2; however, the data from our second series of experiments, in which we used the macrophage Fas induced apoptosis transgenic (Mafia) mice (24), demonstrate that following targeted ablation of macrophages and DCs with the AP20187 dimerizer, LPS-induced neutrophil recruitment to the corneal stroma and development of corneal haze is significantly inhibited compared with untreated mice, and F4/80+ cell infiltration is completely ablated. Although these findings demonstrate an important role for macrophages and DCs in LPS-induced corneal inflammation, it does not eliminate the possibility that corneal epithelial cells also contribute. Firstly, corneal epithelial cells in Mafia mice are not TLR4 deficient, and secondly, we show that neutrophil recruitment to the corneal stroma of AP20187-treated Mafia mice is higher in LPS compared with HBSS treated corneas (Fig. 4B), consistent with elevated IL-1α and CXCL1/KC (Fig. 6). Whether this is a direct response of epithelial cells to LPS, possibly as a result of MD-2 secreted by resident DCs (50), or if corneal epithelial cells are stimulated indirectly by proinflammatory cytokines has yet to be determined. Further indication that DCs have contributed to LPS-induced corneal inflammation stems from our recent observation that these cells produce long, membrane nanotubes that connect to distantly located DCs in the cornea, and that the number of nanotubes increases after trauma and LPS stimulation (16). In vitro studies show that nanotubes can traffic proteins and even organelles between cells (51), and may therefore transmit cytokines to enhance or modulate the inflammatory response in the cornea.
In summary, the present study demonstrates that an important function of resident myeloid lineage cells in the cornea is responding to microbial products by producing proinflammatory and chemotactic cytokines that mediate recruitment of neutrophils and macrophages to the corneal stroma that result in increased corneal thickness and haze. Further investigation is underway to elucidate the relative contributoin of resident DCs vs macrophages in corneal inflammation.
Acknowledgments
We thank Catherine Doller and Scott Howell of the Visual Sciences Research Center Core Facilities at Case Western Reserve University for technical assistance with cryosectioning and imaging.
Footnotes
This work was supported by the National Institute of Health Grants R01EY14362 (to E.P.) EY11373 (Core grant, to E.P.), and K08 EY014912 (to V.L.P.). Additional support for these studies was provided by The Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.
Abbreviations used in this paper: DC, dendritic cell; Mafia, macrophage Fas-induced apoptosis; eGFP, enhanced GFP; HBSS, Hanks’ buffered salt solution.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, Kester M, McMenamin PG. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–116. doi: 10.1016/s1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu FS, Hazlett LD. Toll-like receptors and the eye. Invest Ophthalmol Visual Sci. 2006;47:1255–1263. doi: 10.1167/iovs.05-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of Toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Visual Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 6.Khatri S, Lass JH, Heinzel FP, Petroll WM, Gomez J, Diaconu E, Kalsow CM, Pearlman E. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Visual Sci. 2002;43:2278–2284. [PubMed] [Google Scholar]
- 7.Schultz CL, Morck DW, McKay SG, Olson ME, Buret A. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res. 1997;64:3–9. doi: 10.1006/exer.1996.0190. [DOI] [PubMed] [Google Scholar]
- 8.Carlson EC, Drazba J, Yang X, Perez VL. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Visual Sci. 2006;47:241–248. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- 9.Carlson EC, Lin M, Liu CY, Kao WW, Perez VL, Pearlman E. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 2007;282:35502–35509. doi: 10.1074/jbc.M705823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, Golenbock DT, Fitzgerald KA, Kazura JW, Pearlman E. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. 2007;178:1068–1076. doi: 10.4049/jimmunol.178.2.1068. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 12.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukocyte Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE, Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Visual Sci. 2008 doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Visual Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 16.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Visual Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 19.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukocyte Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 20.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Visual Sci. 2004;45:722–727. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- 21.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Visual Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 22.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Visual Sci. 2002;43:639– 646. [PubMed] [Google Scholar]
- 23.Chinnery HR, Humphries T, Clare A, Dixon AE, Howes K, Moran CB, Scott D, Zakrzewski M, Pearlman E, McMenamin PG. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukocyte Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 25.Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. J Surg Res. 2006;131:296–301. doi: 10.1016/j.jss.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Visual Sci. 2005;46:88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- 28.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niess JH, Reinecker HC. Lamina propria dendritic cells in the physiology and pathology of the gastrointestinal tract. Curr Opin Gastroenterol. 2005;21:687–691. doi: 10.1097/01.mog.0000181710.96904.58. [DOI] [PubMed] [Google Scholar]
- 30.Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, Turner DJ, Sly PD, Stumbles PA, Holt PG. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–5867. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 31.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 32.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med. 2005;171:806– 813. doi: 10.1164/rccm.200407-953OC. [DOI] [PubMed] [Google Scholar]
- 33.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 34.Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, Petroll WM, Cavanagh HD, Takashima A. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology. 2007;120:556–572. doi: 10.1111/j.1365-2567.2006.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He YG, Niederkorn JY. Depletion of donor-derived Langerhans cells promotes corneal allograft survival. Cornea. 1996;15:82–89. [PubMed] [Google Scholar]
- 36.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhans cells in the CD4− but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 37.Hazlett LD, McClellan SA, Rudner XL, Barrett RP. The role of Langerhans cells in Pseudomonas aeruginosa infection. Invest Ophthalmol Visual Sci. 2002;43:189–197. [PubMed] [Google Scholar]
- 38.Hazlett LD, McClellan S, Barrett R, Rudner X. B7/CD28 costimulation is critical in susceptibility to Pseudomonas aeruginosa corneal infection: a comparative study using monoclonal antibody blockade and CD28-deficient mice. J Immunol. 2001;166:1292–1299. doi: 10.4049/jimmunol.166.2.1292. [DOI] [PubMed] [Google Scholar]
- 39.Wuest T, Austin BA, Uematsu S, Thapa M, Akira S, Carr DJ. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179:46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–11138. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Visual Sci. 2006;47:4910– 4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of β-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Visual Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 46.Ueta M, Hamuro J, Kiyono H, Kinoshita S. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–294. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- 47.Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Visual Sci. 2005;46:4235–4244. doi: 10.1167/iovs.05-0543. [DOI] [PubMed] [Google Scholar]
- 48.Ueta M, Nochi T, Jang MH, Park EJ, Igarashi O, Hino A, Kawasaki S, Shikina T, Hiroi T, Kinoshita S, Kiyono H. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–3347. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- 49.Visintin A, Halmen KA, Khan N, Monks BG, Golenbock DT, Lien E. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4) J Leukocyte Biol. 2006;80:1584–1592. doi: 10.1189/jlb.0606388. [DOI] [PubMed] [Google Scholar]
- 50.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci USA. 2001;98:12156–12161. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]


