Abstract
Background
Physical activity may provide benefits for breast cancer survivors in part by reducing systemic inflammation. Physical activity behavior change studies are a type of implementation research in which exercise efficacy information is translated into a “real world” setting, allowing determination of whether physical activity changes are sufficient to improve health outcomes. We hypothesized that breast cancer survivors (BCS) who undertook a physical activity behavior change intervention would have less systemic inflammation and improved cardiorespiratory fitness, muscle strength, body composition, fatigue, and sleep as compared with BCS receiving usual care. The goal of this pilot study was to determine the magnitude and direction of intervention effect sizes for inflammatory related serum markers and relevant health outcomes.
Methods
This randomized controlled trial enrolled 28 Stage I, II, or IIIA BCS who were post-primary treatment and were not regular exercisers. These women were assigned to either a 3-month physical activity behavior change intervention group (ING) or usual care group (UCG). Intervention included supervised aerobic (150 weekly minutes, moderate-intensity) and resistance (two sessions per week) exercise that gradually tapered to home-based exercise. At baseline and after 3 months, health outcomes and serum concentrations of interleukin (IL)-1 beta, IL-6, IL-8, IL-10, tumor necrosis factor (TNF)-alpha, leptin, and adiponectin were measured.
Results
Cardiorespiratory fitness significantly improved in the ING versus UCG (between group difference=3.8 ml/kg/min; d=1.1; P=0.015). Self-reported sleep latency was significantly reduced in the ING versus UCG (between group difference=−0.5; d=−1.2; P=0.02) as was serum leptin (between group difference=−9.0 ng/ml; d=−1.0; P=0.031). Small to medium non-significant negative effect sizes were noted for IL-10 and TNF-alpha and ratios of IL-6:IL-10, IL-8:IL10, and TNF-alpha:IL-10, with non-significant positive effect sizes noted for IL-6 and high molecular weight adiponectin.
Conclusions
Physical activity behavior change interventions in BCS can achieve large effect size changes for several health outcomes. Although effect sizes for inflammatory markers were often small and not significant, changes were in the hypothesized direction for all except IL-6 and IL-10. These preliminary data support larger trials that would more fully examine potential inflammatory changes that accompany physical activity behavior change interventions.
Keywords: exercise, intervention, oncology, cytokine, inflammation, survivorship
Introduction
Physical activity provides multiple psychological and physiologic benefits after a breast cancer diagnosis and is associated with a reduced risk of breast cancer mortality 1–4. Inflammation has been linked to health problems that may improve with physical activity and are particularly troublesome for cancer survivors; these problems include fatigue and sleep dysfunction, which have both been associated with pro-inflammatory cytokines 5–10. Pro-inflammatory cytokines have also been associated with reduced physical functioning in survivors of head and neck cancer 11 and are associated with reduced physical functioning, muscle strength, and/or muscle mass in some cancer-free groups, notably the elderly 12–15. Cytokines may also be markers for risk of breast cancer recurrence as exemplified by an association between chronic elevations of interleukin (IL)-6 and poorer prognosis in breast cancer survivors 16. Thus, inflammation may be a common denominator underlying both the risk of cancer recurrence and the symptoms of fatigue, sleep patterns, and reduced physical functioning 17–19.
Pro-inflammatory cytokines could also link exercise and body composition to health outcomes such as cancer risk via the modulatory effects of adipokines 20. Adipokines are adipocyte-derived chemokines that are over-expressed in obesity and promote the recruitment of macrophages, which in turn release pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, IL-6, and IL-1 beta 21. Obesity is also characterized by increased production of the pro-inflammatory adipokine leptin and by reduced secretion of adiponectin, which has anti-inflammatory effects on macrophages 22. Exercise interventions have been shown to cause beneficial changes in adipokines in some but not all studies of populations both with and without cancer 23–26.
Physical activity behavior change interventions that focus on increasing physical activity behavior in general, as opposed to achieving a specific exercise dose 27, result in improved health outcomes 28–35. We hypothesize that the beneficial effects of these interventions may, in part, be due to a reduction in chronic inflammation 36. Contracting skeletal muscle transiently releases IL-6 during acute exercise, increasing systemic levels of IL-6 up to 100-fold; this marked but transient increase in IL-6, which occurs with each bout of exercise, is theorized to trigger increased IL-10 and IL-1 receptor antagonist, resulting in a greater anti-inflammatory response 37,38, 39.
Although the effect of exercise on systemic markers of inflammation has been studied in a number of populations, few studies have specifically focused on cancer survivors. The inflammatory responses of cancer survivors to exercise may differ from those of other populations because baseline inflammation may be qualitatively or quantitatively different among individuals with chronic disease 40. Furthermore, only a minority of the few studies in cancer survivors have used randomized exercise trials, which provide more rigorous assessment of exercise effects on health outcomes and markers of inflammation in a prospective manner. For example, a randomized, controlled trial of 53 breast cancer survivors assigned to a 15-week vigorous exercise intervention or no exercise found no difference between the groups in pro- and anti-inflammatory cytokine production by unstimulated or phytohemagglutinin (PHA) stimulated peripheral blood mononuclear cells 41. In another study, 28 breast cancer survivors receiving 6 months of resistance training and vigorous aerobic exercise showed no difference in lymphocytic cytokine production or plasma IL-6 as compared with 21 non-exercisers, but did show a significant reduction in plasma interferon (IFN)-gamma at the mid-point of the exercise intervention 42.
Physical activity behavior change studies focus on creating sustained increases in regular exercise behavior whereas exercise efficacy studies focus on health benefits in optimal settings in response to a specific exercise “dose” 27. This difference is important because 1) behavior change studies more closely reflect the translation of exercise efficacy studies into clinical practice and 2) exercise efficacy demonstrated in an optimal setting may not occur in behavior change interventions.
Only a few physical activity behavior change studies have assessed cytokines in cancer survivors. In one study, for example, changes in IL-6 were not detected after participation in behavior change intervention involving a mail delivered diet and physical activity plan; however, this intervention achieved essentially no change in physical activity (< 3 minutes per week on average) 43. Similarly, IL-6 failed to change after a 12-week program of walking for 80 minutes per week in 20 breast cancer survivors receiving hormonal therapy 44. Another study in which breast cancer survivors on adjuvant chemotherapy were randomized to receive diet counseling with and without recommendations for home-based exercise found no change in physical activity or the pro- and anti-inflammatory serum markers IL-1 beta and TNF receptor II 45. However, the amount of exercise may be crucial to the development of changes in inflammation, such that the lack of increase in physical activity in these studies make results inconclusive. For example, C-reactive protein and physical activity were negatively correlated in 741 breast cancer survivors only when patients self-reported over 6 metabolic equivalent (MET) hours per week (i.e., about 90 minutes per week of moderate intensity physical activity) in the year prior to the study assessment 46.
In one of the few studies of resistance training effects on inflammation, 38 breast and prostate cancer survivors were randomized to receive a 4-week home-based physical activity behavior change intervention using pedometers and resistance bands versus the control condition. This study reported a significant negative effect of the intervention on serum IL-6 with no significant difference between the study conditions with regard to TNF-alpha 47. In non-cancer populations, C-reactive protein significantly declined after a one-year resistance training intervention in 28 overweight women 48.
In summary, exercise efficacy and behavior change studies in cancer survivors are consistent with prior studies in healthy adults 38, 39 in that exercise may change serum cytokines without changing cytokine release from blood mononuclear cells in vitro. However, none of the cancer survivor exercise studies has detected an increase in systemic IL-6 due to muscle release of IL-6 as has been reported for healthy adults 38, 39. This discrepancy could be related to the focus of cancer studies on chronic training rather than on acute exercise bouts, which transiently increase IL-6 primarily in the first 24 hours after the acute exercise bout 49. In addition, previous physical activity behavior change studies often may not have achieved exercise levels sufficient to alter inflammatory markers. These issues highlight our need for a better understanding of how physical activity behavior change interventions affect the complex patterns of pro- and anti-inflammatory cytokines and adipokines in breast cancer survivors and how this modulation of systemic inflammation contributes to health concerns such as fatigue and sleep dysfunction.
In preparation for a larger prospective study evaluating the potential inflammatory mechanisms of a physical activity behavior change intervention effects, we chose study outcomes based on the biological framework described in Figure 1. We hypothesized that exercise reduces chronic systemic inflammation (e.g., pro-inflammatory serum cytokines) directly and indirectly through changes in body composition, in turn attenuating fatigue, improving sleep quality, and reducing risk of cancer recurrence. The primary goal of the current study was to obtain preliminary estimates of the magnitude and direction of the exercise intervention effects on serum markers of inflammation (cytokines, adipokines) and relevant health outcomes (cardiorespiratory fitness, muscle strength, body composition, fatigue, and sleep dysfunction) in preparation for a subsequent trial that will be powered to detect changes in serum cytokines and adipokines with the intervention. In particular, we determined preliminary effect sizes to identify those outcomes that are most sensitive to modulation by the intervention and to estimate sample size requirements for subsequent use of these outcome measures. Our intervention goal was 150 minutes of moderate intensity physical activity per week (i.e., equivalent to 7.5 to 12.5 MET hours per week), which we hypothesized would be sufficient to impact systemic inflammation, as assessed using serum markers associated with cancer risk, fatigue, and/or sleep dysfunction (see Table 1). Specifically, we hypothesized that as compared with the usual care group, the physical activity behavior change intervention group would have: 1) lower serum concentrations of pro-inflammatory cytokines (e.g., TNF-alpha, IL-1 beta, IL-6, IL-8) and inflammatory-related markers associated with excess adiposity (e.g., leptin) and 2) higher levels of anti-inflammatory cytokines (e.g., IL-10) and markers associated with lower body weight or fat (e.g., adiponectin). In addition, we expected improvements in all health outcome measures in the intervention group as compared with the usual care group.
Figure 1.
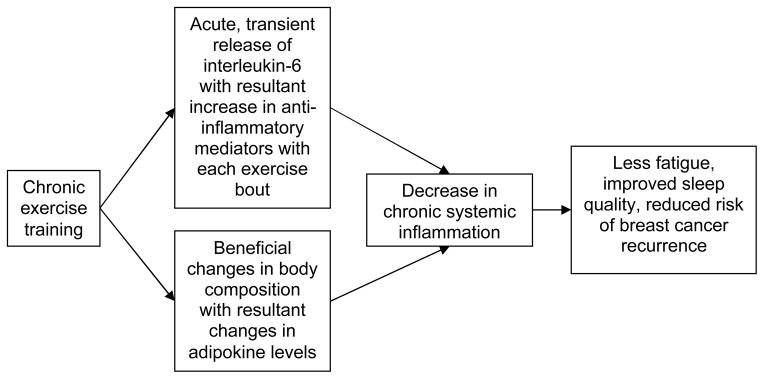
Chronic exercise training, inflammation, and related outcomes: theoretical biological framework
Table 1.
Inflammatory markers: health outcome associated with higher serum levels and expected change with chronic physical activity
| Inflammatory marker | Health outcomes associated with higher levels of inflammatory markera | Expected change with chronic physical activityb |
|---|---|---|
| IL-1 beta | Sleep regulation, cachexia, reduced muscle mass/physical functioning 11, 36, 68, 73–75 | Decrease |
| IL-6 | Sleep dysfunction, fatigue, cachexia, disease risk (cancer and other chronic disease), and reduced muscle mass/physical functioning 9–11, 13, 14, 16, 36, 47, 68, 74, 76 | Decrease36 |
| IL-8 | Sleep dysfunction, poorer breast cancer prognosis 68, 77, 78 | Decrease (in absence of injury) |
| IL-10 | Sleep regulationc36, 68, 73 | Increase |
| TNF-alpha | Sleep dysfunction, cachexia, reduced muscle mass 9, 14, 36, 73, 74 | Decrease |
| Leptin | Associated with body weight and adiposity, increased breast cancer cell proliferation in vitro69 | Decrease |
| Total adiponectin | Associated with body weight and adiposity, reduced breast cancer risk69, 79, 80 | Increase |
| HMW adiponectin | Associated with body weight and adiposity, reduced breast cancer risk69, 79 | Increase |
Certain cytokines may have both pro and anti-inflammatory effects (e.g., IL-6) but only outcomes associated with the primary action are listed.
Does not include muscle cytokine levels in response to acute exercise bouts which may differ from serum levels after exercise training.
Contrary to the other cytokines listed, IL-10 reduces non-rapid eye movements sleep possibly improving sleep quality 68.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; HMW, high molecular weight
Methods
Setting, participants, and study design
The local institutional review board approved the study and all participants provided informed consent before beginning the study. Eligible participants were female, Stage I, II, or IIIA breast cancer survivors between the ages of 18 and 70 years. Other eligibility criteria included: not currently receiving (and not planning to receive during the study duration) chemotherapy or radiation therapy; ≥ 8 weeks post-surgery; English speaking; medical clearance for participation provided by physician. Exclusion criteria were as follows: dementia or organic brain syndrome; medical, psychological, or social characteristic that would interfere with ability to fully participate in study activities (e.g., psychosis, schizophrenia, etc.); contraindication to participation in a regular physical activity program; metastatic or recurrent disease; unable to ambulate; engaged in ≥ 60 weekly minutes of vigorous physical activity or ≥ 150 weekly minutes of moderate plus vigorous activity during the past month (based on self-report); anticipated undergoing elective surgery during the intervention which would interfere with participation (e.g., breast reconstructive surgery); did not live or work within 50 miles of the study site.
This two-arm, randomized controlled pilot study compared an intervention to usual care group. Measurements were obtained at baseline (pre-intervention; M0) and 3 months (post-intervention; M3). Randomization was based on computer generated numbers, performed in blocks of 4, and revealed in the order in which participants completed baseline testing.
Intervention and usual care
The physical activity behavior change intervention was adapted from the previously reported BEAT Cancer program 50–52 by adding strength training using resistance bands. The intervention goal was to gradually increase each participant’s physical activity to 150 weekly minutes of ≥ moderate intensity exercise and two sessions per week on nonconsecutive days of resistance training (i.e., up to 20 repetitions of 8 different exercises using each of the major muscle groups). Participants were tapered from supervised exercise sessions with an exercise specialist to home-based, unsupervised exercise over the first six weeks of the intervention. Participants were given personal heart rate monitors to facilitate achieving target exercise intensity. For behavioral support, participants attended six discussion group sessions with a clinical psychologist over the first nine weeks of the intervention and met with the exercise specialists for a face-to-face “update” counseling session every two weeks in the final six weeks of the intervention.
Because the focus of the intervention was behavior change rather than exercise efficacy 27, the usual care group received written materials from the American Cancer Society which included general information about physical activity and nutrition after a cancer diagnosis but did not include specific recommendations regarding exercise behavior. Participants were told they could receive the intervention at the end of the study, free of charge.
Measures
A self-administered survey was used to assess age, race/ethnicity, marital status, smoking history, alcohol consumption, and medical history. Bilateral arm circumferences were measured on all participants at the mid-point between the shoulder and the elbow (i.e., average of three measurements) to monitor for potential increases related to lymphedema. Because carbohydrate ingestion may influence cytokine response to exercise 36, a 3-day diet record was done prior to the blood draw to determine if between group differences requiring statistical adjustment existed.
Adherence to the intervention physical activity recommendations was measured with 7-day MTI/ActiGraph accelerometer monitoring (aerobic) and exercise log (resistance). Physical activity intensity cutpoints were as follows: light activity = 0–1952, moderate activity = 1953–5724, hard activity = 5725–9468, and very hard activity > 9468 53, 54. Resistance training adherence was recorded by exercise specialists during supervised sessions. Home-based sessions were recorded by the participants; study staff reviewed the log sheets with participants during supervised and follow-up sessions.
A submaximal treadmill test using the Naughton protocol estimated fitness 55. Muscle strength was measured using a back/leg dynamometer. Body mass index (BMI) was calculated from height and weight measured by trained staff. Waist-to-hip ratio was measured using a non-stretching tape measure with the participant standing with abdomen relaxed and arms at sides with no clothing at the waist and only under garments at the hip. Percent body fat was measured by bioelectric impedance (i.e., Quantum X by RJL Systems) in a standardized fashion (i.e., same time of day for each measurement after a ≥ 4 hour fast). Fatigue intensity and interference experienced during the past week was measured with the multi-dimensional fatigue scale (i.e., Fatigue Symptom Inventory; higher score indicates greater fatigue) 56. The following two subscales are reported: 1) fatigue intensity (mean of 4 items, 1 to 10 scale) and 2) fatigue interference (mean of 6 items, 1 to 10 scale). Self-reported sleep dysfunction was measured using the Pittsburgh Sleep Quality Index (PSQI) which was scored according to published protocol (i.e., higher score indicates greater sleep dysfunction) 57. Sleep latency and efficiency were measured objectively using the same accelerometer used for measuring physical activity by transferring to the wrist when in bed. The participant recorded time in and out of bed on a record sheet.
Blood sampling rationale, methods, and assays
Luminex® methodology was chosen because it allows for simultaneous measurement of multiple analytes thereby reducing sample volume requirements and cost related to labor and reagents compared to other single-analyte methods such as enzyme-linked immunosorbent assays (ELISAs). Serum rather than plasma was assayed based on prior comparisons of Luminex® cytokine assays with ELISA measurements that showed good correlation in serum 58, 59 but not in plasma 60. In addition, cytokine levels have been reported to be higher in serum compared with plasma samples among breast and prostate cancer patients 47.
Participants were instructed to abstain from physical activity, smoking, or alcohol; take all medications prescribed for regular daily use; and not take sporadic or “as needed” medications for 24 hours before the blood draw. Participants were asked to fast for 12 hours before the blood draw which was performed by an experienced phlebotomist between 7:45 and 10:00 AM the following morning to minimize variability due to the circadian expression of most cytokines. Blood samples were collected, processed and stored using a standard operating procedure consistent with recommendations of the Standard Operating Procedures Internal Working Group comprised of members from across the Early Detection Research Network 61. For processing serum, the blood sample was collected in a tube designated for serum collection by the manufacturer (i.e., BD Medical # 367815). Sample was kept at room temperature, allowed to clot for 30 minutes, centrifuged at 1500×g for 15 minutes, then immediately placed in labeled polypropylene tubes in 0.5 milliliter aliquots, and stored at −80°C until analysis. Hemolyzed samples were not analyzed and blood samples were redrawn. Repeated freeze-thaw cycles of samples can significantly affect cytokine measurements, however, cytokines have been reported to be stable up to two years when stored at −80°C 62. Therefore, all samples underwent a single freeze-thaw cycle and were batch analyzed within 20 months of the initial sample collection using kits of the same lot numbers to minimize inter-assay variability. All assays were performed by an investigator blinded to the experimental treatment and according to manufacturer’s recommendations. Five cytokines, IL-1 beta, IL-6, IL-8, IL-10, and TNF-alpha, were measured using the MILLIPLEX MAP human high sensitivity cytokine assay (HYCTO-60SK, Millipore Corporation, Billerica, MA). Total adiponectin was measured using adipokine MILLIPLEX panel A (HADK1-61K-A), leptin using MILLIPLEX adipokine panel B (HADK2-61K), and high molecular weight adiponectin using an ELISA kit (EZHMW-64K, Millipore). For MILLIPLEX assays, median fluorescence intensity, calculated from duplicates for each sample, was collected using the Luminex-100 system. BioPlex manager 5.0 software, incorporating a five-parameter logistic curve-fitting method, was used to calculate sample cytokine concentrations for all MILLIPLEX MAP assays. Detection limits were 0.64 pg ml−1 for IL-1 beta, IL-6, IL-10, and TNF-alpha, 0.13 pg ml−1 for IL-8, 85 pg ml−1 for leptin, 145 pg ml−1 for total adiponectin and 0.5ng ml−1 for high molecular weight adiponectin.
Data analysis
The intervention and usual care groups were compared on demographic, medical, and carbohydrate ingestion outcomes at baseline with independent t-test or chi-square, depending on the nature of the variable. For magnitude and direction of intervention effect, M0 was subtracted from M3 for a difference score with the intervention and usual care group compared with independent t-test. An intent-to-treat analysis was performed. The following ratios were calculated to examine the relative pro-inflammatory to anti-inflammatory patterns: IL-6:IL-10, IL-8:IL-10, and TNF-alpha:IL-10. The level of significance was set at a P value < .05. Due to the pilot nature of the study and limited available data related to cytokine response to a physical activity behavior change intervention for breast cancer survivors, we report the effect sizes regardless of statistical significance. Effect sizes were calculated by dividing the between group difference by the standard deviation of the between group difference. Effect sizes were defined as small (.2), medium (.5), or large (.8) 63.
Results
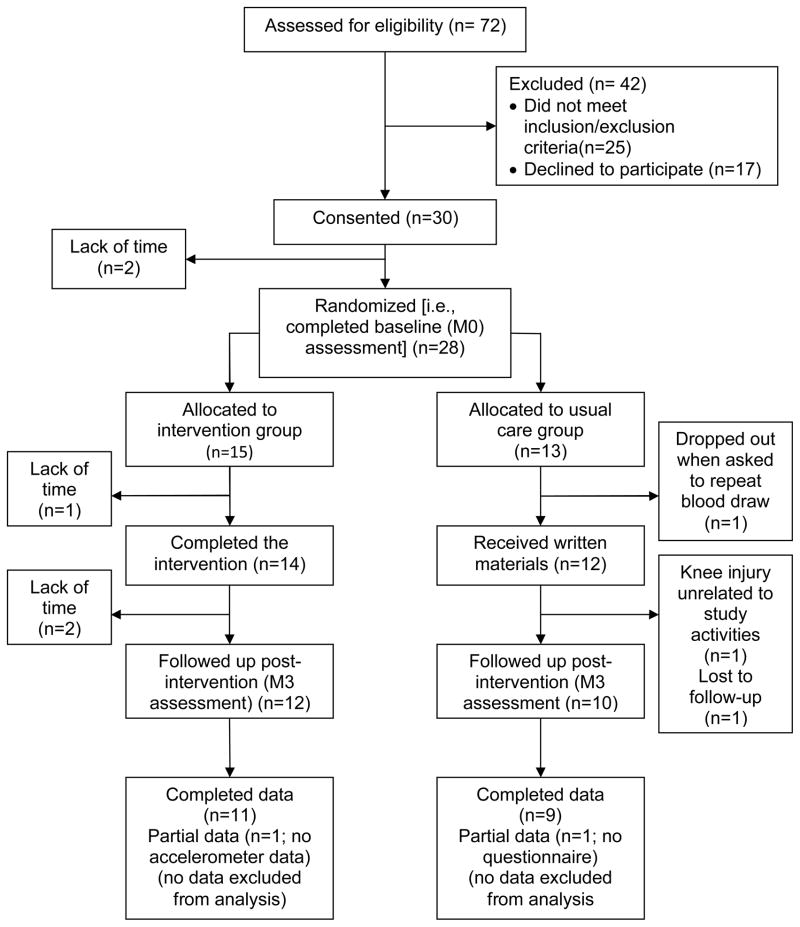
Participant flow and characteristics
Participants were recruited from June 2008 through April 2009. Figure 2 provides the participant flow through the study. Complete M0 assessments were obtained on all 15 intervention participants. Although all usual care participants provided baseline data, the serum sample from one of the usual care participants was not usable due to excessive hemolysis. This participant refused to have repeat phlebotomy done and withdrew from the study. At M3, one participant in the intervention group did not wear the accelerometer long enough to collect 4 valid days of data and one participant in the usual care group did not return a completed survey.
Figure 2.
Participant recruitment, allocation, and retention by study group (M0=month 0, M3=month 3)
Baseline characteristics for all participants combined and stratified by group assignment are provided in Table 2. No statistically significant differences between the study groups at baseline were noted for demographic, medical, and carbohydrate ingestion outcomes with the exception of the usual care group having more mean years of education as compared with the intervention group at baseline (16.3 ± 2.5 versus 14.1 ± 2.9, P = .047) (Table 2).
Table 2.
Baseline characteristics for participants (overall and by group allocation)
| Variable | All (n=28) | Intervention (n=15) | Control (n=13) | P |
|---|---|---|---|---|
| Age, years | 56 ±10.5 (30–69) | 58.0±6.1 (43–67) | 53.7±13.9 (30–69) | 0.317 |
| Race | ||||
| Caucasian | 24 (86%) | 13 (87%) | 11 (85%) | 0.877 |
| African American | 4 (14%) | 2 (13%) | 2 (15%) | |
| Education, years | 15.1±2.9 (9–20) | 14.1±2.9 (9–20) | 16.3±2.5 (12–20) | 0.047 |
| Income (household) | ||||
| <$10,000 | 3 (10.7%) | 2 (13.3%) | 1 (7.7%) | |
| $10,000–$19,999 | 1 (3.6%) | 1 (6.7%) | 0 (0%) | |
| $20,000–$34,999 | 2 (7.1%) | 1 (6.7%) | 1 (7.7%) | 0.754 |
| $35,000–$49,999 | 0 (0%) | 0 (0%) | 0 (0%) | |
| $50,000+ | 22 (78.6%) | 11 (73.3%) | 11 (84.6%) | |
| Months since | ||||
| Diagnosis | 73.9±70.7 (6–242) | 86.9±81.1 (6–242) | 59±55.9 (10–206) | 0.307 |
| Cancer stage | ||||
| Stage 1 | 15 (54%) | 10 (67%) | 5 (39%) | |
| Stage 2 | 9 (32%) | 4 (27%) | 5 (39%) | 0.266 |
| Stage 3 | 4 (14%) | 1 (7%) | 3 (23%) | |
| Smoking (yes) | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Alcohol (yes) | 15 (54%) | 8 (53%) | 7 (54%) | 0.978 |
| Drinks/week | 2.1±4.7 (0–24) | 2.1±6.1 (0–24) | 2.0±2.4 (0–6) | 0.929 |
| Surgery (yes) | 28 (100%) | 15 (100%) | 13 (100%) | 1.00 |
| Chemotherapy (yes) | 21 (75%) | 10 (67%) | 11 (85%) | 0.274 |
| Radiation (yes) | 22 (79%) | 12 (80%) | 10 (77%) | 0.843 |
| Hormonal therapy | ||||
| Aromatase inhibitor | 9 (32%) | 6 (40%) | 3 (23%) | |
| Estrogen receptor | 5 (18%) | 1 (7%) | 4 (31%) | 0.228 |
| None | 14 (50%) | 8 (53%) | 6 (46%) | |
| Lymphedema | ||||
| Yes | 3 (11%) | 1 (7%) | 2 (15.4%) | |
| No | 20 (71%) | 11 (73%) | 9 (69.2%) | 0.743 |
| Not Sure | 5 (18%) | 3 (20%) | 2 (15.4%) | |
| Beta blocker (yes) | 5 (18%) | 2 (13%) | 3 (23%) | 0.502 |
| Post-menopausal (yes) | 24 (86%) | 14 (93%) | 10 (77%) | 0.216 |
| Comorbidity score | 2.7±2.1 (0–8) | 2.7±1.9 (0–6) | 2.7±2.3 (0–8) | 0.938 |
| Left arm circumference (cm) | 34.41±5.9 (24.8–47.0) | 34.6±5.8 (25.67–47.03) | 34.2±6.2 (24.8–46.3) | 0.855 |
| Right arm circumference (cm) | 34.2±5.5 (24.9–47.0) | 34.3±5.3 (25.7–47.0) | 34.2±5.8 (24.9–46.3) | 0.968 |
| Dietary carbohydrates (gm/day) | 211.9±67.8 (105.3–342.9) | 208.6±73.1 (113.7–342.9) | 215.6±64.1 (105.3–293.1) | 0.791 |
Values presented as mean ± SD (range) or n (%)
Exercise adherence, protocol deviations, and adverse events
The 14 participants completing the intervention attended 100% of the supervised exercise sessions (168/168), 100% of the update sessions (42/42), and 73% of the group sessions (61/84). With regard to adherence to aerobic physical activity (based on accelerometer), improvement was noted in the weekly minutes of ≥ moderate intensity physical activity for the intervention versus the usual care group [i.e., +45.4 versus −37.7; mean between group difference = +83.1; effect size (d) =.76; P = .097]. At M3, the mean for ≥ moderate intensity physical activity in the intervention group was 198.4 ± 111.7 minutes per week. With regard to resistance training, the 12 participants in the intervention group providing M3 data completed 21 of the 24 possible resistance exercise sessions over the 12-week period (87.5%) and reported a weekly average of 1.8 sessions per week. During the final 4 weeks of the intervention, the intervention participants completed 5 of the 8 sessions (63%; weekly average = 1.3 sessions).
No protocol deviations occurred. Three adverse events were identified (two related and non-serious in the intervention group and one unrelated and serious in the control group). No significant change in arm circumference for the intervention versus usual care group was noted (left arm: intervention = −.3 cm versus usual care = +.7 cm; between group difference = −1.0 cm; d = −.7; P = .145) (right arm: intervention = −.1 cm versus usual care = +.2 cm; between group difference = −.3 cm; d = −.2; P = .691).
Health outcomes
The magnitude and direction of the effect sizes for the health outcomes are provided in Table 3. Fitness and PSQI (self-reported) sleep latency demonstrated statistically significant effect sizes, all in a beneficial direction (i.e., fitness increased and self-reported sleep latency decreased). Although not statistically significant, a large positive effect size was noted for muscle strength with medium to large negative effect sizes noted for BMI, percent fat mass, PSQI daytime dysfunction subscale, and global PSQI score. Only small effect sizes were found for fatigue and sleep efficiency.
Table 3.
Physical activity, muscle strength, body composition, fatigue, and sleep dysfunction for intervention versus usual care groupa
| Intervention | Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Variable | Mean (M0) | SD | Mean (M3) | SD | Change score | Mean (M0) | SD | Mean (M3) | SD | Change score | Between group difference | P value | d |
| Predicted O2 (ml/kg/min) | 20.2 | 5.2 | 23.9 | 6.2 | 3.7 | 23.9 | 6.0 | 23.9 | 3.8 | −0.1 | 3.8 | 0.015 | 1.1 |
| Back/leg strength (kg) | 55.4 | 18.0 | 68.4 | 15.5 | 13.0 | 62.5 | 18.5 | 67.1 | 16.2 | 4.6 | 8.4 | 0.059 | 0.9 |
| Body mass index (kg/m2) | 33.9 | 7.4 | 33.6 | 7.0 | −0.3 | 30.3 | 7.11 | 30.6 | 7.28 | 0.3 | −0.6 | 0.145 | −0.6 |
| Waist-to-hip ratio | 0.81 | 0.07 | 0.80 | 0.04 | −.01 | 0.83 | 0.06 | 0.83 | 0.05 | 0.00 | −0.01 | 0.429 | −0.3 |
| Fat mass % | 45.3 | 8.5 | 44.2 | 6.7 | −1.1 | 41.6 | 8.0 | 42.3 | 7.7 | 0.6 | −1.7 | 0.163 | −0.6 |
| Fatigue | |||||||||||||
| Intensityb | 4.2 | 2.0 | 4.2 | 1.8 | 0.0 | 3.9 | 1.5 | 4.2 | 1.6 | 0.3 | −0.3 | 0.7 | −0.2 |
| Interference b | 2.6 | 1.6 | 2.6 | 1.5 | 0.0 | 2.6 | 1.5 | 2.4 | 1.1 | −0.2 | 0.2 | 0.7 | 0.2 |
| Sleep (PSQI) | |||||||||||||
| Latencyc | 1.6 | 0.8 | 1.3 | 0.6 | −0.3 | 1.1 | 0.9 | 1.3 | 1.0 | 0.2 | −0.5 | 0.02 | −1.2 |
| Efficiencyc | 0.5 | 0.8 | 0.8 | 1.0 | 0.3 | 0.3 | 0.7 | 0.7 | 0.9 | 0.4 | −0.1 | 0.8 | −0.10 |
| Daytime dysfunctionc | 0.9 | 0.7 | 0.6 | 0.5 | −0.3 | 1.0 | 0.5 | 0.9 | 0.9 | −0.1 | −0.2 | 0.4 | −0.4 |
| Global dysfunction c | 6.5 | 3.1 | 6.3 | 2.7 | −0.2 | 5.4 | 3.4 | 6.2 | 3.2 | 0.8 | −1.0 | 0.3 | −0.4 |
| Sleep (accelerometer) | |||||||||||||
| Efficiency | 81.4 | 10.3 | 79.5 | 8.3 | −1.9 | 80.4 | 9.9 | 83.2 | 10.5 | 2.8 | −4.7 | 0.4 | −0.4 |
| Latency | 8.7 | 6.9 | 7.1 | 3.8 | −1.6 | 9.3 | 5.0 | 9.8 | 5.2 | 0.5 | −2.1 | 0.4 | −0.4 |
Data based on study participants completing both baseline and 3 month follow-up.
Possible range = 1 to 10.
Higher score indicates greater sleep dysfunction; possible range for subscales = 0 to 3 and global dysfunction = 0 to 21. Abbreviations: M0, month 0 (baseline); M3, month 3; SD, standard deviation; d, effect size
Inflammatory related serum markers (cytokines, adipokines)
Except for IL-1 beta, all cytokines evaluated were detectable in ≥ 95% of M0 and M3 samples (Table 4). Due to the high percentage of samples below the level of detection for IL-1 beta, statistical comparisons were not performed. For the remaining cytokines, samples with concentrations below the level of detection were assigned a concentration of one-half the detection limit. This approach is unbiased when the percentages of measurements below the level of detection is less than 10% 64. Leptin, high molecular weight adiponectin, and total adiponectin were detectable in 100% of samples.
Table 4.
Number (percent) of samples with detectable cytokine levels stratified by assessment time point
| Cytokine | Detectable N (%) |
Not detectable N (%) |
|---|---|---|
| Month 0 (total n=27a) | ||
| IL-1 beta (pg/ml) | 15 (56%) | 12 (44%) |
| IL-6 (pg/ml) | 26 (96%) | 1 (4%) |
| IL-8 (pg/ml) | 27 (100%) | 0 (0%) |
| IL-10 (pg/ml) | 26 (96%) | 1 (4%) |
| TNF-alpha (pg/ml) | 27 (100%) | 0 (0%) |
| Month 3 (total n=22) | ||
| IL-1 beta (pg/ml) | 8 (36%) | 14 (64%) |
| IL-6 (pg/ml) | 21 (95%) | 1 (5%) |
| IL-8 (pg/ml) | 22 (100%) | 0 (0%) |
| IL-10 (pg/ml) | 21 (95%) | 1 (5%) |
| TNF-alpha (pg/ml) | 22 (100%) | 0 (0%) |
One participant’s month 0 sample was not useable due to hemolysis. Therefore, total number for month 0 assays was 27.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor
The magnitude and direction of the effect sizes for the inflammatory related serum markers are provided in Table 5. The intervention was associated with a large effect size reduction in leptin as compared with the usual care group and with a medium to large effect size increase in IL-6. Small to medium effect size changes occurred as follows: decrease in IL-10, increase in high molecular weight adiponectin, decrease in total adiponectin, and decreases in the IL-6:IL-10, IL-8:IL-10, and TNF alpha:IL-10 ratios.
Table 5.
Inflammatory related serum markers for intervention versus control groupa
| Intervention | Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Variable | Mean (M0) | SD | Mean (M3) | SD | Change score | Mean (M0) | SD | Mean (M3) | SD | Change score | Between group difference | P value | d |
| IL-6 (pg/ml) | 19.7 | 41.2 | 23.6 | 49.9 | 3.9 | 19.5 | 19.9 | 17.5 | 15.2 | −2.0 | 5.9 | 0.121 | 0.7 |
| IL-8 (pg/ml) | 7.3 | 2.3 | 6.7 | 2.6 | −0.6 | 7.7 | 1.7 | 7.5 | 3.4 | −0.3 | −0.3 | 0.758 | −0.1 |
| IL-10 (pg/ml) | 5.8 | 3.8 | 5.6 | 3.6 | −0.2 | 31.1 | 54.3 | 34.1 | 49.9 | 3.0 | −3.2 | 0.518 | −0.3 |
| TNF-alpha (pg/ml) | 9.6 | 5.6 | 7.0 | 3.3 | −2.6 | 9.6 | 10.7 | 7.7 | 8.9 | −1.9 | −0.8 | 0.690 | −0.2 |
| IL-6:IL-10 ratio | 3.34 | 5.30 | 2.64 | 3.10 | −0.7 | 1.35 | 1.33 | 1.53 | 2.29 | 0.18 | −0.88 | 0.333 | −0.4 |
| IL-8:IL-10 ratio | 1.58 | 0.74 | 1.64 | 0.76 | 0.06 | 1.09 | 0.76 | 1.39 | 1.30 | 0.30 | −0.24 | 0.531 | −0.3 |
| TNF alpha:IL-10 ratio | 1.95 | 1.13 | 1.94 | .98 | −0.01 | .99 | 0.89 | 1.42 | 2.20 | 0.43 | −0.44 | 0.416 | −0.4 |
| Leptin (ng/mL) | 46.5 | 29.1 | 43.9 | 27.8 | −2.6 | 34.4 | 23.1 | 40.8 | 32.2 | 6.4 | −9.0 | 0.031 | −1.0 |
| HMW adiponectin (ug/mL) | 8.8 | 5.4 | 8.7 | 5.2 | −0.01 | 9.0 | 6.2 | 7.6 | 5.5 | −1.4 | 1.41 | 0.361 | 0.4 |
| Total adiponectin (ug/mL) | 19.8 | 10.4 | 19.6 | 10.6 | −0.2 | 17.9 | 9.7 | 18.4 | 11.1 | 0.5 | −0.7 | 0.562 | −0.3 |
Data based on study participants completing both baseline and 3 month follow-up.
Abbreviations: M0, month 0 (baseline); M3, month 3; SD, standard deviation; d, effect size; IL, interleukin; TNF, tumor necrosis factor; HMW, high molecular weight
Discussion
Our physical activity behavior change intervention in breast cancer survivors increased physical activity to a level sufficient to cause significant improvements in fitness, sleep latency, and serum leptin concentrations as compared with usual care. Although not statistically significant, medium to large effect sizes in a beneficial direction occurred for muscle strength, BMI, and percent body fat for the intervention group compared with the usual care group; smaller effect sizes were noted for some but not all dimensions of fatigue and sleep dysfunction. The magnitude of intervention effects on inflammatory markers and ratios of pro:anti-inflammatory cytokines were generally small, although changes in IL-6 and leptin were relatively large. The direction of these effect sizes were as hypothesized with the exception of an increase in IL-6 and decreases in IL-10 and adiponectin in the intervention compared with the usual care group.
The changes we detected in serum markers of inflammation suggest hypotheses and patterns that merit assessment in trials that are powered to detect small effect size changes (e.g., 400 study participants in each study group allocation would be required to detect an effect size of .2 with a power of 80 and p < .05 63). Given that 800 participants would require a costly, multicenter study, a future trial could use a number of strategies to increase effect sizes and improve study power. For example, enrolling a more homogenous sample (e.g., similar level of fatigue, amount of sleep dysfunction, time since cancer diagnosis, age) would reduce variability in response to the intervention, resulting in greater study power. Also, limiting future trials to survivors with higher levels of fatigue and sleep dysfunction at baseline would avoid the chance of smaller effect sizes caused by a “floor effect”. Related to the hypothesized pathways in Figure 1, our intervention may have influenced inflammation, fatigue, and sleep primarily through the direct effect of exercise on chronic systemic inflammation rather than an indirect effect through a change in body composition (i.e., we found minimal changes in body composition in the intervention group). However, lengthening the intervention beyond three months would facilitate greater changes in body composition and possibly increase the effect sizes changes related to inflammation.
Our high percentage of samples with IL-1 beta concentrations below the level of detection is consistent with other studies. For example, IL-1 beta was not detectable in 79% of breast cancer patients on active treatment (i.e., chemotherapy) 65 or in 91% of breast cancer patients with metastatic disease 66, suggesting that IL-1 beta may not increase as a function of therapy or disease severity. The high percentage of non-detectable IL-1 beta in our study population could also reflect relatively good physical functioning in our participants (i.e., they were capable of engaging in moderate intensity exercise). Consistent with this possibility, others detected IL-1 beta in only 0% to 36% of “healthy” subjects when samples were analyzed with multiple different multiplex and ELISA kits including the same high sensitivity Luminex assay used for the current study 67. Finally, we opted to measure IL-1 beta based on its association with poor physical functioning and sleep regulation 11, 68. However, poor sleep quality was not an inclusion criteria for this study.
Our 0.76 effect size increase in weekly minutes of physical activity is consistent with the significant improvement in fitness with the intervention. With regard to resistance exercises, adherence declined during the later weeks of the intervention, which may explain, in part, the lack of significant increase in muscle strength. The significant effect size decrease in leptin was due to both a decrease in the intervention group and an increase in the usual care group. This may be explained, in part, by the patterns of change in physical activity in the study groups (i.e., increased in intervention group and decreased in the usual care group) and the trends noted for BMI, waist-to-hip ratio, and percent body fat. These results are consistent with a previously reported association between lower leptin and higher self-reported physical activity in a cross-sectional study of breast cancer survivors 25. However, we found a significant change in leptin in our behavior change intervention study, whereas an exercise efficacy intervention did not, perhaps because the latter study had an exercise target of 90 minutes per week compared with our target of 150 weekly minutes 26. Consistent with this, two recent randomized exercise efficacy studies in postmenopausal women found significant reductions in leptin after 12 months of 225 weekly minutes of exercise 23, 24. The decrease in leptin represents a valuable health outcome because of its association with lessened risk of breast cancer recurrence and its potential as a therapeutic target 69.
The measured cytokines did not change in a consistently pro-inflammatory or anti-inflammatory manner, perhaps because some cytokines (e.g., IL-6 70) have both pro- and anti-inflammatory properties. The proposed mechanism of exercise effects on cytokines is through a transient increase in IL-6, which is consistent with our effect size of +0.7. We did not expect to find an increase in IL-6 because such changes occur primarily within 24 hours of an acute exercise bout and our participants did not exercise for at least 24 hours before the blood draw. Perhaps over time adequate, repeated and consistent bouts of exercise result in a higher steady state level of serum IL-6 that contributes to a reduction in chronic inflammation via the anti-inflammatory properties of IL-6. Our positive effect on IL-6 differs from a negative effect reported in an intervention study using a home-based pedometer and resistance bands 47. This inconsistency may be due to the fact that cytokine response to exercise may vary based on gender and/or treatment status (i.e., on or off primary cancer treatment). Moreover, the exercise intensity and amount achieved in the previous study may have been lower than that of our study due to minimal contact time with study staff (only one instructional session) and use of perceived exertion rather than target heart rate to insure adequate exercise intensity. However, our ratios of pro:anti-inflammatory cytokines showed negative effect sizes, suggesting that the exercise intervention may change the cytokine “balance” in favor of an overall reduction in inflammatory tone. Such a change in the balance of pro- and anti-inflammatory cytokines could account for the inconsistency between the epidemiologic evidence of reduced breast cancer recurrence and mortality with exercise 1 despite a poorer prognosis in patients with chronic elevations of IL-6 16.
Although the intervention showed little effect on fatigue, a small to medium effect size reduction (i.e., d = −.4) in the PSQI daytime dysfunction subscale is similar to the weighted mean effect size change in fatigue with exercise of −.54 reported in a recent review 4. This suggests that the intervention may improve daytime somnolence not detected by the fatigue measure used. Also, the significant improvement in self-reported sleep latency coupled with the small to medium effect size improvements in the self-reported global sleep score and accelerometer latency suggest that a future, larger trial has the potential to examine the role of pro:anti-inflammatory effects in mediating changes in sleep dysfunction that may occur with a physical activity behavior change intervention for breast cancer survivors.
Although exercise efficacy studies have reported reductions in leptin with exercise training in post-menopausal women 24, our study is unique in reporting that breast cancer survivors show beneficial changes in response to an overall increase in physical activity, as opposed to achieving a specific exercise dose. Our findings are reassuring given the “real world” challenges of achieving specific exercise amounts in broader populations after rigorous randomized exercise efficacy trials identify benefits of specific exercise regimens. Our study is also one of few that describe cytokine responses to a physical activity behavior change intervention in breast cancer survivors. As reviewed in the introduction, behavior change intervention studies have been inconsistent with regard to changes in cytokine markers of inflammation, possibly because of insufficient increases in physical activity. The magnitude and direction of our effect size changes support the possibility that a physical activity behavior change intervention can affect inflammation and related health outcomes. Our study also provides valuable effect size data for power calculations for future trials that may use similar physical activity interventions, inclusion/exclusion criteria, and assay methods. A recent review of exercise and immune function concluded that future studies are needed to identify those inflammatory mediators that are most sensitive to exercise 71. Our study suggests that adipokines may be the most sensitive markers. With regard to serum cytokines other than adipokines, IL-6 may be the most sensitive but its complex effects (i.e., pro- versus anti-inflammatory properties) may complicate interpretation of changes.
Because of its small sample size and lack of study power, our study does not allow definitive conclusions about specific intervention effects. Also, budgetary constraints prevented measurement of percent body fat with dual x-ray absorptiometry, which is considered the gold standard. Lastly, the two study groups differed significantly with regard to education, but the small sample size prevented addressing this difference statistically. However, we expect that the effect of education differences on the study outcomes would be minimal, with the potential bias being toward a smaller effect size change given that higher education may be associated with greater physical activity adherence 72. If that were the case, the adjusted effect size changes would be larger than those we report.
In conclusion, our data show that physical activity behavior change interventions aimed at improving exercise adherence without reaching a specified exercise dose (as in an exercise efficacy study) can have statistically significant beneficial effects on cardiorespiratory fitness, sleep latency, and serum leptin concentrations. The direction and magnitude of changes in serum markers in our study suggest patterns relevant to the interactions of physical activity, inflammation, and fatigue and sleep dysfunction and warrant assessment in larger trials. Further delineating the effects of a physical activity behavior change intervention on individual inflammatory markers and their patterns of change in breast cancer survivors has the potential to increase our understanding of how such interventions might contribute to symptom management.
Acknowledgments
Grant support: The project was supported by the Simmons Cancer Institute at Southern Illinois University School of Medicine Translational Research Award. Dr. Rogers, Hopkins-Price, Vicari, Rao, and Verhulst receive salary support from National Cancer Institute Grant 1R21CA135017. Dr. Rogers, Hopkins-Price, Vicari, and Verhulst also receive salary support from National Cancer Institute Grant 5R01CA136859. Dr. Courneya is supported by the Canada Research Chairs Program and National Cancer Institute Grant 5R01CA136859.
Footnotes
Clinical trial registration: ClinicalTrials.gov NCT00640666
References
- 1.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 4.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 5.Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21:1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- 6.Kirkova J, Walsh D. Cancer symptom clusters--a dynamic construct. Support Care Cancer. 2007;15:1011–1013. doi: 10.1007/s00520-007-0259-2. [DOI] [PubMed] [Google Scholar]
- 7.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 8.Lorton D, Lubahn CL, Estus C, et al. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–374. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- 9.Santos RV, Tufik S, De Mello MT. Exercise, sleep and cytokines: is there a relation? Sleep Med Rev. 2007;11:231–239. doi: 10.1016/j.smrv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29:893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen M, Bruunsgaard H, Weis N, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 16.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 17.Braithwaite D, Satariano WA, Sternfeld B, et al. Long-term prognostic role of functional limitations among women with breast cancer. Journal of the National Cancer Institute. 2010;102:1468–1477. doi: 10.1093/jnci/djq344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of sleep research. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 19.Thornton LM, Andersen BL, Carson WE., 3rd Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer immunology, immunotherapy : CII. 2008;57:1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garaulet M, Hernandez-Morante JJ, de Heredia FP, Tebar FJ. Adiponectin, the controversial hormone. Public health nutrition. 2007;10:1145–1150. doi: 10.1017/S1368980007000638. [DOI] [PubMed] [Google Scholar]
- 23.Frank LL, Sorensen BE, Yasui Y, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 24.Friedenreich CM, Neilson HK, Woolcott CG, et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocrine-related cancer. 2011;18:357–369. doi: 10.1530/ERC-10-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligibel JA, Giobbie-Hurder A, Olenczuk D, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes Control. 2009;20:1523–1528. doi: 10.1007/s10552-009-9358-3. [DOI] [PubMed] [Google Scholar]
- 27.Courneya KS. Efficacy, effectiveness, and behavior change trials in exercise research. Int J Behav Nutr Phys Act. 2010;7:81. doi: 10.1186/1479-5868-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmack Taylor CL, Demoor C, Smith MA, et al. Active for Life After Cancer: a randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psychooncology. 2006;15:847–862. doi: 10.1002/pon.1023. [DOI] [PubMed] [Google Scholar]
- 29.Damush TM, Perkins A, Miller K. The implementation of an oncologist referred, exercise self-management program for older breast cancer survivors. Psycho-Oncol. 2006;15:884–890. doi: 10.1002/pon.1020. [DOI] [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 32.Midtgaard J, Tveteras A, Rorth M, Stelter R, Adamsen L. The impact of supervised exercise intervention on short-term postprogram leisure time physical activity level in cancer patients undergoing chemotherapy: 1- and 3-month follow-up on the body & cancer project. Palliat Support Care. 2006;4:25–35. doi: 10.1017/s1478951506060044. [DOI] [PubMed] [Google Scholar]
- 33.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 34.Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RW, Taliaferro LA, Jacobsen PB. Pilot study of a self-administered stress management and exercise intervention during chemotherapy for cancer. Support Care Cancer. 2006;14:928–935. doi: 10.1007/s00520-006-0021-1. [DOI] [PubMed] [Google Scholar]
- 36.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 37.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57 (Suppl 10):43–51. [PubMed] [Google Scholar]
- 38.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. American journal of physiology. Endocrinology and metabolism. 2002;283:E1272–1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 39.Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer-Kristensen J, Pedersen BK. Bicycle exercise enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or TNF-alpha pre-mRNA in BMNC. Journal of applied physiology. 1994;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]
- 40.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exercise immunology review. 2009;15:6–41. [PubMed] [Google Scholar]
- 41.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol. 2005;98:1534–1540. doi: 10.1152/japplphysiol.00566.2004. [DOI] [PubMed] [Google Scholar]
- 42.Hutnick NA, Williams NI, Kraemer WJ, et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37:1827–1835. doi: 10.1249/01.mss.0000175857.84936.1a. [DOI] [PubMed] [Google Scholar]
- 43.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 44.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W, Case LD, Blackwell K, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8:70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 46.Pierce BL, Neuhouser ML, Wener MH, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprod LK, Palesh OG, Janelsins MC, et al. Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community oncology. 2010;7:463–471. doi: 10.1016/s1548-5315(11)70427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond) 2007;31:996–1003. doi: 10.1038/sj.ijo.0803534. [DOI] [PubMed] [Google Scholar]
- 49.Chatzinikolaou A, Fatouros IG, Gourgoulis V, et al. Time course of changes in performance and inflammatory responses after acute plyometric exercise. Journal of strength and conditioning research / National Strength & Conditioning Association. 2010;24:1389–1398. doi: 10.1519/JSC.0b013e3181d1d318. [DOI] [PubMed] [Google Scholar]
- 50.Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18:1410–1418. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- 51.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 52.Rogers LQ, Vicari S, Courneya KS. Lesson learned in the trenches: facilitating exercise adherence among breast cancer survivors in a group setting. Cancer Nurs. 2010;33:E10–E17. doi: 10.1097/NCC.0b013e3181db699d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Sirard JR, Melanson EL, Li L, Freedson PS. Field evaluation of the Computer Science and Applications, Inc. physical activity monitor. Med Sci Sports Exerc. 2000;32:695–700. doi: 10.1097/00005768-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation including pathophysiology and clinical applications. 3. Baltimore, MD: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 56.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Ray CA, Bowsher RR, Smith WC, et al. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. Journal of pharmaceutical and biomedical analysis. 2005;36:1037–1044. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 59.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 60.Liu MY, Xydakis AM, Hoogeveen RC, et al. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clinical chemistry. 2005;51:1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- 61.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. Journal of proteome research. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC immunology. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 64.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- 67.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. Journal of immunological methods. 2009;350:125–132. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Opp MR. Sleep and psychoneuroimmunology. Neurol Clin. 2006;24:493–506. doi: 10.1016/j.ncl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Ray A, Cleary MP. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin Ther Targets. 14:443–451. doi: 10.1517/14728221003716466. [DOI] [PubMed] [Google Scholar]
- 70.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 71.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: Immune function and exercise. Exercise immunology review. 2011;17:6–63. [PubMed] [Google Scholar]
- 72.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 73.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 74.George J, Cannon T, Lai V, et al. Cancer cachexia syndrome in head and neck cancer patients: Part II. Pathophysiology. Head Neck. 2007;29:497–507. doi: 10.1002/hed.20630. [DOI] [PubMed] [Google Scholar]
- 75.Roubenoff R, Grinspoon S, Skolnik PR, et al. Role of cytokines and testosterone in regulating lean body mass and resting energy expenditure in HIV-infected men. Am J Physiol Endocrinol Metab. 2002;283:E138–145. doi: 10.1152/ajpendo.00426.2001. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 77.Chavey C, Bibeau F, Gourgou-Bourgade S, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 79.Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 80.Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast cancer research : BCR. 2011;13:R34. doi: 10.1186/bcr2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruunsgaard H, Bjerregaard E, Schroll M, Pedersen BK. Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J Am Geriatr Soc. 2004;52:237–241. doi: 10.1111/j.1532-5415.2004.52061.x. [DOI] [PubMed] [Google Scholar]