Abstract
Developing spermatozoa require a series of post-testicular modifications within the luminal environment of the epididymis to achieve maturation; this involves several surface modifications including changes in plasma membrane lipids, proteins, carbohydrates, and alterations in the outer acrosomal membrane. Epididymal maturation can therefore allow sperm to gain forward motility and fertilization capabilities. The objective of this study was to identify maturation dependent protein(s) and to investigate their role with the production of functionally competent spermatozoa. Lectin blot analyses of caput and cauda sperm plasma membrane fractions identified a 17.5kDa Wheat Germ Agglutinin (WGA) binding polypeptide present in the cauda sperm plasma membrane not in the caput sperm plasma membrane. Among the several WGA stained bands, the presence of a 17.5kDa WGA binding polypeptide band was detected only in cauda epididymal fluid not in caput epididymal fluid suggesting that the 17.5kDa WGA-binding polypeptide is secreted from the cauda epididymis and binds to the cauda sperm plasma membrane during epididymal transit. Proteomic identification of the 17.5kDa polypeptide yielded 13 peptides that matched the sequence of peroxiredoxin-5 (PRDX5) protein (Bos Taurus). We propose that bovine cauda sperm PRDX5 acts as an antioxidant enzyme in the epididymal environment, which is crucial in protecting the viable sperm population against the damage caused by endogeneous or exogeneous peroxide.
Keywords: Bovine sperm, Epididymis, Glycoproteins, Peroxiredoxin-5
INTRODUCTION
Fertilization, the union of male and female gametes to create offspring, is an intricate biological process dependent upon several biochemical and physiological events [1, 2]. Spermatozoa leave the testis as morphologically differentiated and functionally immature cells and require a series of post-testicular modifications to become fertilizing competent [3, 4]. The mammalian epididymis represents the site where incompetent testicular sperm undergo maturation and is comprised of three distinct regions: the caput, corpus and cauda [1, 5, 6]. As sperm cells travel through the duct they become mature, acquiring forward motility and the capability to fertilize the ovum [7–11]. During the maturational process plasma membrane proteins undergo several compositional changes via the addition of new components to the sperm surface, the unmasking or modification of preexisting sperm-surface moieties, by protein redistribution between domains, or the loss of sperm-surface components [6, 9, 12]. This membrane reorganization appears crucial to the development of sperm functional capacity. The maturational changes sperm undergo as they travel through the epididymis results from the secretions of several proteins along the epididymal epithelium [6, 13] and the pattern of the region-specific gene expression in the epididymis [14].
Several studies reveal that the mammalian sperm plasma membrane surface is coated with various glycoproteins [15–18]. Sperm surface glycoproteins are thought to induce sperm maturation and fertilizing capacity in the epididymis [17, 19, 20]. The extent to which these surface glycoproteins are altered varies from species to species and differs in each epididymal region [6]. The plasma membrane is a mosaic of distinct domains corresponding to defined segments of the head and flagellum [21]. In rat sperm plasma membrane, several different glycoprotein alterations occur during post-testicular maturation including modifications in their appearance, loss or alteration in staining intensity, and modification of electrophoretic mobility [22]. The mechanisms utilized by sperm to undergo the maturational process are still not completely understood, however, the epididymis does provide sperm with an environment essential for the acquisition of motility and fertilizing ability [1]. Lectins are a class of proteins that can be used to analyze density and distribution variations of exposed saccharides in the sperm’s plasma membrane [17, 23]. Both lectin-like and protein-protein interactions exhibit a potential role in human sperm-egg interactions [24]. The objective of the present study was to identify the maturation-dependent plasma membrane proteins of the bovine sperm and to elucidate their role with the production of functionally competent spermatozoa. Western Blots of SDS-PAGE fractionated bovine sperm plasma membrane samples identified a 17.5kDa WGA binding polypeptide present in the cauda sperm plasma membrane. Proteomic identification of the 17.5kDa polypeptide yielded 13 peptides that matched the sequence of peroxiredoxin-5(PRDX5) protein (BosTaurus). We propose that bovine cauda sperm PRDX5 acts as an antioxidant enzyme in the cauda epididymal environment to protect the viable sperm population against the damage caused by endogeneous or exogeneous peroxide.
MATERIALS AND METHODS
Sperm Preparation
Bovine epididymides were purchased from Martin’s Abattoir in Godwin, North Carolina. Epididymides were stored at 4°C during transit and utilized for sperm preparation within 30 minutes of retrieval. To facilitate sperm release caput and cauda epididymal regions were removed from the organ, minced, and incubated for 5 minutes at 37°C in Hank’s balanced saline solution, pH 7.4, containing 5mM HEPES, 2 mM benzamidine, and 0.05% sodium azide. To evaluate cellular viability, sperm were examined by phase-contrast microscopy. Sperm suspensions were centrifuged at 100 × g for 1 minute to sediment epididymal tubule fragments. Supernatants were centrifuged at 1500 × g for 10 minutes at 4°C using an Eppendorf Centrifuge 5403 (Brinkman Instruments, Inc, Westbury, New York). Pellets were washed 3 times by resuspension in Hanks balanced saline solution, as stated above, following which they were resuspended in a Tris-saline-protease inhibitor solution (TNI) containing 150 mM NaCl, 25 mM Tris-HCl, (pH 7.5), 2 mM benzamidine, 1 µg/mL leupeptin, 1 µg/mL pepstatin, and 0.05% sodium azide and centrifuged at 1500 × g for 10 minutes at 4°C. Resulting pellets were washed 2 more times in TNI as stated above. Caput and cauda sperm plasma membranes were isolated by nitrogen cavitation and discontinuous sucrose gradients centrifugation [25]. To identify the integral membrane protein(s), phase separation analysis of cauda sperm plasma membranes was performed as previously described [26]. Both aqueous and detergent phases were analyzed on 12% SDS-PAGE and transferred to PVDF membranes for lectin blot analysis.
Isolation of Fluid/Particulate Fractions
Caput and cauda epididymides were dissected and minced in TNI at 37°C. Sperm suspensions were centrifuged at 100 × g for 1 minute to sediment any tissue fragments that may be present within the sample. The resulting supernatants were re-centrifuged at 1500 × g for 10 minutes at 4°C, sperm pellets and supernatants were collected. Resulting supernatant fluids were again centrifuged at 100000 × g for 20 minutes to obtain caput and cauda epididymal luminal fluids.
SDS-PAGE, Western Blotting, and Lectin Staining
SDS-PAGE was performed on 12% polyacrylamide gels [27]. Polypeptide bands were stained with silver [28]. Western Blot analysis was performed by the electrophoretic transfer of polypeptides to PVDF membranes [29]. Lectin blot analyses were done as previously described [22]. The specificity of lectin sugar interactions was examined with incubations containing a proper saccharide inhibitor in lectin containing buffer including 0.2 M α-methyl-mannose for Concanavalin A (Con A), 0.2 M N-acetylgalactosamine for Soybean Agglutinin (SBA), 0.2 M N-acetyl glucosamine for Wheat Germ Agglutinin (WGA), and 0.2 M D-galactose for Peanut Agglutinin (PNA) and Ricinus Communis Agglutinin (RCA). Lectin binding polypeptides were visualized by color development using H2O2 and diaminobenzidine. Western blots were stained with phosphotyrosine (P-Tyr) antibody (1:1000 dilution), followed by an affinity purified horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) [30]. Immunoreactive protein bands were identified using enhanced chemiluminescence reagents (Pierce Super Signal, Rockford, IL)
Purification of the 17.5kDa WGA Binding Polypeptide
Bovine cauda plasma membrane samples were extracted in 0.1% Triton X-100 for 1 hour at 4°C and then centrifuged at 100000 × g for 30 minutes at 4°C. The resulting supernatant was dialyzed overnight in a 25 mM Tris-HCl pH 7.5 buffer at 4°C. The dialyzed sample was applied to a DEAE-Sephacel® column (Pharmacia Fine Chemicals AB Uppsala, Sweden) previously equilibrated with 25 mM Tris-HCl, pH 7.5 buffer. Triton extracted plasma membrane samples were added to the column; the collected flow through was again added to the column. To remove any un-bound polypeptides, the column was rinsed with 25 mM Tris-HCl pH 7.5 buffer. Bound polypeptides were eluted with the addition of 0.1 M NaCl and 0.4 M NaCl, both containing 25 mM Tris-HCl, pH 7.5. Eluted samples were concentrated to the desired volume in Pierce® Concentrators, Thermo Scientific (Rockford, IL). The 17.5kDa WGA binding polypeptide was further purified by continuous-elution SDS-PAGE on 12% acrylamide gels using a Model 491 Prep Cell (Bio Rad Laboratories, Hercules, CA). Fractions were analyzed on 12% SDS-PAGE and transferred to PVDF membranes for lectin blot analysis or stained with silver [28].
Proteomic Analysis
Proteomic identification of 17.5kDa WGA binding polypeptide was performed at the Mass Spectrometry Facility of UNC School of Medicine Proteomic Center, Chapel Hill, NC. The 17.5kDa band was subjected to MALDI-TOF-TOF analysis to obtain internal amino acid sequences of several tryptic peptides. Derived peptide sequences were analyzed in the National Center for Biotechnology Information (NCBI) database for the determination of potential functional motifs, including a transmembrane hydrophobic domain, an extracellular domain with consensus glycosylation sites, and to define potential phosphorylation sites and protein interaction domains on its cytoplasmic segment.
Capacitation and Acrosome Reaction
Sperm (4–6 × 10 7/ml) were capacitated in the presence heparin (10 mg/ml) in a modified Tyrode’s medium (pH 7.4) for 4 hours at 39°C with a 95% air: 5% CO2 atmosphere [31]. To initiate the acrosome reaction sperm were incubated with 100µg/mL lysophosphatidyl choline (LPC) for 15 minutes following the end of the 4 hour incubation. Samples were centrifuged at 4000 × g for 10 minutes at 4°C. The pellets and supernatants were adjusted to equal volumes and used for SDS-PAGE and acrosin determination.
Acrosin Assay
Acrosin activity of the pellet and supernatant fractions of capacitated and acrosome reacted spermatozoa were assayed as previously described [32, 33]. One unit of acrosin is defined as the quantity of enzyme required to hydrolyze 1 µm of N-p-tosyl-gly-pro-arg-p-nitroanilide per minute. Proteins were estimated as reported earlier [34].
RESULTS
Glycocalyx Pattern of Bovine Caput and Cauda Sperm Plasma Membrane Fractions
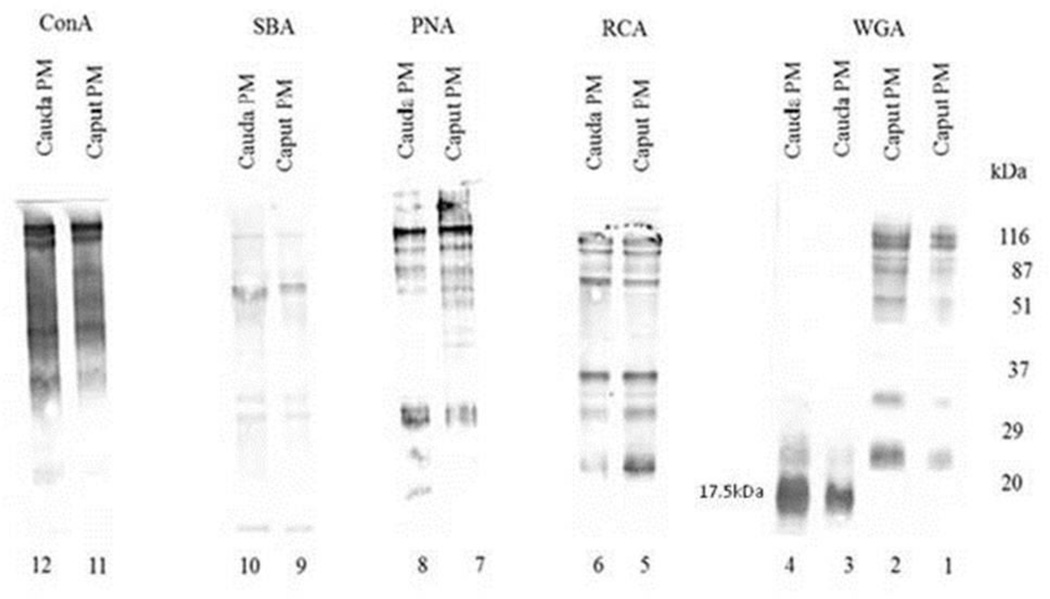
To detect differences among surface glycoproteins in caput and cauda sperm plasma membrane fractions, lectin blot analyses were performed with biotinylated lectins ConA, SBA, PNA, RCA, and WGA. Caput (Fig. 1; lanes 1, 2, 5, 7, 9, 11) and cauda (Fig. 1; lanes 3, 4, 6, 8, 10, 12) sperm plasma membrane fractions stained with RCA (lanes 5 and 6), PNA (lanes 7 and 8), SBA (lanes 9 and 10), and ConA (lanes 11 and 12) did not reveal any significantly diverse banding patterns. However, caput (Fig. 1; lanes 1 and 2) and cauda (Fig. 1; lanes 3 and 4) sperm plasma membrane fractions stained with WGA revealed a noticeably different polypeptide banding pattern; a major 17.5kDa WGA binding polypeptide and two other minor WGA binding polypeptides (approximate molecular weights of 22kDa and 25kDa) were detected in cauda sperm plasma membrane fractions. This 17.5kDa WGA binding polypeptide band was not detected in caput sperm plasma membrane fractions whereas a spectrum of other WGA binding polypeptides (molecular weight ranging between 25kDa-116kDa) was identified with biotinylated WGA. The amount of proteins loaded in each lane was 10 µg, except for lanes 2 and 4. In lanes 2 and 4, 20 µg of caput and cauda sperm plasma membrane proteins were loaded respectively. The 17.5kDa WGA-binding polypeptide was not detected in the presence of 20 µg caput sperm plasma membrane fractions (lane 2). On the contrary, 20 µg cauda sperm plasma membrane fractions (lane 4) revealed the presence of a profoundly stained 17.5kDa WGA binding polypeptide. WGA specifically binds N-acetyl glucosamine and sialic acid residues. The specificity of WGA interaction was evaluated by pre-incubation of 0.2 M N-acetyl glucosamine with biotinylated WGA; no 17.5kDa WGA binding polypeptide was found (data not shown) demonstrating the specificity of the WGA glycoprotein staining. This study suggests that this WGA binding protein is only present in bovine cauda sperm plasma membrane fractions.
Figure 1.
Glycoprotein Pattern of Bovine Caput and Cauda Sperm Plasma Membrane Fractions. Plasma membrane fractions stained with biotinylated ConA, SBA, PNA, and RCA yielded similar major and minor polypeptide binding patterns for caput and cauda epididymal sperm plasma membrane fractions. The amount of proteins loaded in each lane was 10 µg, except for lanes 2 and 4. In lanes 2 and 4, 20 µg of caput and cauda sperm plasma membrane proteins were loaded respectively. Plasma membrane fractions stained with WGA however revealed the presence of a 17.5kDA WGA binding polypeptide in only cauda samples (lanes 3 and 4).
Detection of Wheat Germ Agglutinin Binding Glycoproteins in Cauda Epididymal Spermatozoa and Epididymal Fluid
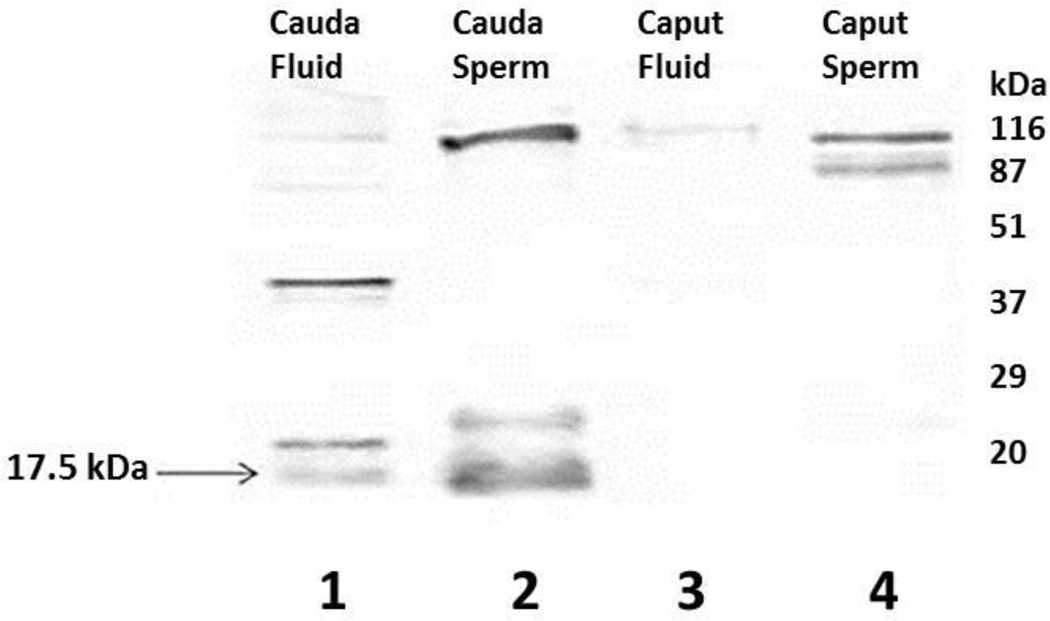
To examine the presence of the 17.5kDa WGA binding polypeptide in epididymal spermatozoa and epididymal fluid, lectin blots of caput (Fig. 2, lane 3) and cauda (Fig. 2, lane 1) epididymal luminal fluid and spermatozoa from the caput (Fig. 2, lane 4) and cauda (Fig. 2, lane 2) epididymis were stained with biotinylated WGA. Several WGA-stained bands were detected in cauda epididymal fluid (Fig. 2, lane 1) and sperm (Fig. 2, lane 2) including the 17.5kDa polypeptide. On the contrary, caput epididymal fluid displayed only one faint WGA-stained (~116kDa) band (Fig. 2, lane 3) and caput epididymal sperm (Fig. 2, lane 4) revealed few WGA stained high molecular weight bands. Among the several WGA stained bands, the presence of a 17.5kDa WGA binding polypeptide band was detected in both cauda epididymal fluid and spermatozoa, not in caput epididymal spermatozoa or epididymal fluid suggesting that the 17.5kDa WGA binding polypeptide is only present in the mature caudal region of the bovine epididymis.
Figure 2.
Wheat Germ Agglutinin Binding Glycoproteins in Plasma Membranes of Caput and Cauda Epididymal Spermatozoa and Epididymal Fluid. The 17.5kDa WGA binding polypeptide was detected in both cauda epididymal fluid and epididymal sperm. This 17.5kDa band was not detected in caput epididymal fluid or in caput epididymal sperm.
Phase Separation Analysis of 17.5kDa Wheat Germ Agglutinin Binding Polypeptide
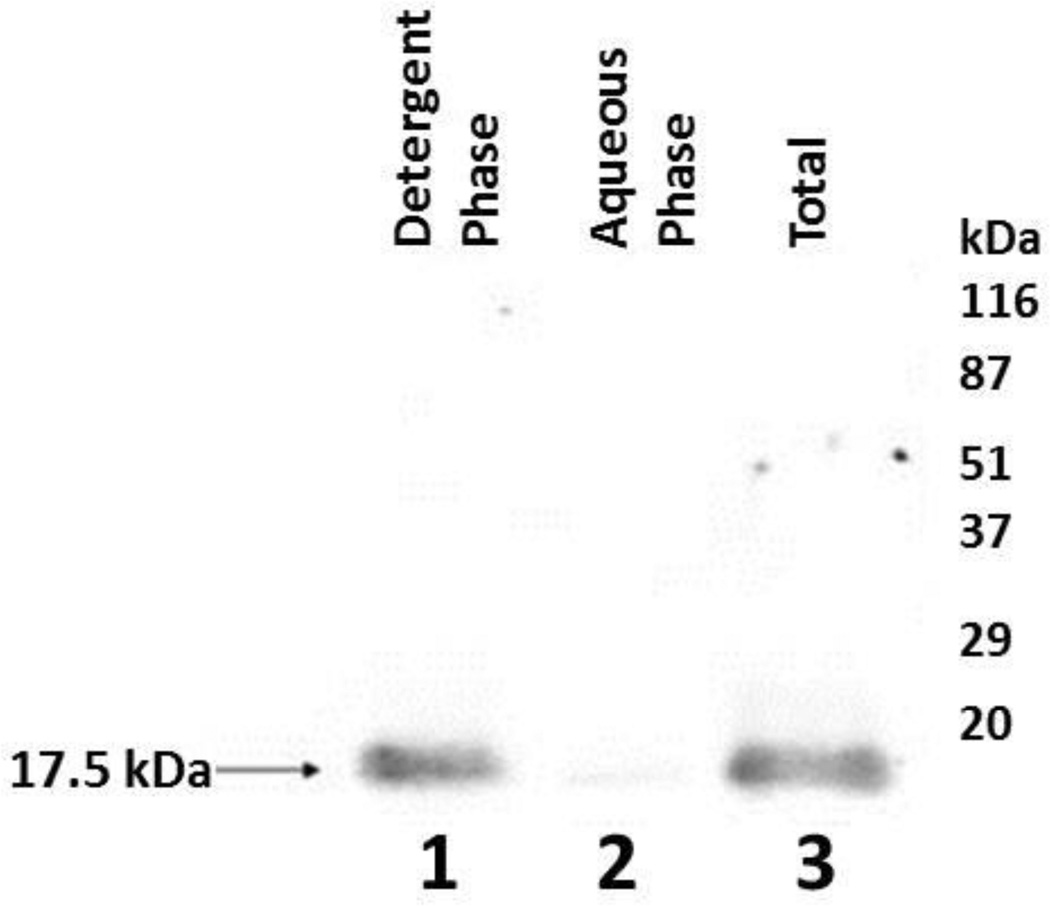
To determine if the 17.5kDa WGA binding polypeptide is an integral or peripheral membrane protein, cauda plasma membrane fractions (3.4 µg protein/µl) were extracted in 1 % Triton X-114. Resulting aqueous and detergent phases were collected and SDS-PAGE was performed on 12% gels. Lectin blot analysis with biotinylated WGA revealed the presence of a 17.5kDa WGA binding polypeptide in the detergent phase (Fig. 3, lane 1). Since this polypeptide was not extensively detected in the aqueous phase (Fig. 3, lane 2) following the analysis, it strongly confirms that the 17.5kDa polypeptide is an integral membrane protein. This study has revealed that the 17.5kDa polypeptide is embedded within the lipid bilayer of cauda sperm plasma membrane.
Figure 3.
Triton X-114 Phase Separation Analysis. Lane 1: Detergent phase containing 17.5kDa WGA binding polypeptide. Lane 2: Aqueous phase. Lane 3: Total bovine cauda sperm plasma membrane protein prior to phase separation analysis.
Purification of Bovine Cauda 17.5kDa WGA Binding Polypeptide and Proteomic Analysis of 17.5kDa WGA Binding Polypeptide
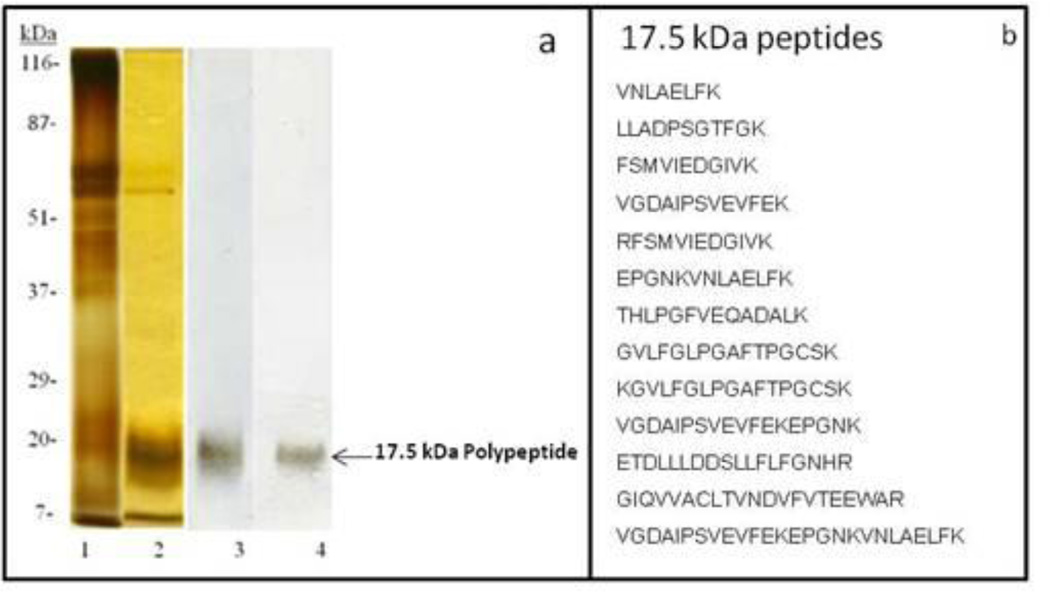
To purify the 17.5kDa WGA binding polypeptide, the Triton X-100 soluble fraction of bovine cauda sperm plasma membrane was fractionated by DEAE-Sepharose chromatography and continuous-elution SDS-PAGE on 12% polyacrylamide gels as stated in the materials and methods. Silver stained gels (Fig. 4a, lane 1) and lectin blots stained with WGA (Fig. 4a, lane 3) detected the presence of a 17.5kDa WGA binding polypeptide following elution with buffer containing 0.4 NaCl (DEAE- Sepharose Chromatography). To isolate the 17.5kDa WGA binding polypeptide from this 0.4 M NaCl eluted sample, continuous elution SDS-PAGE was performed. The 17.5kDa WGA binding polypeptide (Fig. 4a, lanes 2 and 4) was well separated from other polypeptides and the 17.5kDa WGA binding polypeptide fractions were pooled and used for proteomic analysis. These results confirm the purity of the fractions from the continuous-elution experiment. Proteomic identification of the 17.5kDa WGA binding polypeptide by MALDI-TOF-TOF analysis yielded 13 peptides (Figure 4b) that matched the NCBI database sequence of peroxiredoxin-5 (PRDX5) protein (Bos Taurus).
Figure 4.
a: Purification of 17.5kDa WGA Binding Polypeptide. Silver stained 12% SDS-PAGE (lanes 1–2). Lectin blot stained with biotinylated WGA (lanes 3–4). Lanes 1and 3: 0.4 M NaCl eluted fraction of DEAE- Sepharose Chromatography of Triton X-100 soluble fraction of bovine cauda sperm plasma membrane. Lanes 2 and 4: Pooled fractions of continuous elution SDS-PAGE containing17.5kDa WGA binding polypeptide. The purified 17.5kDa WGA binding polypeptide was subjected to proteomic analysis.
b: Tryptic peptides of the 17.5 kDa polypeptide identified by MALDI-TOF-TOF proteomic analysis
Fate of 17.5kDa WGA Binding Polypeptide During Capacitation and Acrosome Reaction
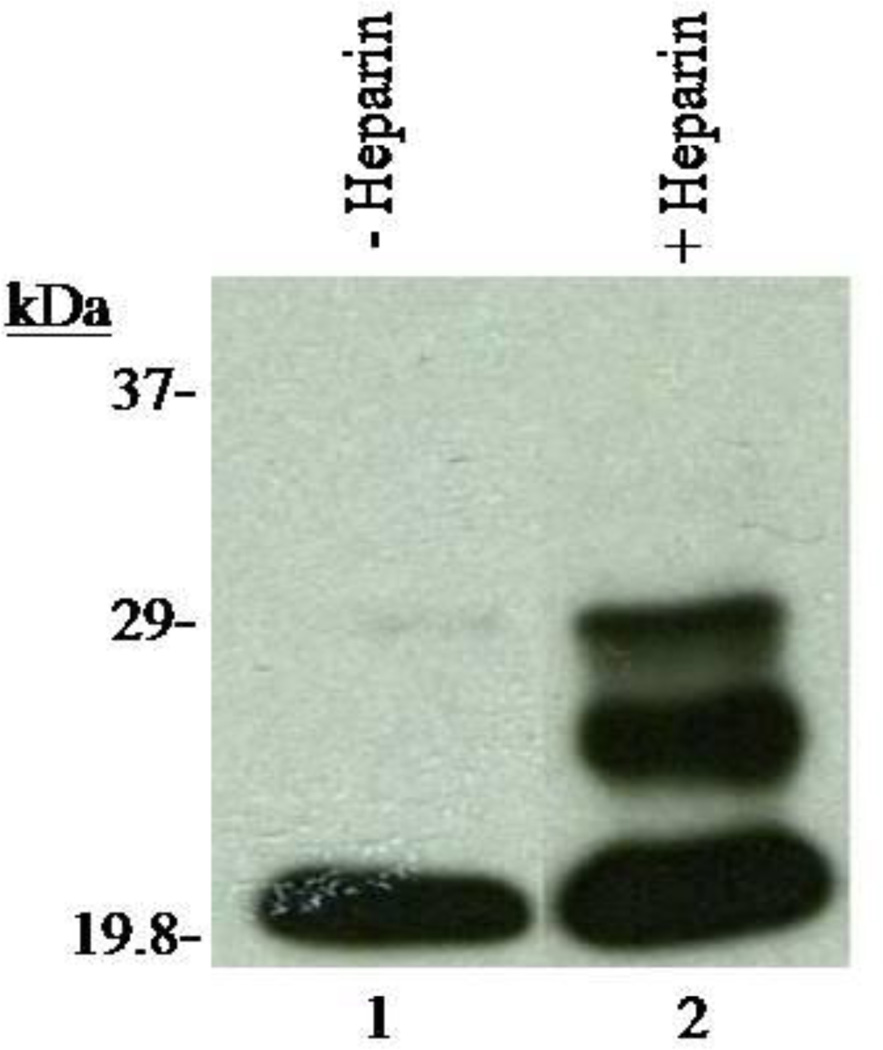
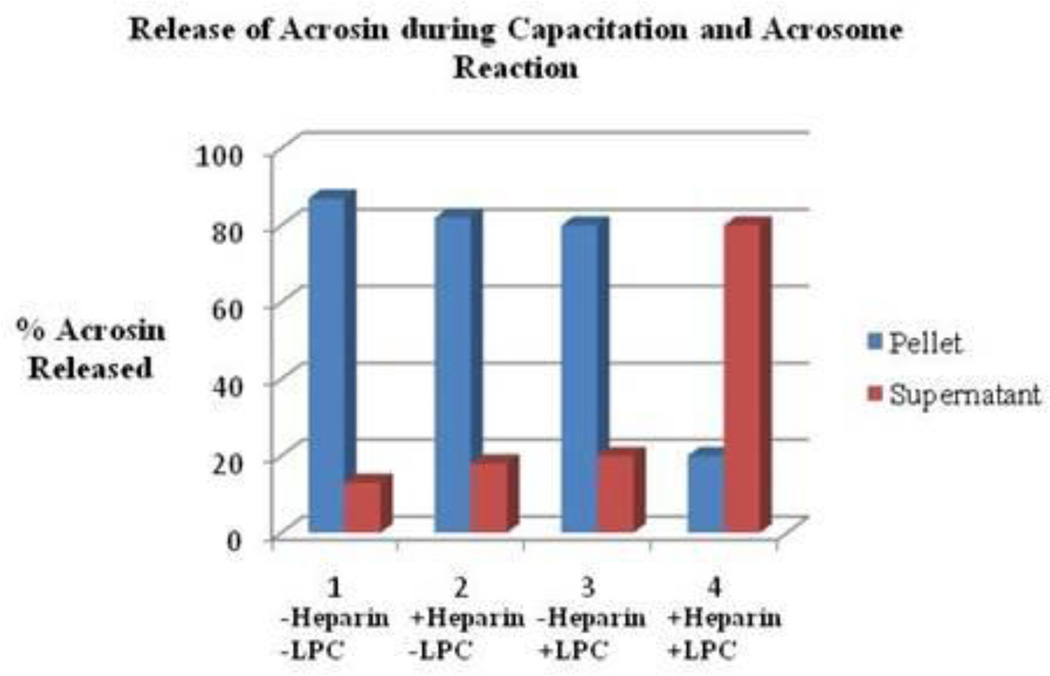
To determine if the 17.5kDa WGA binding polypeptide is retained or released following capacitation and the acrosome reaction, Western blots of 12% SDS-PAGE were stained with biotinylated WGA. As demonstrated in Figure 5 (lanes 2, 4, 6, and 8) several WGA binding polypeptides were retained in the pellet during both capacitation and the acrosome reaction, including the 17.5kDa WGA binding polypeptide. Protein tyrosine phosphorylation is a key biochemical event accompanying sperm capacitation [35–37]. To examine whether capacitation successfully occurred in our current protocol, we analyzed our capacitated sperm fraction with immunoblots stained with α-phospho-tyrosine antibody (Fig. 6). We observed that the 29 and 26 kDa polypeptides were phosphorylated during capacitation (Fig. 6, lane 2). Since the 20kDa phosphorylated polypeptide was present in both the control and capacitated sperm fractions, it suggests that the 20kDa is a non-specific band. Phosphorylation of the bovine cauda polypeptide during capacitation confirms the efficacy of our procedure. Acrosin levels were analyzed in the pellet and supernatant fractions of all samples to confirm the occurrence of an LPC-induced acrosome reaction. As illustrated in Figure 7, fractions incubated in the presence of both heparin and LPC (Set 4) demonstrated a lower percentage (approximately20%) of acrosin in pelleted fractions and a higher percentage (approximately80%) of acrosin in supernatant fractions. This study revealed that most of the acrosin present in cauda sperm was released in the supernatant fraction. All results support the proficiency of our acrosome reaction model. Previously, comparable results were also reported in bovine ejaculated sperm [38]. This data further suggests that the 17.5kDa polypeptide remains anchored to the plasma membrane even after the release of acrosomal contents (acrosomal exocytosis).
Figure 5.
Fate of 17.5kDa WGA Binding Polypeptide during Capacitation and Acrosome Reaction. Lectin blots stained with biotinylated WGA revealed the retention of several polypeptides in pelleted sperm fractions, including the 17.5kDa protein (lanes 2, 4, 6, and 8). Thus, the 17.5kDa WGA binding polypeptide is retained during capacitation and acrosome reaction and therefore must be integrated within the structure of a sperm cell’s plasma membrane.
Figure 6.
Detection of Phosphorylation in Capacitated Sperm Fractions. X-ray film of capacitated sperm fractions incubated in the absence and presence of heparin. Sperm fractions incubated in the presence of heparin (lane 2) underwent phosphorylation as opposed to those incubated in the absence of heparin (lane 1). These results provide biochemical evidence that the capacitation reaction was properly executed.
Figure 7.
Release of Acrosin Following Acrosome Reaction. Set 1: Pellet and supernatant fractions incubated in the absence of heparin and LPC. Set 2: Pellet and supernatant fractions incubated in the presence of heparin and the absence of LPC. Set 3: Pellet and supernatant fractions incubated in the presence of LPC and the absence of heparin. Set 4: Pellet and supernatant fractions incubated in the presence of both heparin and LPC. The percent of acrosin released in Set 4 was significantly higher than that of Sets 1–3 resulting in a successful execution of the acrosome reaction. The data are representative of three experiments.
DISCUSSION
Epididymal maturation of mammalian spermatozoa is mediated by the protein components of the epididymal lumen [19, 39–42]. Several studies suggest that the sperm plasma membrane undergoes extensive biochemical changes, including organization and modification of surface glycoproteins, as sperm travel through the proximal to the distal epididymis. One of the modifications is the adsorption of glycoproteins from the luminal fluid to the sperm plasma membrane. Lectin blot analyses demonstrates the modifications of sperm plasma membrane glycoproteins during maturation in several mammalian species, including rat, hamster, guinea pig, ram, and chimpanzee [10, 17, 22, 43–50]. Thus several studies suggest that the modifications of plasma membrane proteins are prerequisite for successful mammalian fertilization. In the present study, we reported the association of glycoproteins on sperm plasma membrane during the process of bovine sperm maturation in the epididymis and furthermore provide evidence for different mechanisms that may cause these modifications.
By comparing and contrasting the binding specificity of caput and cauda plasma membrane fractions with the use of several lectins, we observed a maturation-associated appearance of a 17.5kDa WGA binding polypeptide in bovine cauda sperm plasma membrane fractions (Figure 1). In the bull [51] and the ram [17], a WGA-binding glycoprotein is involved in gaining fertilizing capacity of sperm during epididymal transport. In the feline, WGA binding glycoproteins secreted from the epididymis and bind to epididymal sperm [52]. Several investigators also reported that the epididymal secretory proteins attached to sperm surface during maturation in the epididymis, such as, aosteopontin [53], one of the members of the - defensin superfamily (24 kDa glycoprotein) [54], isoantigen (E-3) with defensin- and lectin-like motifs [55] in rat, and CD52-like molecule in bovine [56]. Our data is in agreement with other epididymal secretory proteins that have been reported previously in several species. We conclude that the 17.5kDa WGA-binding polypeptide is secreted from the cauda epididymis and binds to the cauda sperm plasma membrane during epididymal transit.
Proteomic analyses of the 17.5kDa polypeptide with MALDI-TOF-TOF yielded 13 peptides that matched the NCBI database sequence of peroxiredoxin-5(PRDX5) protein (Bos Taurus). PRDX5 belongs to the peroxiredoxin superfamily of thiol-dependent peroxidases and is able to reduce hydrogen peroxide, alkyl hydroperoxides and peroxynitrite. PRDX5 is a cytoprotective antioxidant enzyme acting against endogeneous or exogeneous peroxide attacks rather than as a redox sensor [57–59]. PRDX5 is widely expressed in tissues having large subcellular distribution. In mammalian cells, it is present in mitochondria, peroxisomes, the cytosol, and the nucleus [57, 59]. During epididymal transit, spermatozoa are exposed to various oxidative stresses [58, 60]. Reactive oxygen species (ROS) can have both beneficial and detrimental effects on sperm function. For example, high ROS can cause sperm membranes to undergo lipid peroxidation, leading to changes in membrane fluidity, decreased fertilizing capacity, and even sperm death [60–65] whereas a low concentration of ROS is necessary for initiation of capacitation and the acrosome reaction [59, 66–69]. Epididymal spermatozoa have enzymatic antioxidant defenses against ROS-induced damage. PRDX5 is absent in caput membranous fraction of boar spermatozoa whereas a high level of expression was observed in cauda membranous fraction [70]. Our study is in consistent with the previous results in boar that PRDX5 is only present in the bovine sperm cauda plasma membrane fraction. It has been proposed that epididymosomes (prostasome-like particles) may be related to the quantitative increases of PRDX5 in boar epididymal spermatozoa [70]. Epididymosomes secreted by the epididymis play a vital role in mammalian male reproductive physiology [42, 71]. The present study reveals that PRDX5 is secreted from cauda epidiymis and binds to the sperm plasma membrane. Alternatively it could be possible that bovine epididymosomes may be related to the transfer of PRDX5 in cauda sperm. Future studies will address this issue. We propose that bovine cauda sperm PRDX5 acts as an antioxidant enzyme in the epidiymal environment, which is crucial in protecting the viable sperm population against the damage caused by endogeneous or exogeneous peroxide.
Following capacitation and the acrosome reaction, retention of the PRDX5 polypeptide in the cauda sperm hybrid membrane complex suggests that PRDX5 plays an important role in antioxidant defenses against ROS-induced damage during capacitation. It may be possible that the phosphorylation of bovine sperm PRDX5 occurs during capacitation. Future studies will examine this physiological event. At present the localization of PRDX5 in bovine sperm is not well studied. The high metabolic turnover occurs in the midpiece of the tail where the mitochondria are localized. Thus it will allow us to suggest that the bovine sperm PRDX5 secreted from cauda epididymis binds to the tail of sperm. In addition present study will not rule out the bovine sperm PRDX5 glycosylation sites and the potential functional motifs including a transmembrane hydrophobic domain and an extracellular domain with consensus glycosylation sites. Additional studies are needed to resolve these issues. Our future studies will also explore the mechanism of binding of PRDX5 to the bovine sperm plasma membrane.
ACKNOWLEDGEMENTS
Supported by #NIH/NIGMS 5SC3GM 096875-03 and NSF HBCU-UP Award #1036257
REFERENCES
- 1.Yanagimachi R. Mammalian Fertilization. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1993. pp. 189–317. [Google Scholar]
- 2.Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol. 2013;757:321–350. doi: 10.1007/978-1-4614-4015-4_11. [DOI] [PubMed] [Google Scholar]
- 3.Aitken RJ, Nixon B, Minjie L, Koppers AJ, Baker MA. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian Journal of Andrology. 2007;9:554–564. doi: 10.1111/j.1745-7262.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 4.Baker MA, Nixon B, Naumovski N, Aitken RJ. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58:211–217. doi: 10.3109/19396368.2011.639844. [DOI] [PubMed] [Google Scholar]
- 5.Gatti JL, Castella S, Dacheux F, Ecroyd H, Metayer S, Thimon V, Dacheux JL. Post-testicular sperm environment and fertility. Animal Reproduction Science. 2004;82–83:321–339. doi: 10.1016/j.anireprosci.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Dacheux JL, Belleannee C, Jones R, Labas V, Belghazi M, Guyonnet B, Druart X, Gatti JL. Mammalian epididymal proteome. Molecular and Cellular Endocrinology. 2009;306:45–50. doi: 10.1016/j.mce.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Bedford JM. Maturation, transport, and fate of spermatozoa in the epididymis. In: Greep RO, Astwood EB, editors. Handbook of Physiology: Endocrinology, Male Reproductive System. Washington DC: Waverly Press; 1975. pp. 303–317. [Google Scholar]
- 8.Hamilton DW. Structure and function of the epithelium lining the ductuli efferentes, ductus epididymidis, and ductus deferens in the rat. In: Greep RO, Astwood EB, editors. Handbook of Physiology: Endocrinology, Male Reproductive System. Washington DC: Waverly Press; 1975. pp. 259–301. [Google Scholar]
- 9.Orgebin-Crist M-C, Danzo BJ, Davies J. Endocrine control of the development and maintenance of sperm fertilizing ability in the epididymis. In: Greep RO, Astwood EB, editors. Handbook of Physiology: Endocrinology, Male Reproductive System. Washington DC: Waverly Press; 1975. pp. 319–338. [Google Scholar]
- 10.Olson GE, Orgebin-Crist MC. Sperm surface changes during epididymal maturation. Ann NY Acad Sci. 1982;383:372–391. doi: 10.1111/j.1749-6632.1982.tb23179.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper TG. Sperm maturation in the epididymis: a new look at an old problem. Asian Journal of Andrology. 2007;9:533–539. doi: 10.1111/j.1745-7262.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 12.Eddy EM, O’Brien DA, Welch JE. Mammalian sperm development in vivo and invitro. In: Om W, editor. Elements of Mammalian fertilization. Boca Raton: CRC Press; 1991. pp. 1–28. [Google Scholar]
- 13.Dacheux JL, Dacheux F, Paquignon M. Changes in sperm surface membrane and luminal protein fluid content during epididymal transit in the boar. Biol Reprod. 1989;40:633–651. doi: 10.1095/biolreprod40.3.635. [DOI] [PubMed] [Google Scholar]
- 14.Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012;349:717–731. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 15.Geussova G, Peknicova J, Capkova J, Kalab P, Moos J, Philimonenko VV, Hozak P. Monoclonal antibodies to canine intra-acrosomal proteins recognizing acrosomal status during capacitation and acrosome reaction. Andrologia. 1997;29:261–268. doi: 10.1111/j.1439-0272.1997.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 16.Harayama H, Watanabe S, Masuda H, Kannan Y, Miyake M, Kato S. Lectin-binding characteristics of extracts from epididymal boar spermatozoa. J Reprod Dev. 1998;44:21–27. [Google Scholar]
- 17.Magargee SF, Kunze E, Hammerstedt RH. Changes in lectin-binding features of ram sperm surfaces associated with epididymal maturation and ejaculation. Biol Reprod. 1988;38:667–685. doi: 10.1095/biolreprod38.3.667. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud A, Parrish JJ. Changes in lectin binding to bovine sperm during heparin-induced capacitation. Mol Reprod Dev. 1996;44:525–532. doi: 10.1002/(SICI)1098-2795(199608)44:4<525::AID-MRD12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Jones R. Plasma membrane structure and remodelling during sperm maturation in the epididymis. J Reprod Fert Suppl. 1998;53:73–84. [PubMed] [Google Scholar]
- 20.Phopin K, Nimlamool W, Lowe-Krentz LJ, Douglass EW, Taroni JN, Bean BS. Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol Reprod Dev. 2013;80:273–285. doi: 10.1002/mrd.22164. [DOI] [PubMed] [Google Scholar]
- 21.Eddy EM, O'Brien DA. The Spermatozoon. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1993. pp. 29–77. [Google Scholar]
- 22.Srivastava A, Olson GE. Glycoprotein changes in the rat plasma membrane during maturation in the epididymis. Molecular Reproduction and Development. 1991;1991;29:357–364. doi: 10.1002/mrd.1080290407. [DOI] [PubMed] [Google Scholar]
- 23.Hammerstedt RH, Hay SR, Amann RP. Modification of ram sperm membranes during epididymal transit. Biol Reprod. 1982;27:745–754. doi: 10.1095/biolreprod27.3.745. [DOI] [PubMed] [Google Scholar]
- 24.Clark GF. The role of carbohydrate recognition during human sperm-egg binding. Hum Reprod. 2013;28:566–577. doi: 10.1093/humrep/des447. [DOI] [PubMed] [Google Scholar]
- 25.NagDas SK, Winfrey VP, Olson GE. Identification of Ras and Its Downstream Signaling Elements and Their Potential Role in Hamster Sperm Motility. Biol Reprod. 2002;67:1058–1066. doi: 10.1095/biolreprod67.4.1058. [DOI] [PubMed] [Google Scholar]
- 26.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 29.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NagDas SK, Winfrey VP, Olson GE. Tyrosine Phosphorylation Generates Multiple Isoforms of the Mitochondrial Capsule Protein, Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPx), During Hamster Sperm Capacitation. Biol Reprod. 2005;72:164–171. doi: 10.1095/biolreprod.104.033530. [DOI] [PubMed] [Google Scholar]
- 31.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod. 1988;38:1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- 32.Lottenberg R, Christensen U, Jackson CM, Coleman PL. Assay of coagulation proteases using chronogenic and fluorogenic substrates. Meth Enzymol. 1981;80:341–361. doi: 10.1016/s0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- 33.NagDas SK, Winfrey VP, Olson GE. Identification of hydrolase binding activities of the acrosomal matrix of hamster spermatozoa. Biol Reprod. 1996;55:1405–1414. doi: 10.1095/biolreprod55.6.1405. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;1121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 36.Porambo JR, Salicioni AM, Visconti PE, Platt MD. Sperm phosphoproteomics: historical perspectives and current methodologies. Expert Rev Proteomics. 2012;9:533–548. doi: 10.1586/epr.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saccary L, She YM, Oko R, Kan FW. Hamster oviductin regulates tyrosine phosphorylation of sperm proteins during in vitro capacitation. Biol Reprod. 2013;89:38. doi: 10.1095/biolreprod.113.109314. [DOI] [PubMed] [Google Scholar]
- 38.Olson GE, Winfrey VP, Neff JC, Lukas TJ, NagDas SK. An antigenically related polypeptide family is a major structural constituent of a stable acrosomal matrix assembly in bovine spermatozoa. Biol Reprod. 1997;57:325–334. doi: 10.1095/biolreprod57.2.325. [DOI] [PubMed] [Google Scholar]
- 39.Khole V. Epididymis as a target for contraception. Indian J Exp Biol. 2003;41:764–772. [PubMed] [Google Scholar]
- 40.Dacheux JL, Belleannee C, Guyonnet B, Labas V, Teixeira-Gomes AP, Ecroyd H, Druart X, Gatti JL, Dacheux F. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58:197–210. doi: 10.3109/19396368.2012.663233. [DOI] [PubMed] [Google Scholar]
- 41.Dun MD, Aitken RJ, Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum Reprod Update. 2012;18:420–435. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 43.Nicolson GL, Usui N, Yanagimachi R, Yanagimachi H, Smith JR. Lectin-binding sites on the plasma membranes of rabbit spermatozoa. Changes in surface receptors during epididymal maturation and after ejaculation. J Cell Biol. 1977;74:950–962. doi: 10.1083/jcb.74.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolson GL, Bronginski AB, Beattie G, Yanagimachi R. Cell surface changes in the proteins of rabbit spermatozoa during epididymal passage. Gamete Res. 1979;2:153–163. [Google Scholar]
- 45.Olson GE, Hamilton DW. Characterization of the surface glycoproteins of rat spermatozoa. Biol Reprod. 1978;19:26–35. doi: 10.1095/biolreprod19.1.26. [DOI] [PubMed] [Google Scholar]
- 46.Koehler JK. Lectins as probes of the spermatozoan surface. Arch Androl. 1981;6:197–217. doi: 10.3109/01485018108987531. [DOI] [PubMed] [Google Scholar]
- 47.Olson GE, Danzo BJ. Surface changes in rat spermatozoa during epididymal transit. Biol Reprod. 1981;24:431–443. doi: 10.1095/biolreprod24.2.431. [DOI] [PubMed] [Google Scholar]
- 48.Young LG, Gould KG, Hinton BT. Lectin binding sites on the plasma membrane of epididymal and ejaculated chimpanzee sperm. Gamete Res. 1986;14:75–87. [Google Scholar]
- 49.Lee SH, Ahuja KK. An investigation using lectins of glycocomponents of mouse spermatozoa during capacitation and sperm zona binding. J Reprod Fertil. 1987;80:65–74. doi: 10.1530/jrf.0.0800065. [DOI] [PubMed] [Google Scholar]
- 50.Rankin TL, Holland MK, Orgebin-Crist MC. Lectin binding characteristics of mouse epididymal fluid and sperm extracts. Gamete Res. 1989;24:439–452. doi: 10.1002/mrd.1120240410. [DOI] [PubMed] [Google Scholar]
- 51.Medeiros CMO, Parrish JJ. Changes in lectin binding to bovine sperm during heparin-induced capacitation. Mol Reprod Dev. 1996;44:525–532. doi: 10.1002/(SICI)1098-2795(199608)44:4<525::AID-MRD12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 52.Toyonaga M, Morita M, Hori T, Tsutsui T. Distribution of glycoproteins on feline testicular sperm, epididymal sperm and ejaculated sperm. J Vet Med Sci. 2011;73:827–829. doi: 10.1292/jvms.10-0400. [DOI] [PubMed] [Google Scholar]
- 53.Siiteri JE, Ensrud KM, Moore A, Hamilton DW. Identification of osteopontin (OPN) mRNA and protein in the rat testis and epididymis, and on sperm. Molecular Reproduction and Development. 1995;40:16–28. doi: 10.1002/mrd.1080400104. [DOI] [PubMed] [Google Scholar]
- 54.Zanich A, Pascall JC, Jones R. Secreted glycoprotein 2D6 that binds to the sperm’s plasma membrane is a member of the β-defensin superfamily of pore-forming glycopeptides. Biol Reprod. 2003;69:1831–1842. doi: 10.1095/biolreprod.103.018606. [DOI] [PubMed] [Google Scholar]
- 55.Rao J, Herr JC, Reddi PP, Wolkowicz MJ, Bush LA, Sherman NE, Black M, Flickinger CJ. Cloning and characterization of a novel sperm-associated isoantigen (E-3) with defensin- and lectin-like motifs expressed in rat epididymis. Biol Reprod. 2003;68:290–301. doi: 10.1095/biolreprod.102.005983. [DOI] [PubMed] [Google Scholar]
- 56.Michalkova K, Simon M, Antalikova J, Klima J, Horovska L, Jankovicova J, Hluchy S. Identification of bovine CD52-like molecule by monoclonal antibody IV A-543: distribution of CD52-like molecule in the bull genital tract. Theriogenology. 2010;74:1066–1074. doi: 10.1016/j.theriogenology.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Knoops B, Goemaere J, Eecken VV, Declercq J-P. Peroxiredoxin 5: Structure, Mechanism, and Function of the Mammalian Atypical 2-Cys Peroxiredoxin. Antioxidants & Redox Signaling. 2011;15:817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 58.Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004;216:31–39. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 59.O'Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod. 2011;84:238–247. doi: 10.1095/biolreprod.110.085712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oehninger S, Blackmore P, Mahony M, Hodgen G. Effects of hydrogen peroxide on human spermatozoa. J Assist Reprod Genet. 1995;12:41–47. doi: 10.1007/BF02214128. [DOI] [PubMed] [Google Scholar]
- 62.Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995;16:464–468. [PubMed] [Google Scholar]
- 63.Hsieh YY, Chang CC, Lin CS. Seminal malondialdehyde concentration but not glutathione peroxidase activity is negatively correlated with seminal concentration and motility. Int J Biol Sci. 2006;2:23–29. doi: 10.7150/ijbs.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tremellen K. Oxidative stress and male infertility - a clinical perspective. Hum Reprod. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 65.Espino J, Bejarano I, Ortiz A, Lozano GM, Garcia JF, Pariente JA, Rodriguez AB. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil Steril. 2010;94:1915–1917. doi: 10.1016/j.fertnstert.2009.12.082. [DOI] [PubMed] [Google Scholar]
- 66.Zini A, Garrels K, Phang D. Antioxidant activity in the semen of fertile and infertile men. Urology. 2000;55:922–926. doi: 10.1016/s0090-4295(00)00453-2. [DOI] [PubMed] [Google Scholar]
- 67.O’Flaherty C, Breininger E, Beorlegui N, Beconi MT. Acrosome reaction in bovine spermatozoa: role of reactive oxygen species and lactate dehydrogenase C4. Biochim Biophys Acta. 2005;1726:96–101. doi: 10.1016/j.bbagen.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Awda BJ, Mackenzie-Bell M, Buhr MM. Reactive oxygen species and boar sperm function. Biol Reprod. 2009;81:553–561. doi: 10.1095/biolreprod.109.076471. [DOI] [PubMed] [Google Scholar]
- 69.de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46:502–510. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Park K, Jeon S, Song Y-J, Yi LSH. Proteomic analysis of boar spermatozoa and quantity changes of superoxide dismutase 1, glutathione peroxidase, and peroxiredoxin 5 during epididymal maturation. Animal Reproduction Science. 2012;135:53–61. doi: 10.1016/j.anireprosci.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 71.Belleannee C, Calvo E, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]