Abstract
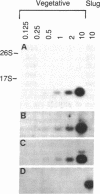
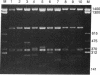
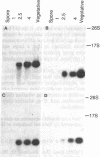
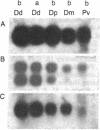
Few eukaryotic genes are expressed only during cell growth and division. We found that the slime mold Dictyostelium discoideum is unusual in that it expresses many genes only during proliferation. Thirty-two percent (304/950) of the sequences in a cDNA library made from vegetative mRNA were homologous to RNAs that are present at high levels during growth but at low or undetectable levels during differentiation when no cell growth occurs. In vitro translation assays confirmed that one-third of the vegetative cell mRNAs decreased in steady-state levels during differentiation. These vegetative cell-specific transcripts identified a diverse coordinately regulated class of genes: (i) 9 of the 10 cDNAs tested hybridized to unique small transcripts ranging from 400 to 620 bases long; (ii) the sequences showed various degrees of homology to related species; (iii) transcript levels synchronously fell by a factor of greater than 20 during development and synchronously increased during germination. This class of genes may play important roles in normal cell proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay S. L., Meller E. Efficient transformation of Dictyostelium discoideum amoebae. Mol Cell Biol. 1983 Dec;3(12):2117–2130. doi: 10.1128/mcb.3.12.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Complexity of nuclear and polysomal RNAs in growing Dictyostelium discoideum cells. Dev Biol. 1980 Aug;78(2):268–284. doi: 10.1016/0012-1606(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Properties of germinating spores of Dictyostelium discoideum. J Bacteriol. 1968 Nov;96(5):1680–1689. doi: 10.1128/jb.96.5.1680-1689.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine K. M., Morrissey J. H., Loomis W. F. Differential synthesis of spore coat proteins in prespore and prestalk cells of Dictyostelium. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7361–7365. doi: 10.1073/pnas.79.23.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston A. J., Vork F. The spatial pattern of DNA synthesis in Dictyostelium discoideum slugs. Exp Cell Res. 1978 Sep;115(2):454–457. doi: 10.1016/0014-4827(78)90308-7. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Bonner J. Characterization of the genome of the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1972 May 28;66(3):339–361. doi: 10.1016/0022-2836(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M., Part D., Felenbok B. Changes in the polyadenylated messenger RNA population during development of Dictyostelium discoideum. Dev Biol. 1981 Jan 15;81(1):155–166. doi: 10.1016/0012-1606(81)90358-4. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Trevan D. J. Dictyostelium amoebae can differentiate into spores without cell-to-cell contact. J Embryol Exp Morphol. 1981 Apr;62:369–378. [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. A family of short, interspersed repeat sequences at the 5' end of a set of Dictyostelium single-copy mRNAs. Cell. 1979 Apr;16(4):787–796. doi: 10.1016/0092-8674(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Kopachik W. J., Dhokia B., Kay R. R. Selective induction of stalk-cell-specific proteins in Dictyostelium. Differentiation. 1985;28(3):209–216. doi: 10.1111/j.1432-0436.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr Sensitivity of Dictyostelium discoideum to nucleic acid analogues. Exp Cell Res. 1971 Feb;64(2):484–486. doi: 10.1016/0014-4827(71)90107-8. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr Temperature-sensitive mutants of Dictyostelium discoideum. J Bacteriol. 1969 Jul;99(1):65–69. doi: 10.1128/jb.99.1.65-69.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Zahn K. The synthesis of ninety proteins including actin throughout the HeLa cell cycle. J Cell Biol. 1978 Dec;79(3):833–838. doi: 10.1083/jcb.79.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Reed S. I. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2119–2123. doi: 10.1073/pnas.77.4.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Fractionation and functional analysis of newly synthesized and decaying messenger RNAs from vegetative cells of Dictyostelium discoideum. J Mol Biol. 1979 Mar 5;128(3):371–395. doi: 10.1016/0022-2836(79)90093-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M. Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol. 1979 Mar 25;129(1):19–35. doi: 10.1016/0022-2836(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Zada-Hames I. M., Ashworth J. M. The cell cycle and its relationship to development in Dictyostelium discoideum. Dev Biol. 1978 Apr;63(2):307–320. doi: 10.1016/0012-1606(78)90136-7. [DOI] [PubMed] [Google Scholar]