Abstract
Purpose
To investigate the role of the TLR4/MD-2 antagonist eritoran tetrasodium in a murine model of contact lens–associated corneal infiltrates.
Methods
C57BL/6 mouse corneas were abraded and treated with eritoran tetrasodium or placebo, either before or after stimulation with either LPS, the TLR2 ligand Pam3Cys, or antibiotic-killed Pseudomonas aeruginosa. A 2-mm punch from a silicon hydrogel contact lens was used to cover the corneal surface throughout the inhibition and stimulation period. Corneal infiltrates were detected by in vivo confocal microscopy and by immunohistochemistry for neutrophils. The effect of eritoran tetrasodium on stimulated human corneal epithelial cells (HCECs), macrophages, and neutrophils was also assessed.
Results
Eritoran tetrasodium significantly inhibited CXC chemokine production in the cornea and development of corneal infiltrates, specifically neutrophils, in response to stimulation with LPS (TLR4), but not Pam3Cys (TLR2). When the antagonist was applied after LPS stimulation, neutrophil infiltration was also inhibited, although a higher concentration was needed. Furthermore, IL-8 production by TLR4- but not TLR2-stimulated HCECs, macrophages and neutrophils was also significantly reduced. Corneal inflammation induced by P. aeruginosa in the presence of tobramycin was found to be dependent on expression of TLR4 and MD-2 and is inhibited by eritoran tetrasodium.
Conclusions
Eritoran tetrasodium is a highly effective antagonist of TLR4/MD-2-dependent corneal inflammation.
Pseudomonas aeruginosa is a major cause of bacterial keratitis in the United States and worldwide, and a predominant risk factor is contact lens wear.1,2 In addition to infectious keratitis, contact lens wear is also associated with sterile, culture-negative clinical manifestations, including contact lens–associated red eye (CLARE), and contact lens peripheral ulcers (CLPU).3,4 Although symptoms are less severe than for infectious keratitis, affected individuals experience pain, redness, blurred vision, and severe discomfort. The number of contact lens wearers exceeds 34 million in the United States and 140 million worldwide, and the incidence of corneal infiltrative events has increased with the predominant use of silicon hydrogel lenses.3,4
The cause of these sterile infiltrates has been difficult to assess unequivocally, but a strong indication that microbial products are involved comes from examination of corneal biopsies from sites of infiltrates, which show neutrophils in corneal infiltrates of patients with CLPU,5 that corneal (fluorescein) staining, indicative of epithelial abrasions, appears to be a risk factor for infiltrative events, and also that bacteria can be isolated from contact lenses and lens cases.2,4,6,7 Using animal models, we and others demonstrated that exposure of the abraded corneal surface to LPS or other bacterial products induces neutrophil recruitment to the corneal stroma.8–12 We therefore propose that corneal infiltrates occur as a consequence of minor, contact lens–associated corneal abrasions and stimulation of resident cells in the epithelium by dead or noninvasive bacteria or by bacterial products such as LPS.
Bacterial cell wall components such as LPS can activate the host innate immune response by activation of the Toll-like receptor (TLR) family of pathogen recognition molecules,13 which therefore represent a potential target for immune intervention. TLR4 is the most complex and sensitive member of this receptor family, an accessory molecules lipid A binding protein, CD14 and MD-2 to detect picomolar levels of the lipid A moiety LPS,14 and is the only member of the TLR family that activates both the MyD88 and the TRIF intracellular signaling pathways.15 Antagonistic rather than agonistic lipid A activity on TLR4, was initially demonstrated for Rhodobacter sphaeroides lipid A (RSLA), which has five acyl chains compared with six chains on lipid A from most Gram-negative bacteria. RSLA also has pronounced antagonistic activity for LPS Gram-negative bacteria, and has only minor agonist activity on some cell types.16–18 The structure of RSLA has therefore provided the basis for generating synthetic lipid A antagonists,19 one of which is eritoran tetrasodium. This compound, which has four acyl chains, inhibits LPS-induced monocyte activation, LPS endotoxemia, and LPS-induced chronic airway disease and is undergoing clinical trials for bacterial sepsis.20–22 Furthermore, the compound has successfully undergone phase I clinical trials and was shown to be safe and nontoxic in normal volunteers.23–25 Recent analysis of the crystal structure of TLR4 shows that eritoran tetrasodium binds to the MD-2 accessory molecule associated with TLR426 and is consistent with the mode of action being directly antagonistic to lipid A binding, as the tetra-acylated eritoran tetrasodium does not initiate TLR4 dimerization or activation of the signaling cascade.19,26
In our current studies, we identified a potent and specific anti-inflammatory activity of this antagonist by inhibiting TLR4-, but not TLR2- induced CXC chemokine production recruitment of neutrophils to the corneal stroma. Eritoran tetrasodium also specifically inhibits CXC chemokine production by corneal epithelial cells, macrophages, and neutrophils, thereby demonstrating the antagonistic effect of this reagent on multiple cell types associated with development of corneal infiltrates. Furthermore, we show that eritoran tetrasodium inhibits TLR4/MD-2-dependent corneal inflammation induced by P. aeruginosa in the presence of tobramycin.
Methods
Source of Animals
Six- to 8-week-old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). TLR4−/− mice were provided by Shizao Akira (Osaka University, Japan), and MD-2−/− mice were obtained from Christopher Karp (University of Cincinnati) with permission from Ke Miyaki (University of Tokyo, Japan).
Antagonist Preparation
Eritoran tetrasodium (1.1 mg) or placebo was obtained from Eisai Research Institute (Andover, MA) and reconstituted in endotoxin-free water (Sigma-Aldrich, Monticello, IA). The reagents were stored at −80°C and sonicated before use.
Cell Lines and In Vitro Stimulation
The SV-40-transfected human corneal epithelial cell line (HCE-T) was obtained from ATCC (American Type Culture Collection, Manassas, VA), and was grown to 80% confluency in keratinocyte serum-free medium (KSFM) containing 0.05 mg/mL bovine pituitary extract and 5 ng/mL epidermal growth factor in collagen-coated plates. Before stimulation, the cells underwent epidermal growth factor starvation overnight, as described.27,28 HCE cells were plated into 48-well plates and preincubated with 200 ng/mL MD-2 (R&D Systems, Minneapolis, MN) for 1 hour. The human neutrophil-like cell line (HL-60) was maintained in RPMI with 10% FBS and incubated 5 days in 1.2% DMSO to generate the neutrophil phenotype. The U937 macrophage-cell line was cultured in RPMI medium (Invitrogen-Gibco, Grand Island, NY) with 10% FBS, and 5 × 104 cells/well were added to 96-well plates.
All cells were incubated with eritoran tetrasodium or placebo, followed by stimulation with ultrapure LPS (TLR4-specific, Escherichia coli K12; InVivogen, San Diego, CA) or with the TLR2 ligand Pam3CysK4 (EMC Microcollections, Tübingen, Germany). After 3 hours, cell-free supernatants were collected, and CXCL8/IL-8 was measured by ELISA (R&D Systems).
Mouse Model of Contact Lens–Associated Corneal Inflammation
C57BL/6 mice (6–8 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were maintained in specific pathogen-free conditions in microisolator cages and were treated in accordance with the guidelines provided in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The mice were anesthetized by intraperitoneal injection of 0.4 mL (5%) 2,2,2-tribromoethanol (TBE). Three parallel abrasions were made in the corneal epithelium with a 26-gauge needle. Eritoran tetrasodium or placebo was added to the corneal surface, and a 2-mm diameter punch from a normal contact lens (Lotrafilcon A; Ciba Vision, Duluth, GA) was placed on the corneal surface. After one hour, we added 40 µg LPS or 10 µg Pam3Cys in 2 µL. The contact lens was removed after another hour, when the mice recovered from anesthesia at 2 hours. In some experiments, the order of agonist and antagonist was reversed.
Pseudomonas aeruginosa–Induced Corneal Inflammation
Pseudomonas aeruginosa strain ATCC 19660 was obtained directly from ATCC and maintained in stocks at −80°C. Bacteria were grown overnight (18 hours) in tryptic soy broth (TSB), and aliquots from these stationary cultures were diluted 1:100 and grown in TSB until OD650 = 0.2 (1 × 108 CFU/mL). The bacteria were centrifuged, washed in PBS, and resuspended at 2 × 109 bacteria/mL in 0.3% tobramycin in PBS (Sigma-Aldrich). Bacterial killing was confirmed by absence of growth on TSB agar plates. The corneas were abraded by three parallel scratches, and a 5-µL bacterial suspension containing 1 × 107 organisms was placed on the corneal surface and covered by a 2-mm diameter punch from a silicon hydrogel contact lens (Lotrafilcon; Ciba Vision), as just described.
In Vivo Confocal Microscopy Analysis of Corneal Thickness and Haze
In vivo analysis of cellular infiltration was accomplished with a confocal microscope (Confoscan; Nidek, Gamagori, Japan), as described previously.8 Briefly, the mice were anesthetized and immobilized, and the cornea was examined using a 40× objective with a transparent gel (Genteal; Novartis Ophthalmics, Duluth, GA) as a medium. A series of images of the entire cornea was captured (Navis software; Zebra Technologies, Vernon Hills, IL), and stromal thickness (the area between the basal epithelium and corneal endothelium) was measured directly with the software. To measure total infiltrate (termed corneal haze), the light intensity readout of each 1- to 2-µm image of the corneal stroma was exported (Prism; Graph Pad Software, San Diego, CA), and the total area under the curve was then calculated as previously described.8,12
Immunohistochemistry
Eyes were snap frozen in liquid nitrogen, and 5-µm sections were incubated 2 hours with anti-neutrophil antibody NIMP-R14 diluted 1:100 in 1% fetal calf serum in TBS (1%FCS/TBS), as described.8,9 After washing, corneal sections were incubated with FITC-conjugated rabbit anti-rat antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 in 1% FCS/TBS. Slides were mounted in antifade medium containing DAPI (Vectashield; Vector), and the number of neutrophils in each section was examined by fluorescence microscopy and quantified by direct counting.
Apoptosis Assay
Cell viability was measured in vitro measured by trypan blue exclusion. The corneal sections were incubated with terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) reagents according to the manufacturer’s directions (Roche, Penzberg, Germany). TUNEL-positive cells were detected and quantified by fluorescence microscopy.
Statistics
Statistical analyses significance was determined by ANOVA followed by Tukey post hoc analysis (Prism; Graph Pad Software). A value of P < 0.05 was considered significant.
Results
Effect of Eritoran Tetrasodium on LPS-Induced CXC Chemokine Production in the Cornea
Neutrophils are the predominant cell type in CLPU,5 and they mediate changes in corneal structure in animal models of LPS-induced corneal inflammation.9,11 Furthermore, neutrophil recruitment to the corneal stroma is mediated by CXCL1/KC, which is elevated early in LPS-induced corneal inflammation,8,9,29 and which we found to have an important role in neutrophil recruitment.8,9,29 To determine the role of eritoran tetrasodium in LPS-induced corneal inflammation, we anesthetized the mice, abraded the corneas, and applied 1 µL eritoran tetrasodium topically. A 2-mm diameter punch from soft contact lens was then placed on the corneal surface. After 1 hour, the lens was removed, and either 40 µg LPS or 10 µg of the TLR2 agonist Pam3Cys in 2 µL was added for an additional hour. After 3 hours (total of 4 hours after stimulation), corneas were dissected, homogenized, and centrifuged, and CXCL1/KC in the soluble fraction was measured by ELISA.
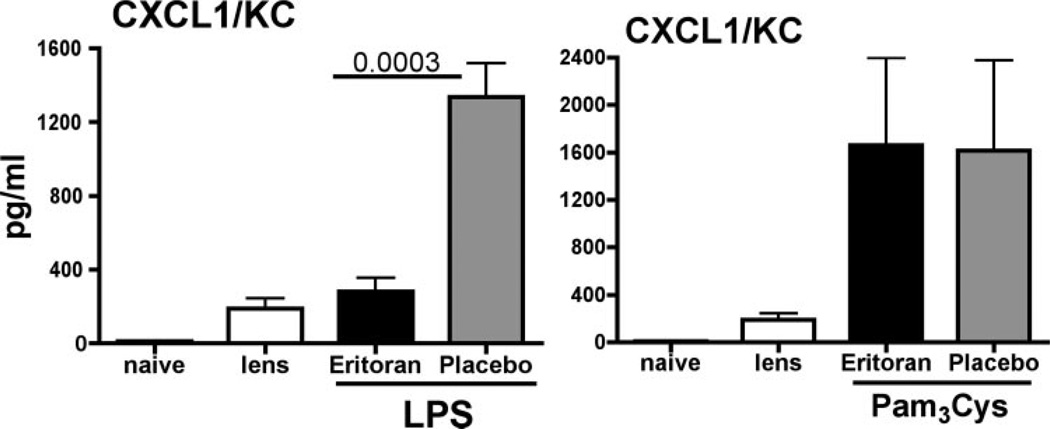
Figure 1 shows that CXCL1/KC was not detected in corneas incubated with contact lenses alone. However, in corneas treated with LPS (and placebo), CXCL1/KC was significantly elevated. When the corneas were pretreated with eritoran tetrasodium before LPS, chemokine production was significantly reduced, demonstrating the antagonistic effect of eritoran tetrasodium in this model.
Figure 1.
CXC chemokine production in corneas treated with the lipid A antagonist eritoran tetrasodium. Corneas of C57BL/6 mice were abraded with three parallel, superficial scratches. Eritoran tetrasodium (2 µL of 1.1 mg/mL) or placebo was added topically, and a 2-mm diameter punch from a lotrafilcon silicon hydrogel contact lens was placed on the corneal surface. After 1 hour, the lens was removed, 2 µL LPS (40 µg) or Pam3Cys (10 µg) was added topically, and the lens was replaced. After 1 hour, the lens was removed, and 3 hours later (4 hours after stimulation), corneas were dissected and homogenized, and CXCL1/KC was measured by ELISA. LPS-treated corneas: P = 0.0003 for 1.1 mg/mL eritoran versus placebo; Pam3Cys-stimulated corneas: P > 0.05 between Eritoran and placebo. This experiment is representative of two repeat studies with five mice per group.
Our previous studies showed that topical application of the synthetic lipopeptide Pam3Cys induces CXC chemokine production and corneal inflammation that is dependent on TLR2 and MyD88.8 To determine the effect of eritoran tetrasodium, corneas were abraded and treated with the antagonist before stimulation with Pam3Cys. Figure 1 also shows that Pam3Cys induced similar levels of CXCL1/KC in the cornea as LPS; however, chemokine production was unaffected by pretreatment of eritoran tetrasodium, thereby demonstrating the antagonist effect of eritoran tetrasodium for TLR4- but not TLR2-induced corneal inflammation.
Effect of Eritoran Tetrasodium on Cellular Infiltration of the Corneal Stroma
The corneal stroma is composed of antiparallel rows of collagen fibrils, regularly spaced by intermediate proteoglycans, primarily keratan sulfate. The predominant cell types present are keratocytes, which are quiescent, translucent cells organized in the stroma as a regular array, and occasional macrophages and dendritic cells. When examined by vivo confocal microscopy, which scans the entire corneal stroma (~80 µm), the normal transparent corneal stroma shows no infiltrating cells, whereas our previous findings showed that topical exposure to LPS stimulates a pronounced cellular infiltrate detected by their high reflectivity in the cornea.8,9
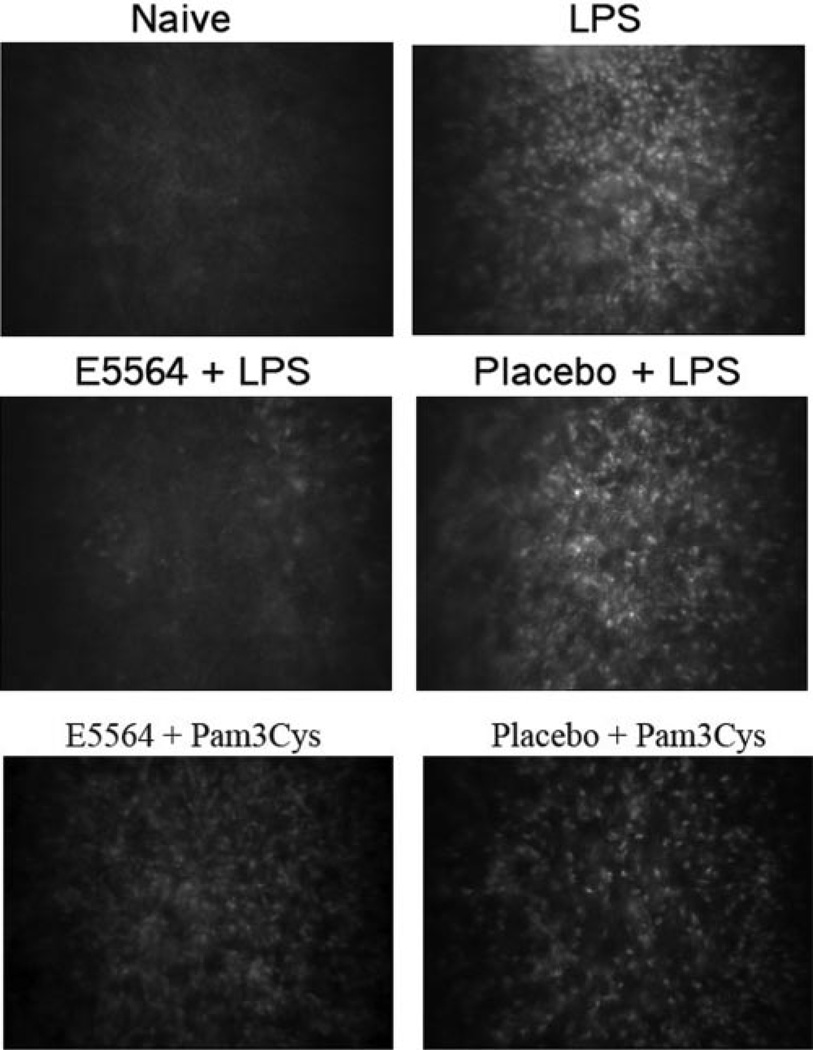
To determine the effect of eritoran tetrasodium on cellular infiltration to the corneal stroma, corneas were abraded and treated with eritoran tetrasodium or placebo as described above. After 2 hours, the contact lens was removed, and after 24 hours, corneas were examined by in vivo confocal microscopy (Confoscan; Nidek), and images of the central corneal stroma were captured. As shown in Figure 2, there are no infiltrating cells in corneas of naïve mice, whereas LPS pretreated corneas show an intense cellular infiltrate in the central corneal stroma (detected as small, light reflective cells) 24 hours after contact lens associated exposure to LPS either alone or in the presence of placebo. In marked contrast, corneas treated with eritoran tetrasodium showed minimal cellular infiltrate in the central corneal stroma. There was no effect of eritoran tetrasodium on Pam3Cys/TLR2–induced corneal inflammation (Fig. 2, bottom panels), further demonstrating the selective effect of this antagonist.
Figure 2.
Effect of eritoran tetrasodium on corneal infiltrates. Corneas of C57BL/6 mice were abraded and treated with eritoran tetrasodium or placebo before LPS or Pam3Cys stimulation. After 24 hours, which is the peak of neutrophil infiltration, corneas were examined by in vivo confocal microscopy. Representative images of the central corneal stroma reveal cellular infiltration in LPS-treated corneas either alone or with placebo, but not in eritoran tetrasodium-treated corneas. In contrast, the cellular infiltrate was present in antagonist-treated and placebo-treated corneas stimulated with Pam3Cys.
Effect of Pretreatment with Eritoran Tetrasodium on LPS-Induced Contact Lens–Associated Neutrophil Recruitment and Development of Corneal Haze
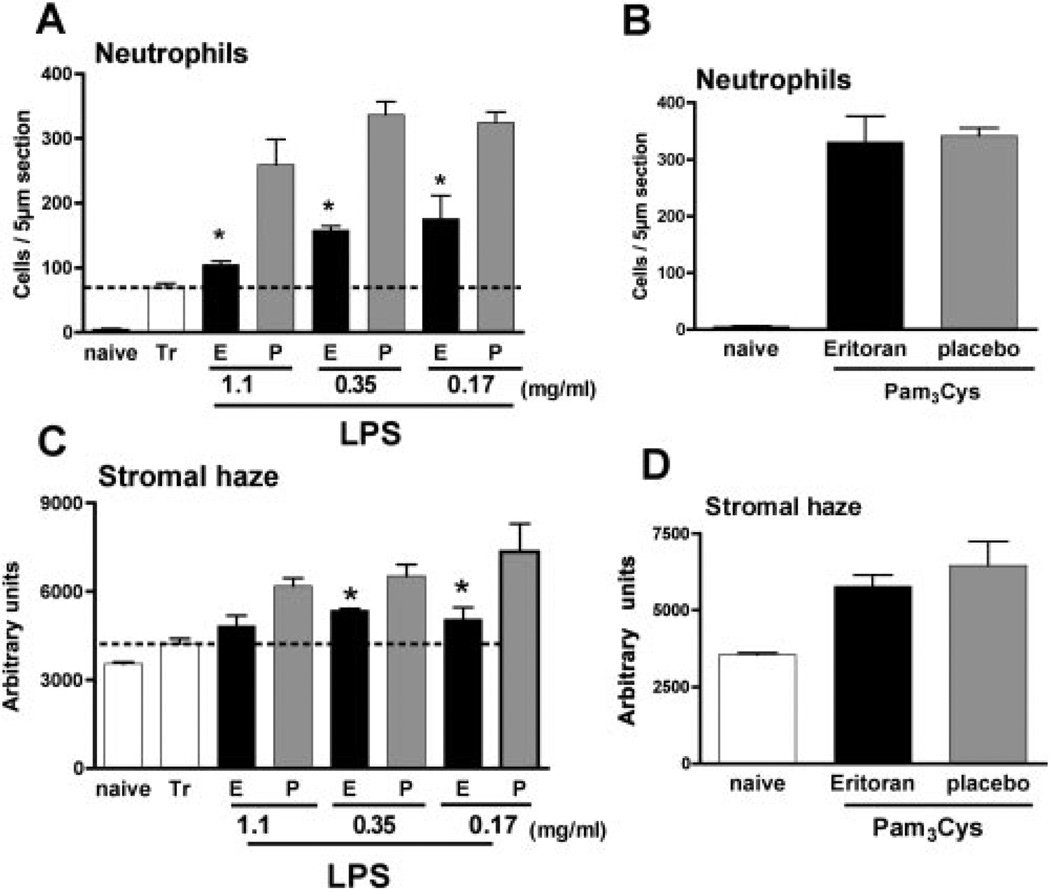
To quantify the inhibitory effect of eritoran tetrasodium on cellular infiltrates to the cornea after 24 hours, we measured the total infiltrate by reflectivity in the corneal stroma. As each 1-µm section from anterior to posterior stroma is measured in terms of light reflectivity, these were used to generate a curve, and the area under the curve represents the total reflectivity. In addition, as most of the corneal infiltrate is neutrophils,8,9 the number of these cells per 5 µm corneal section was directly counted.
As shown in Figure 3, corneas that were abraded and incubated with contact lenses alone had ~50 neutrophils per section, whereas LPS-treated corneas had >300 neutrophils per corneal section. Mice given topical application of eritoran tetrasodium before LPS showed a dose-dependent reduction in the number of neutrophils. LPS-induced cellular infiltrate, measured by total reflectivity, was also reduced in corneas pretreated with eritoran tetrasodium, although ID50 was threefold higher (1.1 mg/mL).
Figure 3.
Neutrophil recruitment and quantification of corneal infiltrates after topical application of eritoran tetrasodium. Corneas were treated as described in the legend to Figure 1, examined by in vivo confocal microscopy, and processed for immunohistochemistry, to assess the neutrophil infiltrate. Corneas treated with 2 µL eritoran tetrasodium (E) or placebo (P) at indicated concentrations, and stimulated with either LPS (A, C) or Pam3Cys (B, D). Neutrophil recruitment and stromal haze were measured as described in the text. *Significant differences (P < 0.05) between antagonist and placebo; there was no significant effect of this antagonist on Pam3Cys-induced corneal inflammation.
Figure 3B, 3D shows neutrophil infiltration and reflectivity in corneas stimulated with Pam3Cys. As before, TLR2 activation induces neutrophil infiltration and development of corneal haze8; however, pretreatment with the highest concentration of eritoran tetrasodium had no effect on either neutrophil infiltration or total reflectivity. Taken together, these findings demonstrate that eritoran tetrasodium has an antagonistic effect on TLR4- but not TLR2-induced corneal inflammation.
Effect of Eritoran Tetrasodium Applied Topically after LPS-Induced Corneal Inflammation
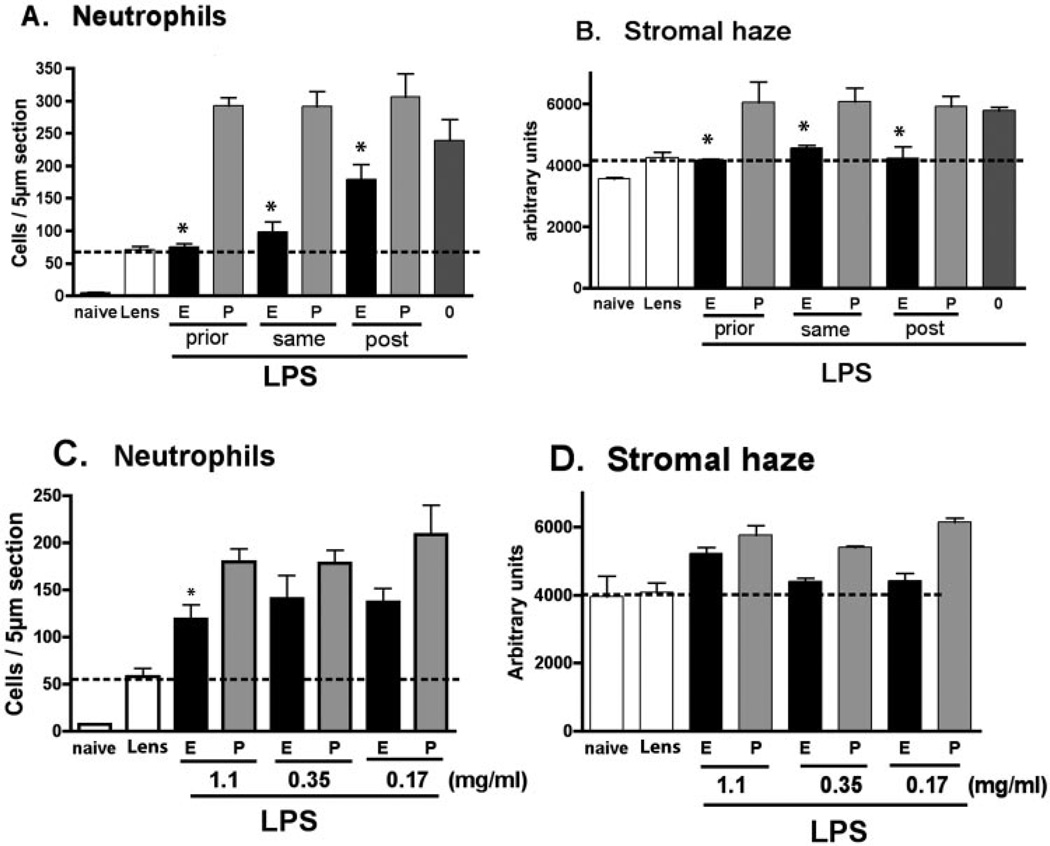
To determine whether eritoran tetrasodium can inhibit corneal inflammation after the response has been initiated, corneas were abraded and stimulated with LPS as described earlier. Our previous studies showed that activation of TLR2 induces MAP kinase and IκB phosphorylation in the cornea within 1 hour.30 We therefore added eritoran tetrasodium 1 hour after LPS stimulation, and compared with mice either pretreated with eritoran tetrasodium or given eritoran tetrasodium and LPS simultaneously. Neutrophil infiltration and corneal haze were examined as before.
As shown in Figure 4, LPS-induced neutrophil infiltration and corneal haze were significantly reduced in all groups treated with eritoran tetrasodium compared with placebo, indicating that antagonism remains effective even after the inflammatory response has been initiated. In some experiments, a second application of the antagonist was applied after 3 hours (when the contact lens was no longer present). We found that there was no significant difference between this protocol and single applications of eritoran (data not shown).
Figure 4.
Comparison of eritoran tetrasodium given before and after LPS-induced corneal inflammation. Mouse corneas were abraded as described in the legend to Figure 1. (A, B) Corneas were treated with 2 µL of 1.1 mg/mL eritoran tetrasodium or placebo either before LPS (prior), at the same time as LPS (same), or 1 hour after LPS stimulation (post). After 24 hours, neutrophil infiltration and corneal haze were measured as before. *Significant differences (P < 0.001) for prior and same treated with eritoran tetrasodium versus placebo; P < 0.05 for post treatment with eritoran versus placebo. (C, D) The dose–response of antagonist given as a therapeutic (posttreatment) 1 hour after LPS stimulation. These experiments were repeated three times with similar results. Tr, trauma control; 0: LPS only.
Effect of Eritoran Tetrasodium on Apoptosis in the Corneal Epithelium
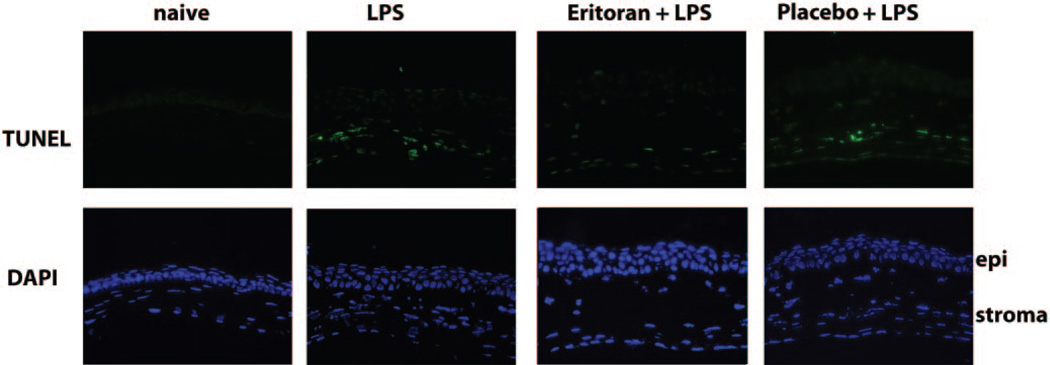
To determine whether there is any cytotoxic effect of eritoran tetrasodium on corneal epithelium, mice were treated as described above, and corneal sections were examined after 24 hours. We used a TUNEL assay on eritoran-treated corneas to identify apoptotic cells, and total cells were identified with the DAPI nuclear stain. As shown in Figure 5, there were no TUNEL-positive cells in the corneal epithelium in which eritoran tetrasodium was applied, either in the presence or absence of LPS. TUNEL-positive cells were detected in the corneal stroma of LPS-treated corneas, either alone or with placebo, which corresponds to the presence of neutrophils (not shown). These observations demonstrate that there is no proapoptotic effect of eritoran tetrasodium on corneal epithelial cells.
Figure 5.
Absence of apoptosis in eritoran tetrasodium–treated corneal epithelium. Mice were pretreated with 2.2 µg antagonist before LPS, as described in Figure 4. The eyes were snap frozen and 5-µm sections were stained using the TUNEL assay and counterstained with DAPI to identify individual cells. Note that the only TUNEL-positive cells are in the corneal stroma and correspond to the presence of neutrophils.
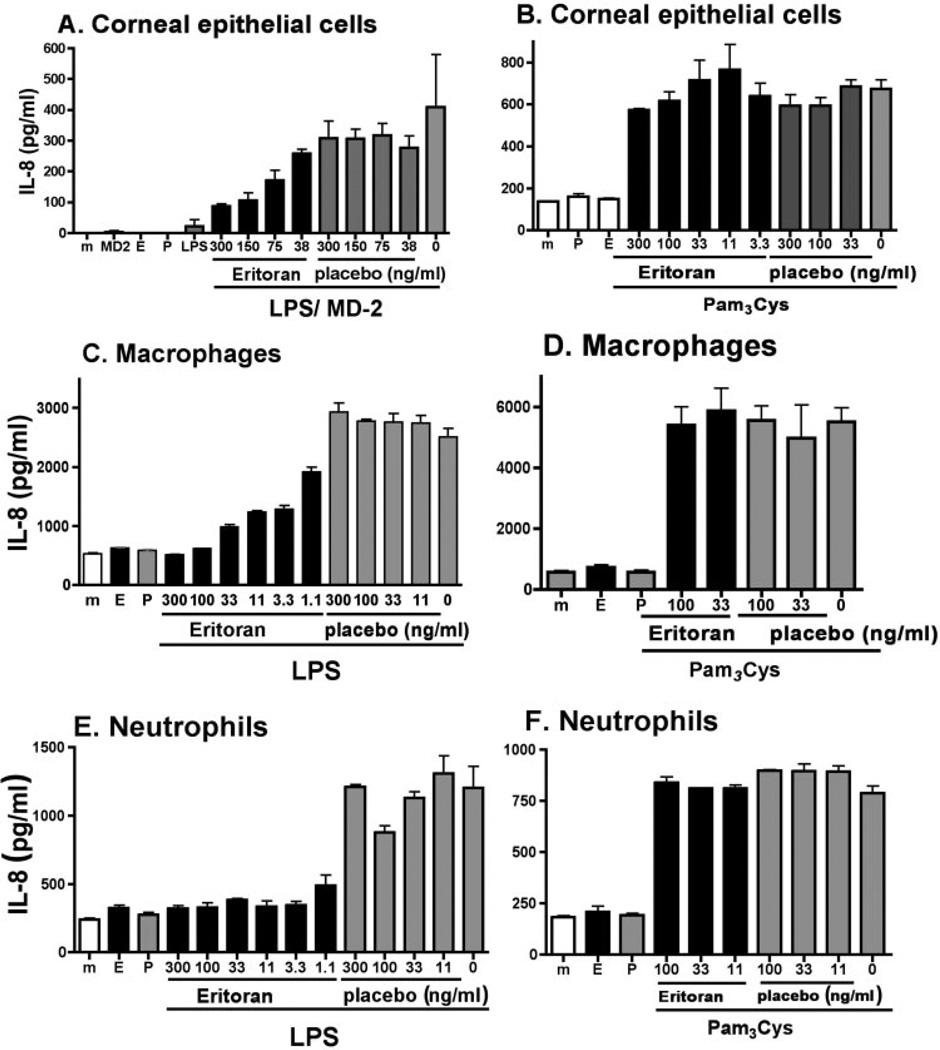
Effect of Eritoran Tetrasodium on LPS-Induced IL-8 Production by Human Corneal Epithelial Cells, Macrophages and Neutrophils
As eritoran tetrasodium has an inhibitory role in vivo, we next examined the effect of eritoran tetrasodium on specific cell types involved in corneal inflammation, including corneal epithelial cells, macrophages, and neutrophils.
Human macrophage, neutrophil, and corneal epithelial cell lines were treated with increasing concentrations of antagonist or placebo before stimulation with LPS or Pam3Cys. After 3 hours, the concentration of the neutrophil chemokine IL-8 in the culture supernatant was measured by ELISA. As shown in Figure 6, all three cell types produced IL-8 in response to LPS. Furthermore, eritoran tetrasodium inhibited LPS-induced IL-8 production in a dose-dependent manner in each cell type, whereas there was no effect of the antagonist on Pam3Cys-induced responses. The macrophage and neutrophil cell lines produced high levels of IL-8, which were inhibited by 1 ng/mL eritoran tetrasodium. In contrast, human corneal epithelial cells, which respond to LPS only in the presence of exogenous MD-2,31 produced less IL-8 and required a higher concentration of eritoran tetrasodium to inhibit cytokine production.
Figure 6.
Effect of eritoran tetrasodium on TLR2- and TLR4-stimulated human corneal epithelial, macrophage, and neutrophil cell lines. Cell lines derived from human corneal epithelial cells (HCE-T), macrophages (U937), and neutrophils (HL-60) were stimulated with LPS (A, C, E) or Pam3Cys (B, D, F) in the presence of antagonist or placebo. After 3 hours (U937 and HL-60 cells) or 24 hours (HCE-T cells), IL-8 levels in culture supernatants was quantified by ELISA. Data shown are the mean ± SEM of three wells per sample. Experiments were repeated three times with similar results. (A) P < 0.01 for 300 ng/mL eritoran versus placebo; P < 0.05 for 150 ng/mL eritoran versus placebo. P > 0.05 for 75 ng/mL eritoran versus placebo with LPS stimulation; no difference between eritoran versus placebo with Pam3Cys stimulation. (C, E) P < 0.001 for 11 ng/mL with LPS stimulation for macrophages and neutrophils. (E, F) P < 0.001 for 11 ng/mL with LPS stimulation. There was no significant difference between antagonist and placebo in Pam3Cys stimulated cells (B, D, F).
We therefore conclude that the antagonistic effect of eritoran tetrasodium is not due to its effect on a single cell type, but likely inhibits TLR4 activation by resident and infiltrating cells.
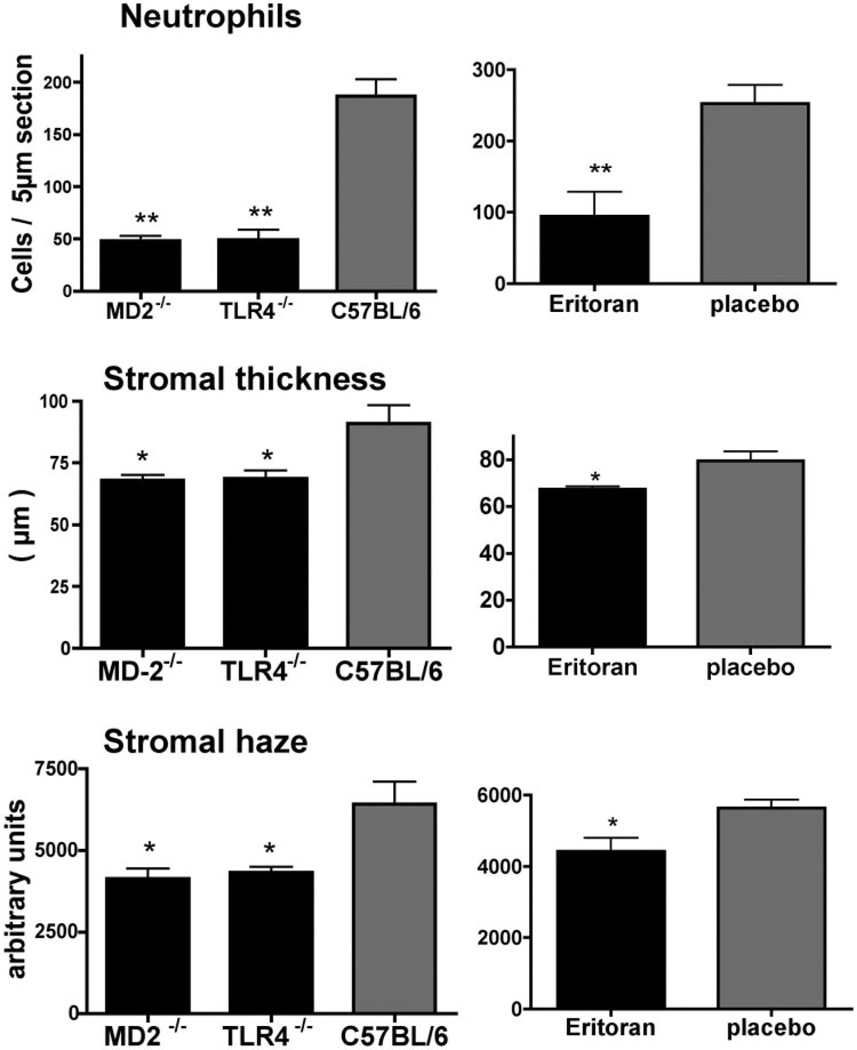
Corneal Inflammation Induced by Antibiotic-Killed P. aeruginosa is TLR4/MD-2-dependent and Is Inhibited by Eritoran Tetrasodium
As P. aeruginosa is a major cause of contact lens–related bacterial keratitis, we also examined the effect of eritoran tetrasodium in a model of P. aeruginosa-induced corneal inflammation. Our preliminary studies found no difference in corneal inflammation induced by P. aeruginosa killed either by heat or after brief incubation with tobramycin (data not shown). To determine the role of TLR4 and MD-2 in P. aeruginosa-induced corneal inflammation, we incubated P. aeruginosa in tobramycin for 30 minutes to kill the bacteria (confirmed after plating) and added 2 µL bacterial suspension containing 1 × 107 organisms (in the presence of antibiotic) to the abraded corneal surface of C57BL/6, TLR4−/− and MD-2−/− mice. Bacteria were covered with a 2 mm diameter punch from a silicon hydrogel contact lens for 2 hours as described. After 24 hours, corneal inflammation was examined as before. Figure 7A shows P. aeruginosa-treated C57BL/6 corneas had a pronounced neutrophil infiltration to the corneal stroma; however, neutrophil infiltration, corneal thickness and corneal haze were significantly lower in TLR4−/− and MD-2−/− corneas compared with C57BL/6 corneas. Similar results were found for LPS (not shown).
Figure 7.
Effect of eritoran tetrasodium on corneal inflammation induced by antibiotic-killed P. aeruginosa. P. aeruginosa strain ATCC 19660 was killed by 30 minutes incubation with 0.3% tobramycin, and 1 × 107 bacteria were added to the abraded corneal surface of C57BL/6, TLR4−/−, and MD-2−/− mice, and a 2 mm diameter punch from a silicon hydrogel was added. Corneas were treated with 2.2 µg eritoran tetrasodium in 2 µL H2O or with placebo. After 24 hours, neutrophil infiltration, and corneal thickness and haze were measured as before. Data were analyzed by ANOVA and statistically significant differences (*P < 0.05; **P < 0.001) were noted for all parameters between C57BL/6 mice compared with TLR4−/− and MD-2−/− mice (A), and between eritoran tetrasodium compared with placebo. These data are the mean ± SD of five mice per group, and the experiments were repeated twice with similar results.
When tobramycin-killed P. aeruginosa were added to corneas in the presence of eritoran tetrasodium (2,2 g in 2L), each of these markers of corneal inflammation were significantly inhibited compared with placebo (Fig. 7B). Together, these findings support our observations using ultrapure LPS, and indicate that corneal inflammation induced by antibiotic-killed P. aeruginosa is TLR4/MD-2 dependent and can be inhibited by eritoran tetrasodium.
Discussion
Bacterial LPS has a variable polysaccharide region (which is the o-antigen, a core ketodeoxyoctulosonic acid (KDO) polysaccharide region, and a lipid A region. The biological activities of LPS, including induction of proinflammatory and chemotactic cytokines, in addition to pyrogenicity and cytotoxicity properties are due to the lipid A region.13 The TLR4/MD-2 antagonist eritoran tetrasodium is stable in vitro and in animal models, and has no agonistic activity.19 This lipid A antagonist also inhibits ischemia reperfusion injury and acute lung injury in mice.32,33
In the present study, we demonstrate that eritoran tetrasodium has potent antagonist activity in murine models of LPS and P. aeruginosa-induced corneal inflammation in which contact lenses are used to retain LPS at the corneal surface. This model, while clearly limited by short-term exposure of agonists and antagonists at the corneal surface, is an important first step in examining anti-inflammatory agents in the context of contact lens–associated corneal inflammation.
In addition to TLR4, TLR2, TLR3, TLR5, and TLR9 activation can activate human corneal epithelial cells and initiate an inflammatory response in the corneal epithelium.8,30,34–37 However, TLR4 is likely the most sensitive receptor in this family, due to sequential interactions with accessory proteins. Gioannini et al.14 showed that LBP and CD14 combine to extract single endotoxin molecules from endotoxin aggregates or from bacterial outer membrane and form monomeric endotoxin:CD14 complexes. LPS is then transferred from CD14 to MD-2, and this monomeric endotoxin:MD-2 complex binds TLR4 at picomolar concentrations and induces TLR4 homodimerization and cell signaling. LBP and CD14 gene-knockout mice show partial reduction in LPS responsiveness, whereas MD-2−/− mice have the same nonresponsive phenotype as TLR4−/− mice,38,39 indicating an essential role for this coreceptor. Furthermore, the crystal structure demonstrates that this antagonist binds to MD-2 rather than TLR426; therefore, the TLR4/MD-2 complex is now thought to be the LPS receptor, with MD-2 as the binding protein and TLR4 the signaling protein. Finally, TLR4 is also unique among the TLRs in stimulating both the MyD88- and the TRIF-dependent intracellular signaling pathways, which together enhance the intensity and diversity of responses and can be activated and dimerized by picomolar levels of LPS or lipid A.14 Both pathways are functional in the cornea,8,12,34 and we recently demonstrated that MyD88 has a novel inhibitory role on TRIF signaling in corneal epithelial cells.34
Results from the present study also demonstrate that eritoran tetrasodium has inhibitory activity on IL-8 production by three cell types associated with corneal inflammation: human corneal epithelial cells, macrophages, and neutrophils. Our findings with the U937 macrophage cell line are consistent with a previous report showing the effect of proinflammatory cytokine production in LPS-stimulated human blood monocytes.40 Similar concentrations of antagonist block LPS-induced IL-8 production in the human HL-60 neutrophil cell line. As neutrophils express TLR4 and respond to low levels of LPS, this finding adds to our previous understanding of this inflammatory process by demonstrating that eritoran tetrasodium can function on neutrophils by inhibiting IL-8 production, which would lead to further neutrophil recruitment. Future studies will examine the effect of this antagonist on neutrophil activation and release of cytotoxic mediators.
Although we demonstrated that highly purified LPS induces corneal inflammation, there has been some question of the responsiveness of human corneal epithelial cells to LPS. An earlier report by Song et al.41 showed that corneal epithelial cells do respond to LPS; however, studies by Ueta et al.28 showed that TLR4 was intracellular and did not respond to LPS, and Blais et al.42 showed that accessory molecules LBP, CD14, and MD-2 in tears enhance HCEC responsiveness; however Visintin et al.31 showed conclusively by addition of exogenous MD-2 or by transfection of an MD-2 plasmid that expression of this accessory molecule is essential for LPS activation by corneal epithelial cells,31 which we also demonstrate in the current study. The recent crystal structure analysis of TLR4/MD-2 shows that eritoran tetrasodium binds to MD-2, not TLR4,26 supporting the notion that MD-2 expression on corneal epithelial cells is essential for LPS activation.
In the present study, corneal inflammation induced by killed P. aeruginosa was completely dependent on TLR4, as gene-knockout mice did not develop corneal inflammation. This observation is consistent with the reported role for TLR4 in P. aeruginosa keratitis,43 although our findings also demonstrate an essential role for MD-2 using gene-knockout mice and inhibition with eritoran tetrasodium.
Anti-inflammatory therapy of patients with corneal infiltrates is limited to steroid use, which has as a side effect increased ocular pressure; therefore, one goal of our studies on corneal inflammation is to identify potential targets for anti-inflammatory therapy. We recently demonstrated that C6 ceramide in liposomal formulation inhibits corneal inflammation induced by LPS or by killed Staphylococcus aureus, which is TLR2 dependent,12 by blocking C-Jun NH2 terminal kinase (JNK) phosphorylation.44 In the present study, we showed that the TLR4 antagonist eritoran tetrasodium inhibits LPS- and P. aeruginosa-induced corneal inflammation, but not TLR2-induced inflammation, and is therefore a more selective antagonist for corneal infiltrates caused by Gram-negative bacteria.
Acknowledgments
The authors thank Fabian Gusovsky (Eisai Research Institute of Boston, Inc.) for providing eritoran tetrasodium and placebo and Catherine Doller for excellent histology.
Supported by a sponsored Research Agreement with Eisai Research Institute, and further supported by NIH Grants R01EY14362 and P30EY11373 (EP) and by Research to Prevent Blindness and the Ohio Lions Eye Research Foundation.
Footnotes
Disclosure: Y. Sun, Eisai Pharmaceuticals of Boston, Inc. (F), P; E. Pearlman, Eisai Pharmaceuticals of Boston, Inc. (F), P
References
- 1.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–272. doi: 10.1097/OPX.0b013e3180485d5f. [DOI] [PubMed] [Google Scholar]
- 4.Szczotka-Flynn L, Debanne SM, Cheruvu VK, et al. Predictive factors for corneal infiltrates with continuous wear of silicone hydrogel contact lenses. Arch Ophthalmol. 2007;125:488–492. doi: 10.1001/archopht.125.4.488. [DOI] [PubMed] [Google Scholar]
- 5.Holden BA, Reddy MK, Sankaridurg PR, et al. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: histopathologic observations on the nature and type of corneal infiltrate. Cornea. 1999;18:538–543. [PubMed] [Google Scholar]
- 6.Holden BA, Sankaridurg PR, Sweeney DF, Stretton S, Naduvilath TJ, Rao GN. Microbial keratitis in prospective studies of extended wear with disposable hydrogel contact lenses. Cornea. 2005;24:156–161. doi: 10.1097/01.ico.0000138844.90668.91. [DOI] [PubMed] [Google Scholar]
- 7.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Johnson AC, Heinzel FP, Diaconu E, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 9.Khatri S, Lass JH, Heinzel FP, et al. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–2284. [PubMed] [Google Scholar]
- 10.Schultz CL, Buret AG, Olson ME, Ceri H, Read RR, Morck DW. Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect Immun. 2000;68:1731–1734. doi: 10.1128/iai.68.3.1731-1734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz CL, Morck DW, McKay SG, Olson ME, Buret A. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res. 1997;64:3–9. doi: 10.1006/exer.1996.0190. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2008;83:481–489. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 14.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 16.Kutuzova GD, Albrecht RM, Erickson CM, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J Immunol. 2001;167:482–489. doi: 10.4049/jimmunol.167.1.482. [DOI] [PubMed] [Google Scholar]
- 17.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 18.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59:441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs. 2005;6:496–502. [PubMed] [Google Scholar]
- 20.Hawkins LD, Christ WJ, Rossignol DP. Inhibition of endotoxin response by synthetic TLR4 antagonists. Curr Top Med Chem. 2004;4:1147–1171. doi: 10.2174/1568026043388123. [DOI] [PubMed] [Google Scholar]
- 21.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8:483–488. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 22.Savov JD, Brass DM, Lawson BL, McElvania-Tekippe E, Walker JK, Schwartz DA. Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. Am J Physiol. 2005;289:L329–L337. doi: 10.1152/ajplung.00014.2005. [DOI] [PubMed] [Google Scholar]
- 23.Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2007;29:467–509. [PubMed] [Google Scholar]
- 24.Bennett-Guerrero E, Grocott HP, Levy JH, et al. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2007;104:378–383. doi: 10.1213/01.ane.0000253501.07183.2a. [DOI] [PubMed] [Google Scholar]
- 25.Rossignol DP, Wasan KM, Choo E, et al. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother. 2004;48:3233–3240. doi: 10.1128/AAC.48.9.3233-3240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Kruszewski FH, Walker TL, DiPasquale LC. Evaluation of a human corneal epithelial cell line as an in vitro model for assessing ocular irritation. Fundam Appl Toxicol. 1997;36:130–140. [PubMed] [Google Scholar]
- 28.Ueta M, Nochi T, Jang MH, et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–3347. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- 29.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are produced selectively by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81(3):786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of corneal inflammation induced by toll like receptor 2. 2008;83(4):991–997. doi: 10.1189/jlb.1107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visintin A, Halmen KA, Khan N, Monks BG, Golenbock DT, Lien E. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4) J Leukoc Biol. 2006;80:1584–1592. doi: 10.1189/jlb.0606388. [DOI] [PubMed] [Google Scholar]
- 32.Shimamoto A, Chong AJ, Yada M, et al. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Kozlowski J, Schuster DP. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med. 2005;172:344–351. doi: 10.1164/rccm.200503-343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF induced corneal inflammation by inhibiting activation of c-jun n-terminal kinase (JNK) J Biol Chem. 2008;283(7):3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 39.Yang KK, Dorner BG, Merkel U, et al. Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J Immunol. 2002;169:4475–4480. doi: 10.4049/jimmunol.169.8.4475. [DOI] [PubMed] [Google Scholar]
- 40.Czeslick E, Struppert A, Simm A, Sablotzki A. E5564 (Eritoran) inhibits lipopolysaccharide-induced cytokine production in human blood monocytes. Inflamm Res. 2006;55:511–515. doi: 10.1007/s00011-006-6057-3. [DOI] [PubMed] [Google Scholar]
- 41.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- 42.Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–4244. doi: 10.1167/iovs.05-0543. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Fox T, Adhikary G, Kester M, Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008;83:1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]