Abstract
Metacognition—the ability to monitor and control one’s own cognition—is a sophisticated ability that reveals humans’ reflective mind and consciousness. Researchers have begun to explore whether animals share humans’ metacognitive capacity. This article reprises the original study that explored metacognition across species. A captive dolphin performed an auditory pitch-discrimination task using High/Low discrimination responses and an Uncertainty response with which he could decline to complete any trials he chose. He selectively declined the difficult trials near his discriminative threshold—just as humans do. This comparative exploration of metacognition required a trial-intensive titration of perceptual threshold and the training of a distinctive behavioral response. It could not have been conducted in the wild, though the naturalistic observation of dolphin uncertainty behaviors and risk-management strategies would no doubt yield complementary insights. The dolphin study inaugurated a new area of cross-species research. This research area opens a new window on reflective mind in animals, illuminates the phylogenetic emergence of metacognition, and may reveal the antecedents of human consciousness.
Keywords: uncertainty monitoring, metacognition, dolphin cognition, comparative psychology, decision making under uncertainty
Humans feel uncertainty and doubt. They know when they do not know or do not remember. They often (not always!) respond appropriately to these feelings by deferring response while they seek additional guidance and information. These adaptive responses are the focus of the expansive literature on metacognition and uncertainty monitoring (Benjamin, Bjork, & Schwartz, 1998; Dunlosky & Bjork, 2008; Flavell, 1979; Koriat, 1993; Koriat & Goldsmith, 1996; Nelson, 1992; Scheck & Nelson, 2005; Schwartz, 1994; Serra & Dunlosky, 2005). The essential idea in this field is that some minds have a cognitive executive that monitors cognition to evaluate its progress and guides cognition to improve its prospects. These monitoring/control functions are explored empirically by collecting humans’ feelings of knowing, judgments of learning, and tip-of-the-tongue states.
Human metacognition is crucial to all aspects of humans’ learning, thinking, and comprehension. Moreover, metacognition reveals sophisticated aspects of mind. It shows a hierarchical organization of cognition in humans, because metacognitive processes regulate lower-level perceptual and cognitive processes (Nelson & Narens, 1990). It shows humans’ conscious awareness of their cognition, because humans can introspect and verbally report those states (Nelson, 1996; Koriat, 2007). It shows humans’ self-awareness (Gallup, 1982), because uncertainty is experienced as a personal, self-owned cognitive experience (“I don’t know”; “I can’t tell”). In short, metacognition is one of humans’ highest-level cognitive abilities that could even be uniquely human.
Thus, it is an important question within comparative psychology whether nonhuman animals share this capacity with humans (Kornell, 2009; Smith, 2009; Smith, Beran, & Couchman, in press). The answer could bear on animals’ consciousness and self-awareness, too. Indeed, metacognition—given its centrality in reflective mind—might have a potential to reveal cognitive (dis)continuities between humans and animals that is rivaled only by language use and tool manufacture.
Given the question’s importance, Smith and his colleagues initiated the cross-species study of uncertainty monitoring and metacognition by asking whether a captive bottlenosed dolphin (Tursiops truncatus) might share humans’ capacity for cognitive monitoring and cognitive self-regulation (Smith, Schull, Strote, McGee, Egnor, & Erb, 1995). The study was conducted with the dolphin Natua (Figure 1) at the Dolphin Research Center in Grassy Key, Florida. This article describes that study retrospectively. It made a significant contribution to comparative psychology. It inaugurated a new domain of cross-species research, one that has implications regarding animal mind and intelligence and one that is still an active focus of empirical research and theoretical development.
Figure 1.
The dolphin participant in the study of Smith et al. (1995). Photograph Credit: Dolphin Research Center, Inc., Grassy Key, Florida. Used with permission.
Researchers exploring metacognition across species faced a difficult challenge. The typical metacognition paradigms used in human research were not applicable to animals, because they depended so heavily on conscious introspection and explicit (verbal!) reports about judgments of learning or feelings of knowing. The problem was to create paradigms that might tap the same cognitive capacities purely behaviorally and strictly nonverbally.
One basic requirement of these paradigms was that they create trial difficulty for the animal. Difficulty is necessary to arouse something like an uncertainty state in animals which they may monitor or respond adaptively to. To meet this requirement, Smith et al. adopted the psychophysical procedures commonly used in perceptual research with humans and animals. These procedures are specifically designed to create carefully titrated difficulty for the subject. They present perceptual discriminations, but then they deliberately narrow the contrast between the discriminative stimuli, forcing observers to make difficult discriminations near their perceptual limit or threshold (Au & Moore, 1990; Blough, 1958; Schusterman & Barrett, 1975; Yunker & Herman, 1973).
Accordingly, Smith et al. gave the dolphin an auditory psychophysical discrimination. The dolphin was to press the High response paddle whenever a repeating 2,100-Hz tone occurred. The dolphin was to press the alternative Low response paddle whenever a repeating tone of any lower pitch occurred (1,200–2,099 Hz). Initially, the animal performed the easy task of discriminating 2,100-Hz tones from 1,200-Hz tones. Then, the difficulty of the discrimination was raised by raising the pitch of the below-2,100 Hz tones until the dolphin was struggling to distinguish 2,100-Hz tones from tones near 2,085 Hz. At mature performance, the difficulty of the trials was titrated based on the dolphin’s performance to hold the task within the near-threshold region of the dolphin’s discriminative capacity and thus sustain its level of difficulty. The dolphin received fish rewards for correct responses, and brief trial-less timeout periods for incorrect responses. During many sessions, the dolphin’s enclosure was opened to the Gulf of Mexico, in case the ongoing level of trial difficulty recommended to the dolphin a change of pace or a road trip.
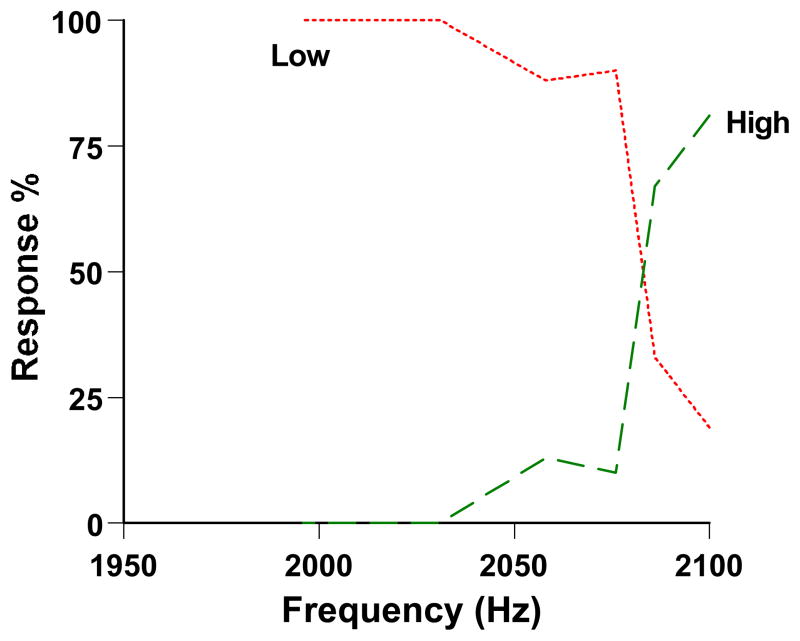
Figure 2 shows the dolphin’s performance in the High-Low discrimination. He often made correct High responses to 2,100-Hz trials. He often made correct Low responses to trials below about 2,075 Hz. But the trials surrounding 2,085 Hz, his known threshold relative to 2,100 Hz (Herman & Arbeit, 1972) and just 0.11 semitones from the standard 2,100-Hz tone, produced near-chance performance. The dolphin was performing at his true psychophysical limit. The task was causing him the intended difficulty.
Figure 2.
Performance by a dolphin in the auditory discrimination of Smith et al. (1995). The horizontal axis indicates the frequency (Hz) of the trial. The High response was correct for tones at 2,100 Hz—these trials are represented by the rightmost data point for each curve. All lower-pitched tones deserved the Low response. The green-dashed and red-dotted lines, respectively, show the dolphin’s percentage of High and Low responses at each frequency level. From “The Uncertain Response in the Bottlenosed Dolphin (Tursiops truncatus),” by J. D. Smith, J. Schull, J. Strote, K. McGee, R. Egnor, and L. Erb, 1995, Journal of Experimental Psychology: General, 124, p. 399. Copyright 1995 by the American Psychological Association. Reprinted with permission.
However, remember that the important question for metacognition research is whether animals can monitor the psychological signal of this difficulty and respond adaptively to it – that is, whether psychophysical procedures can leverage trial difficulty by generating useable uncertainty states in animals. The task described so far might have been creating the uncertainty states the comparative metacognition researcher seeks to study. The dolphin might have been prepared to report on that uncertainty or to act adaptively in managing it. The dolphin might have been having an internal soliloquy: to respond High or not to respond High, that is the question. But the threshold task alone cannot show whether the animal senses the difficulty or could manage the uncertainty. These capacities are hidden by allowing only two responses that map to the two input classes (2,100-Hz tones and lower tones) and by denying the animal any way to comment on uncertainty or respond adaptively to it.
Thus, one sees that that the second requirement of a cross-species metacognition paradigm is that it provide a behavioral (i.e., nonverbal) response that lets the animal comment on or cope adaptively with uncertainty states. In fact, humans in early psychophysical studies were often allowed to respond Uncertain when they felt they could not answer difficult discriminations (Angell 1907; Fernberger 1914, 1930; George 1917; Watson, Kellogg, Kawanishi, & Lucas, 1973; Woodworth 1938). Some questioned including uncertainty responses in psychophysical tasks because those responses seemed to be particularly temperamental, changeable, and psychologically distinctive. In fact, some believed that uncertainty responses were on a different cognitive level than the primary perceptual responses because they were a meta-comment on the subject’s failure to successfully classify a stimulus. For example, Brown (1910) and Jastrow (1888) suggested that uncertainty responses were less perceptual-classification responses and more confidence-rating responses. Likewise, Boring (1920) and George (1917) concluded that uncertainty responses depended on non-sensory attitudes whereas the primary perceptual responses depended on sensory states. This controversy actually sharpens the interest in the uncertainty response if it can be successfully incorporated into comparative paradigms. For that response might be meta- to animals’ primary perceptual responses, too. It might represent their comment or report on indeterminacy and difficulty.
Accordingly, Smith et al. gave the dolphin an uncertainty response with which he could decline to complete any trials of his choosing. The Uncertain paddle did not offer the animal any concrete reward. Instead, it advanced him, following a substantial delay, into an easy, low-pitched trial that was rewarded when completed with the Low response. In essence, the dolphin received a delayed, easy next trial following uncertainty responses. For this reason, uncertainty responses were non-optimal whenever the dolphin knew the discrimination trial’s answer. Then, the faster route to reward was to make the correct response. Uncertainty responses were optimal when the animal did not know the answer, because then the possibility of a timeout was averted, even though this route to reward was relatively slow.
It was an important feature of Smith et al.’s design that they not train the dolphin how or when to use the uncertainty response. Therefore, it was introduced in the following way. On a proportion of trials, both primary responses were disabled electronically, so that responses to those two paddles were futile. The trial then had to be “repaired” using the uncertainty response. These disabling events occurred randomly with no regard to the trial’s pitch level or High–Low status. It had to be left completely to the dolphin to realize that the uncertainty response would not only let broken trials be repaired, but it would also let difficult trials be declined. It is intriguing that the dolphin carried out this qualitative functional transfer fairly easily, though this period of training was suspenseful for the researchers who could not intervene.
Figure 2 showed the dolphin’s two-response performance with the uncertainty response disallowed. High and Low responses mapped to 2,100 Hz tones and below-2,100 Hz tones, respectively, with these response curves crossing near the dolphin’s threshold at the level of chance performance. The crucial question, not illuminated by Figure 2, was how the animal would behave at threshold when allowed to respond Uncertain.
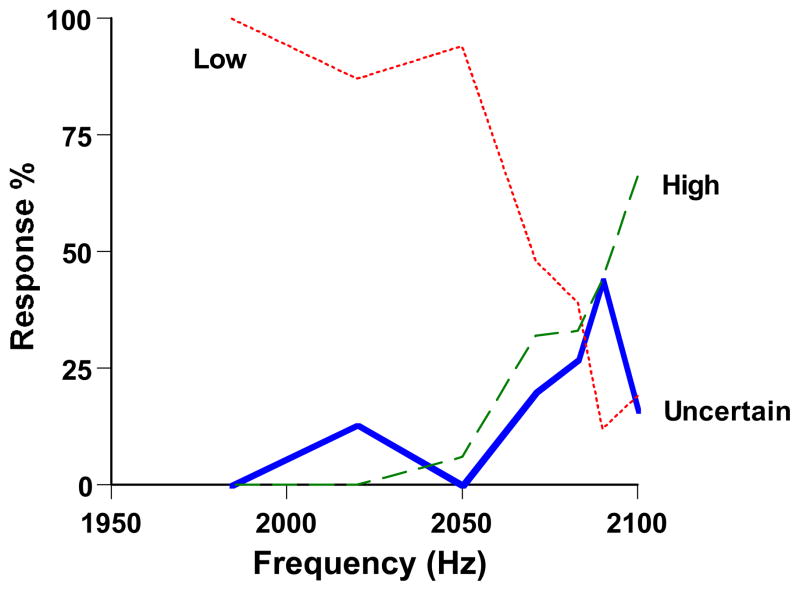
Figure 3 answers this question. The dolphin’s primary discrimination performance was the same, but now he used the uncertainty response selectively for the difficult trials near his discrimination threshold. That is, he assessed correctly when he was at risk for error in the primary discrimination and he adaptively declined those trials. His uncertainty responses peaked near 2,086 Hz, 14 Hz (0.0067%) away from the standard High tone. The dolphin was performing at his true perceptual limit and he evidently knew he was.
Figure 3.
Performance by a dolphin in the auditory discrimination of Smith et al. (1995). The horizontal axis indicates the frequency (Hz) of the trial. The High response was correct for tones at 2,100 Hz—these trials are represented by the rightmost data point for each curve. All lower-pitched tones deserved the Low response. The green-dashed and red-dotted lines, respectively, show the dolphin’s percentage of High and Low responses at each frequency level. The blue-solid line shows the dolphin’s percentage of Uncertainty responses at each frequency level. From “The Uncertain Response in the Bottlenosed Dolphin (Tursiops truncatus),” by J. D. Smith, J. Schull, J. Strote, K. McGee, R. Egnor, and L. Erb, 1995, Journal of Experimental Psychology: General, 124, p. 399. Copyright 1995 by the American Psychological Association. Reprinted with permission.
In related research, humans have used the uncertainty response in a strikingly similar fashion. Moreover, they attribute those uncertainty responses to their conscious, metacognitive states of not knowing. Indeed, it is a persistent and thought-provoking aspect of research in this area that humans attribute their use of the two primary discrimination responses (i.e., High and Low) to the prevailing stimulus conditions (It is High; It is Low), but they attribute their uncertainty responses inwardly to conscious states of not knowing (I don’t know; I can’t tell). As the early psychophysicists suggested, for humans the uncertainty response has a qualitatively distinctive psychological status. It is interesting to consider whether it may also have this status for the dolphin.
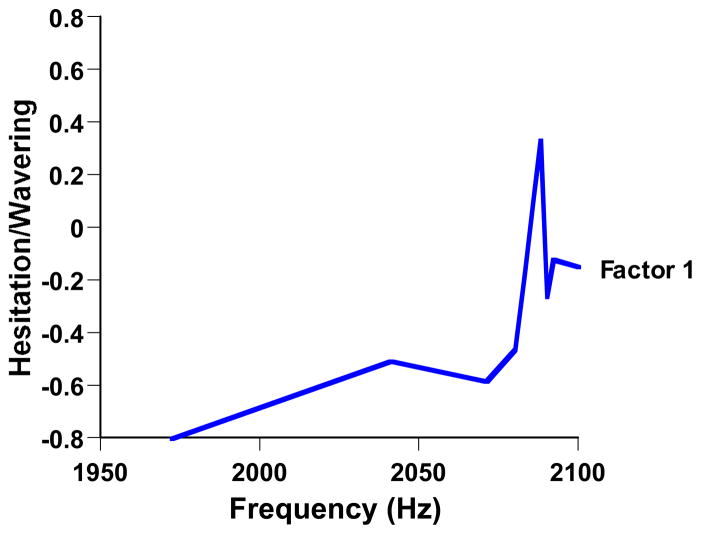
In fact, Smith et al. found that the dolphin’s own class of uncertainty behaviors attended his uncertainty responses near threshold. He sometimes slowed approaching the response paddles, or wavered among them, or swept his head from side to side, or opened and closed his mouth rhythmically. To discover the eliciting trial contexts for these behaviors, we had raters judge for each trial of video-taped sessions the intensity of the behaviors. Then, a factor analysis evaluated the latent structure behind the correlations among these behavioral-rating variables. The strongest behavioral factor was allied to hesitation/wavering by the dolphin. Figure 4 shows for trials at different frequency levels the intensity of these Factor 1 behaviors. These behaviors were most intense at 2,087 Hz and they were distributed along the pitch continuum like the dolphin’s uncertainty responses were (Figure 3). These Factor 1 behaviors are intuitive symptoms of uncertainty that reinforce an uncertainty-based interpretation of the animal’s performance.
Figure 4.
Ancillary behaviors by a dolphin during performance in the auditory discrimination of Smith et al. (1995). The horizontal axis indicates the frequency (Hz) of the trial. The dolphin’s weighted overall Factor 1 behavior (hesitancy, slowing, wavering) is shown for each frequency level. From “The Uncertain Response in the Bottlenosed Dolphin (Tursiops truncatus),” by J. D. Smith, J. Schull, J. Strote, K. McGee, R. Egnor, and L. Erb, 1995, Journal of Experimental Psychology: General, 124, p. 402. Copyright 1995 by the American Psychological Association. Reprinted with permission.
If the dolphin’s uncertainty responses do reflect his metacognitive monitoring of uncertainty, then they are illuminating behavioral ambassadors that bear on reflective mind in marine mammals. The correct interpretation of these responses, and the correct level of cognitive sophistication to grant them, has naturally been actively considered.
It is difficult to explain the dolphin’s performance using low-level, associative mechanisms. The dolphin’s entire mature trial landscape spanned less than one Just Noticeable Difference. He was barely able to respond High at 2,100 Hz and barely able to respond Low at 2,070 Hz. There was no psychological room for a third stimulus class between 2,100 and 2,070 Hz. So there was no distinctive middle stimulus to which a Middle response could be mapped. There was nothing between High and threshold Low except High-Low indeterminacy. Thus, the dolphin’s uncertainty responses were probably about resolving indeterminacy.
His distinctive behaviors at threshold support this interpretation. If he were simply making a Middle response to middle stimuli (e.g., a C# response between C and D responses), no such behaviors would be expected. Indeed, one would then expect hesitancy/wavering behaviors at the two problematic boundaries between the middle region and the outer perceptual regions. It is broadly acknowledged, even by associative-behavioral theorists, that psychological processing is distinctive near the breakpoint of a discrimination because the forces of stimulus control and stimulus-response association break down there (Boneau & Cole, 1967; Commons, Nevin, & Davison, 1991; Davison, McCarthy, & Jensen, 1985; Miller, Saunders, & Bourland, 1980; Pavlov, 1927; Terman & Terman, 1972). Threshold uncertainty responding is qualitatively different from conditioned responding, and the dolphin’s data pattern urges the development of sophisticated models and theories to explain it.
From the perspective of Shiffrin and Schneider (1977, also Atkinson & Juola, 1974), one might say that the dolphin’s trial-by-trial uncertainty awareness illustrates a form of controlled cognitive processing. Threshold stimuli—definitionally—are indeterminate mental representations that map unreliably and inconsistently onto behavioral responses. Facing this indeterminacy, the organism must recruit higher levels of cognition to resolve it. Therefore, the dolphin’s uncertainty responses probably represent a controlled decision to decline the trial when the dolphin is at the threshold of perception. This description is the minimum cognitive sophistication that one must grant the dolphin’s uncertainty responses. Even skeptical treatments of animal metacognition endorse this level of sophistication (Carruthers, 2008). They accept that animals’ uncertainty-monitoring systems are higher-level and cognitive. They even accept that animal have a meta-gatekeeper in their cognitive system (definitely not verbal and perhaps not conscious) that prevents response when indeterminacy arises so the organism can resolve it adaptively.
Tolman went a step farther. He was intrigued by the ancillary uncertainty behaviors exhibited by animals when they face difficult discriminations and choicepoints. The dolphin’s hesitation/wavering behaviors typify this class of behavior. Tolman (1927) called these uncertainty behaviors “lookings or runnings back and forth.” He thought these behaviors could operationalize animal consciousness for the behaviorist. This remarkable claim illustrates well the classic idea in cognitive science that conscious metacognition and self-regulation are particularly fostered by difficulty and indeterminacy. Karoly [66] proposed that conflicted conditions initiate self-regulation. James [63] thought that consciousness assists hesitant nerve processes. Dewey [64] thought that self-awareness is heightened when the world resists our understanding. Gray [67] analyzed from a neuroscience perspective the brain circuits that respond to difficulty with attentional resources and arrested behavior (also Smith, 1995).
Thus, a psychological analysis of the dolphin’s performances reveals that uncertainty responses have a complex and sophisticated psychology behind them that probably is grounded in controlled cognitive processing that resolves indeterminacy at difficult decisional choice-points. Though, of course, one does not have to attribute full consciousness to the dolphin to explain its behavior, it is clear why there has been sharp theoretical interest in this possibility.
For this reason among others, the results from the dolphin experiment and the questions about animal mind that it raises have resonated broadly through comparative psychology. There is now comparative metacognition research on many species that has been conducted by many laboratories (reviews in Smith, 2009; Smith, Beran, & Couchman, in press). The research area inaugurated by the dolphin project has become an influential sub-discipline in the field of comparative psychology.
This represents a strong and substantive contribution by a project with a captive dolphin. Moreover, the project would not have been possible observationally or in the wild. The titration of the animal’s threshold—so critical to carefully controlling the level of trial difficulty presented—was too trial intensive. The training of the distinctive uncertainty response was too complex. We think it is possible that the dolphins’ ancillary uncertainty behaviors (hesitation, wavering, lateral head movements, mouth movements) might be used to study naturalistically dolphins’ reactions to the uncertain situations they must surely face (Griffin, 2003). Those observations doubtless could provide complementary insights about dolphins’ risk-management and decision-making strategies. However, this possibility only augments the value of the Grassy Key project. For it demonstrated the character of dolphins’ uncertainty behaviors under carefully controlled conditions, grounding and easing future naturalistic study.
The dolphin project made an additional contribution, too. The consensus of the literature is that the study of animal metacognition should probably not rely on ancillary behaviors to convey information about animals’ uncertainty processes. These uncertainty behaviors may not occur, they may not be easily observable if they do occur, they may be poorly interpretable or measurable, and they may defeat comparative research because animals in different species may react qualitatively differently when facing uncertainty and indeterminacy (e.g., compare Gisiner & Schusterman, 1992; Herman, Kuczaj, & Holder, 1993). Accordingly, the most constructive empirical approach to comparative metacognition research will be to give animals of different species the same concrete response that lets them report on or deal with the difficult situation. This is the approach that has been used now in research on pigeons and multiple species of apes and monkeys, but was pioneered successfully in testing with the dolphin Natua.
The dolphin project also bears on the evolutionary emergence of reflective mind within the vertebrates. There are diverse aspects to reflective mind in animals, including metacognition, self-awareness, consciousness, and theory of mind. Gallup (1982) used the mirror-recognition test to study the emergence of these capacities in primates. His well-known results—that some apes, but no monkeys, showed mirror self-recognition—led him to two influential conclusions. First, he concluded that successful mirror recognition also indicated animals’ self-awareness, reflective consciousness, and metacognition. Indeed, all aspects of reflective mind were linked cognitively and evolutionarily for Gallup. Second, he concluded that all these aspects of reflective mind emerged together only once in cognitive evolution, in the ancestral ape lineage.
The dolphin project contributes to this area because the metacognition paradigm complements Gallup’s mirror-recognition paradigm. Mirror recognition alone cannot confirm any relation between bodily mirror recognition and consciousness / metacognition. An independent measure of cognitive self-awareness, as instantiated by the metacognition paradigm, helps fill this gap. The dolphin’s results also suggestively falsify Gallup’s hypothesis. They suggest that some aspects of reflective mind exist outside the ape lineage. The data showing dolphin metacognition contribute to this area similarly to the data showing dolphin mirror recognition (Reiss & Marino, 2001).
The dolphin data should also be understood relative to the broader phylogenetic distribution of metacognition. Research from multiple laboratories / paradigms has confirmed that pigeons either do not have, or do not express, any metacognitive capacity (Inman & Shettleworth, 1999; Roberts; Feeney, McMillan, MacPherson, & Musolino, 2009; Sutton & Shettleworth, 2008). This suggests that metacognition is not a basic component of the vertebrate cognitive system. Research from multiple laboratories / paradigms has confirmed that apes and macaques do express a metacognitive capacity (Call, 2010; Call & Carpenter, 2001; Couchman, Coutinho, Beran, & Smith, in press; Hampton, 2001; Washburn, 2009) whereas capuchin monkeys (a New World primate species) do not (Basile, Hampton, Suomi, & Murray, 2009; Beran, Smith, Coutinho, Couchman, & Boomer, 2009; Fujita, 2009; Paukner, 2006).
Together with the dolphin project, the current data suggest that the largest-brained and most cognitively sophisticated species—in divergent vertebrate lineages—developed the capacity for metacognition. This species overview would suggest that metacognition evolved convergently multiple times during cognitive evolution.
Deciding this issue now would be premature, and additional cross-species research will be valuable. In particular, research on the corvids—birds known for their cognitive sophistication—would provide a third critical test of this hypothesis (in addition to macaques/apes and dolphins). It will also be constructive to test marine mammals on additional metacognition paradigms, including the metamemory paradigms featured in Hampton (2001), Roberts et al. (2009), and Sutton and Shettleworth (2008).
It will be a profoundly important conclusion if cognitive evolution toward higher levels generally and inherently produces forms of metacognition and cognitive self-awareness. And of course it will be a profoundly different conclusion than the one that has dominated comparative psychology for many years.
Acknowledgments
The preparation of this article was supported by Grant 1R01HD061455-01A1 from NICHD and by Grant BCS-0956993 from NSF.
References
- Angell F. On judgments of “like” in discrimination experiments. American Journal of Psychology. 1907;18:253. [Google Scholar]
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Luce RD, Suppes P, editors. Learning, Memory, and Thinking. San Francisco, CA: W. H. Freeman; 1974. pp. 243–293. [Google Scholar]
- Au WW, Moore PW. Critical ratio and critical bandwidth for the Atlantic bottlenose dolphin. Journal of the Acoustical Society of America. 1990;88:1635–1638. doi: 10.1121/1.400323. [DOI] [PubMed] [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AS, Bjork RA, Schwartz BL. The mismeasure of memory: When retrieval fluency is misleading as a metacognitive index. Journal of Experimental Psychology: General. 1998;127:55–68. doi: 10.1037//0096-3445.127.1.55. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JC, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough DS. A method for obtaining psychophysical threshold from the pigeon. Journal of the Experimental Analysis of Behavior. 1958;1:31–43. doi: 10.1901/jeab.1958.1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneau CA, Cole JL. Decision theory, the pigeon, and the psychophysical function. Psychological Review. 1967;74:123–135. doi: 10.1037/h0024287. [DOI] [PubMed] [Google Scholar]
- Boring EG. The control of attitude in psychophysical experiments. Psychological Review. 1920;27:440–452. [Google Scholar]
- Brown W. University of California Publications in Psychology. Vol. 1. Berkeley CA: The University Press; 1910. The judgment of difference; pp. 1–71. [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Call J. Do apes know that they could be wrong? Animal Cognition. 2010 doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Carruthers P. Meta-cognition in Animals: a skeptical Look. Mind and Language. 2008;23:1, 58–89. [Google Scholar]
- Commons ML, Nevin JA, Davison MC, editors. Signal detection: mechanisms, models, and applications. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Couchman JJ, Coutinho MVC, Beran MJ, Smith JD. Beyond stimulus cues and reinforcement signals: A new approach to animal metacognition. Journal of Comparative Psychology. doi: 10.1037/a0020129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, McCarthy D, Jensen C. Component probability and component reinforcer rate as biasers of free-operant detection. Journal of the Experimental Analysis of Behavior. 1985;44:103–120. doi: 10.1901/jeab.1985.44-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J. Art as experience. New York: Perigee Books; 1934/1980. [Google Scholar]
- Dunlosky J, Bjork RA, editors. Handbook of memory and metamemory. New York: Psychology Press; 2008. [Google Scholar]
- Fernberger SW. The effect of the attitude of the subject upon the measure of sensitivity. American Journal of Psychology. 1914;25:538–543. [Google Scholar]
- Fernberger SW. The use of equality judgments in psychophysical procedures. Psychological Review. 1930;37:107–112. [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Self-awareness and the emergence of mind in primates. American Journal of Primatology. 1982;2:237–248. doi: 10.1002/ajp.1350020302. [DOI] [PubMed] [Google Scholar]
- George SS. Attitude in relation to the psychophysical judgment. American Journal of Psychology. 1917;28:1–38. [Google Scholar]
- Gisiner R, Schusterman RJ. Sequence, syntax, and semantics: Responses of a language-trained seal lion (Zalophus californianus) to novel sign combinations. Journal of Comparative Psychology. 1992;106:78–91. [Google Scholar]
- Gray JA. The contents of consciousness: a neuropsychological conjecture. Behavioral and Brain Sciences. 1995;18:659–722. [Google Scholar]
- Griffin DR. Significant uncertainty is common in nature. Behavior and Brain Sciences. 2003;26:346. doi: 10.1017/S0140525X03290087. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman LM, Arbeit WR. Frequency difference limens in the bottlenose dolphin: 1–70 kc/s. Journal of Auditory Research. 1972;2:109–120. [Google Scholar]
- Herman LM, Kuczaj SA, Holder MD. Responses to anomalous gestural sequences by a language-trained dolphin: Evidence for processing of semantic relations and syntactic information. Journal of Experiment Psychology: General. 1993;122:184–194. doi: 10.1037//0096-3445.122.2.184. [DOI] [PubMed] [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:389–395. [Google Scholar]
- James W. Great Books of the Western World. Vol. 53. Chicago: University of Chicago Press; 1890/1952. The principles of psychology. [Google Scholar]
- Jastrow J. A critique of psycho-physic methods. American Journal of Psychology. 1888;1:271–309. [Google Scholar]
- Karoly P. Mechanisms of self-regulation: a systems view. Annual Review of Psychology. 1993;44:23–52. [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling of knowing. Psychological Review. 1993;100:609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Koriat A. Metacognition and consciousness. In: Zelazo PD, Moscovitch M, Thompson E, editors. The Cambridge handbook of consciousness. Cambridge, UK: Cambridge University Press; 2007. pp. 289–325. [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation of memory accuracy. Psychological Review. 1996;103:490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Kornell N. Metacognition in humans and animals. Current Directions in Cognitive Science. 2009;18:11–15. [Google Scholar]
- Miller JT, Saunders SS, Bourland G. The role of stimulus disparity in concurrently available reinforcement schedules. Animal Learning & Behavior. 1980;8:635–641. [Google Scholar]
- Nelson TO, editor. Metacognition: Core readings. Toronto: Allyn and Bacon; 1992. [Google Scholar]
- Nelson TO. Consciousness and metacognition. American Psychologist. 1996;51:102–116. [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. The Psychology of Learning and Motivation. 1990;26:125–141. [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Anrep GV, editor. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proceedings of the National Academy of Sciences of the USA. 2001;98:5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E. Do pigeons (Columba livia) study for a test? Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Scheck P, Nelson TO. Lack of pervasiveness of the underconfidence-with-practice effect: Boundary conditions and an explanation via anchoring. Journal of Experimental Psychology: General. 2005;134:124–128. doi: 10.1037/0096-3445.134.1.124. [DOI] [PubMed] [Google Scholar]
- Schusterman RJ, Barrett B. Detection of underwater signals by a California sea lion and a bottlenose porpoise: variation in the payoff matrix. Journal of the Acoustical Society of America. 1975;57:1526–1537. doi: 10.1121/1.380595. [DOI] [PubMed] [Google Scholar]
- Schwartz BL. Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychonomic Bulletin and Review. 1994;1:357–375. doi: 10.3758/BF03213977. [DOI] [PubMed] [Google Scholar]
- Serra MJ, Dunlosky J. Does retrieval fluency contribute to the underconfidence-with-practice effect? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1258–1266. doi: 10.1037/0278-7393.31.6.1258. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Smith JD. The homunculus at home. Commentary on J. A. Gray, The contents of consciousness: a neuropsychological conjecture. Behavioral and Brain Sciences. 1995;18:697–698. [Google Scholar]
- Smith JD. The study of animal metacognition. Trends in Cognitive Sciences. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Couchman JJ. Animal metacognition. To appear. In: Zentall T, Wasserman E, editors. Comparative cognition: Experimental explorations of animal intelligence. Oxford, UK: Oxford University Press; in press. [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) Journal of Experimental Psychology: General. 1995;124:391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman J. Concurrent variation of response bias and sensitivity in an operant-psychophysical test. Perception & Psychophysics. 1972;11:428–432. [Google Scholar]
- Tolman EC. A behaviorist’s definition of consciousness. Psychological Review. 1927;34:433–439. [Google Scholar]
- Washburn DA, Gulledge JP, Beran MJ, Smith JD. With his memory magnetically erased, a monkey knows he is uncertain. In press. Biology Letters. 2009 doi: 10.1098/rsbl.2009.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Kellogg SC, Kawanishi DT, Lucas PA. The uncertain response in detection-oriented psychophysics. Journal of Experimental Psychology. 1973;99:180–185. [Google Scholar]
- Woodworth RS. Experimental psychology. New York: Holt; 1938. [DOI] [PubMed] [Google Scholar]
- Yunker MP, Herman LM. Discrimination of auditory temporal differences by the bottlenose dolphin and by the human. Journal of the Acoustical Society of America. 1974;56:1870–1875. doi: 10.1121/1.1903525. [DOI] [PubMed] [Google Scholar]