Abstract
Much evidence suggests that oxidative stress plays a role in schizophrenia pathogenesis. Major oxidative stress sources include hydrogen peroxide and biogenic aldehydes that are mainly cleared in vivo by glutathione peroxidase (GPX) and aldehyde dehydrogenase (ALDH), respectively. Both enzymes are richly expressed in brain. Schizophrenia patients have significantly increased plasma levels of malondialdehyde and glutathione, combined with decreased GPX activity and ALDH1 mRNA levels in the ventral tegmental area. Absence of Aldh1a1 (murine homolog of ALDH1) gene causes increased basal extracellular dopamine concentrations, a common characteristic of schizophrenia. Studies investigating association between gene polymorphisms of GPX1 (the most abundant form of GPX) or ALDH1A1 with schizophrenia also have not clearly demonstrated whether ALDH1A1 or GPX1 is involved in pathogenesis of schizophrenia. To investigate possible contributions of ALDH and GPX to pathological behaviors associated with schizophrenia, we generated mice with both Aldh1a1 and Gpx1 gene deletions (KO). Aldh1a1/Gpx1 KO and wild type (WT) mice had similar number of novel entry and alteration in Y-maze test, suggesting no cognition deficit in KO. Furthermore, KO and WT displayed similar social interaction and novelty preferences in three chambered tests. Overall, KO and WT had similar activity levels, as indicated by their entries in the Y-maze and sociability tests. Furthermore both genotypes buried a similar percentage of marbles in a 30 min marble-burying task. In summary, homozygous deletion of Gpx1 and Aldh1a1 genes was not associated with schizophrenia–like behavioral phenotypes including anxiety, hyperactivity, cognitive deficit or social disability. Our findings suggest that constitutive absence of these genes alone is unlikely to give rise to common behavioral schizophrenia symptoms. However, these mice may be highly sensitive to oxidative challenges during critical stages of prenatal or juvenile brain development.
Keywords: oxidative stress, glutathione peroxidase 1 (Gpx1), aldehyde dehydrogenase 1a1 (Aldh1a1), schizophrenia, gene deletion
1. Introduction
Schizophrenia is a devastating form of mental illness with cognitive, positive and negative symptoms expressed as abnormal mental function and disturbed behavior [1]. The onset of symptoms typically occurs in young adulthood [2]. About 24 million people worldwide are affected by schizophrenia (http://www.who.int/mental_health/management/schizophreni a/en/). The global lifetime prevalence of schizophrenia reaches up to 3.5% [3]. Schizophrenia patients die 12–15 years earlier than the average population [4]. Increased dopamine synthesis, dopamine release, and basal synaptic dopamine concentration has been shown to be associated with schizophrenia by neuroimaging studies [5, 6]. Nevertheless, the pathogenesis of schizophrenia is still unknown.
There is some evidence suggesting that oxidative stress and damage play important roles in pathogenesis of schizophrenia [1, 7–9]. Reactive oxygen species, such as hydrogen peroxide and biogenic aldehydes are sources of oxidative stress capable of causing significant cellular inflammation and damage. Neuroinflammation has been suggested to play an important role in schizophrenia and other psychiatric illness, including autism and depression [10–12]. In terms of defending cells against oxidative stress, glutathione peroxidase (GPX) is a major enzyme clearing hydrogen peroxide, and aldehyde dehydrogenase (ALDH) is a major enzyme clearing biogenic aldehydes in vivo.
GPX reduces free radicals to protect cells against oxidative stress, by oxidizing glutathione (GSH) to Glutathione disulfide (GSSG) at the same time [13]. GPX1, the most abundant form of GPX, is expressed in kidney, liver, erythrocytes, brain, lung and heart [14]. Gpx1 plays a limited role during normal development and under physiological conditions, which is evidenced by the fact that Gpx1 null mice are healthy and fertile and do not show any histopathology up to 15 months of age [14, 15]. However, Gpx1 null mice show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide [16]. Gpx1 null mice have increased levels of lipid peroxides and their liver mitochondria have increased hydrogen peroxide release [17]. Besides, although many studies have been conducted to associate GPX1 gene polymorphism with schizophrenia, it is still not clear whether GPX1 gene polymorphism is involved in pathophysiology of schizophrenia due to limitations of the studies [12, 18–20]. Furthermore, decreased GPX activity, low plasma GPX levels and increased plasma GSH levels were detected in schizophrenia patients as compared to age-matched controls [21–23]. GPx1 is predominately localized to microglia with lower levels in neurons in both rat brain [24] and human brain [25]. Deletion of GPx1 gene might lead to microglia activation, a marker of neuroinflammation, which further causes schizophrenia–like phenotypes.
ALDH is an important enzyme for dopamine metabolism. Dopamine is metabolized by monoamine oxidase to 3,4-Dihydroxyphenylacetaldehyde (DOPAL), which then is metabolized to 3,4-Dihydroxyphenylacetic acid by ALDH. Plasma levels of malondialdehyde (MDA) were significant elevated in schizophrenia patients [23]. Furthermore, despite reported studies linking ALDH1A1 polymorphisms with schizophrenia [26], contribution of ALDH1A1 to pathogenesis of schizophrenia still need to be further confirmed. Also, ALDH1 mRNA levels in the ventral tegmental area of schizophrenia patients were significantly reduced as compared to controls [27]. Aldh1a1, the murine homolog of human ALDH1, is expressed in brain of mice and rats [28]. Absence of the Aldh1a1 gene significantly increased basal extracellular dopamine levels [29], which mimics increased extracellular basal dopamine concentrations in schizophrenia.
Given this evidence, we hypothesized that constitutive absence of both Gpx1 and Aldh1a1 genes producing key enzymes responsible for clearing 2 important forms of oxidative stress: aldehyde and hydrogen peroxide, might produce schizophrenia–like behavioral phenotypes. To test the hypothesis, we employed several widely-used murine behavior tests to characterize schizophrenia-like phenotypes: Y-maze, 3-chamber sociability, and marble burying. These tests can reveal behavioral tendencies in mice that resemble cognitive and negative symptoms of schizophrenia. This study is unique in using mice with homozygous deletion of Gpx1 and Aldh1a1 genes to study the pathological roles of oxidative stress in pathogenesis of schizophrenia.
2. Materials and methods
2.1. Animals
Mice with homozygous homozygous deletion of Aldh1a1 and Gpx1 genes (Aldh1a1/Gpx1 KO) were generated by crossing mice lacking the Aldh1a1 gene (supplied by courtesy of Dr. G. Duester) with mice lacking the Gpx1 gene (supplied by courtesy of Dr. Holly Van Remmen). Details regarding the generation and characterization of these two mouse models have been previously described [14,30]. The corresponding wild type control mice of each genotype were crossed to generate the wild type control for Aldh1a1/Gpx1 KO mice (WT). Mice were genotyped as described before for Aldh1a1 gene [29] and Gpx1 gene [14]. Age-matched of Aldh1a1/Gpx1 KO and WT mice (3.1–3.6 months) were used for several behavioral tests. These behavioral tests were chosen because they are widely used to evaluate schizophrenia–like behavioral phenotypes in mouse schizophrenia models. Animal experiments were conducted according to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
2.2. Y-maze test
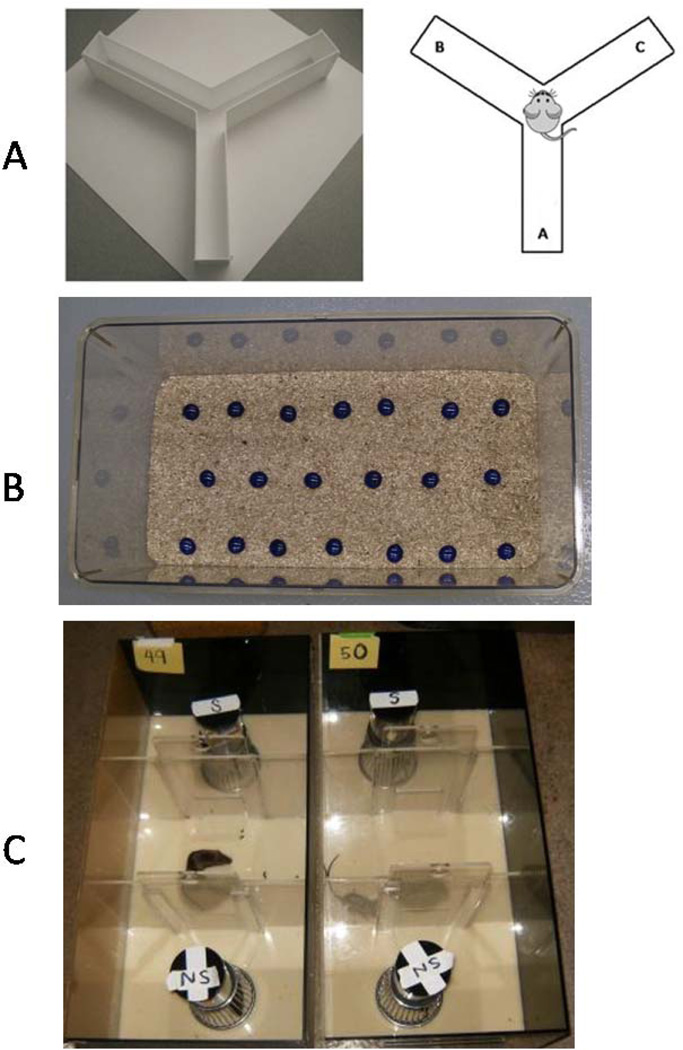
A three-arm Y-maze (arm A, B and C, Figure 1A (http://sbfnl.stanford.edu/cs/bm/lm/bml_ymaze.html),) was used as a measure of cognitive function, memory and general locomotor activity. Mice were pre-trained with one arm blocked (the novel arm: arm A) and allowed to move in the other two arms freely. Mice were then placed at the end of one of the two (not novel, arm B or C) arms and allowed to move freely through the 3 arms of the maze during a 5-min session and the series of arm entries (arm A or B or C) was recorded visually. Alternation was defined as successive entry into the three arms, on overlapping triplet sets.
Figure 1.
Behavioral test instruments. A Y-maze, B Marble bury test, C Social interaction test.
2.3. Marble bury test
Mice were individually placed for a 30-min session in a large clean plastic rat housing cage covered with sawdust to a depth of 5 cm. Twenty clean blue glass marbles were evenly spaced apart in three rows of 6–7 marbles and topped with a filter lid for 30 min (Figure 1B). The number of marbles buried was counted at the end of the session. Percentage of marbles buried or the number of marbles buried was plotted. Buried was defined by having over two thirds of the total top surface of the marble covered by bedding [31].
2.4. Three-chamber sociability tests
Social interaction and social novelty tests were conducted following the same protocols using the same custom-made three-chambered rectangular plastic testing arenas (Figure 1C) as described previously [31]. Similar size of male SR mice with a mixed background of Swiss, C57BL and 129Svev housed were used as stranger mice, which were generously given by Dr. Paul Hasty at the University of Texas Health Science Center at San Antonio. The SR mice were housed in the same facility but had no prior contact with test mice. Briefly, before testing, each test mouse was introduced into the central chamber with both side compartment doors shut for 10 min, and then the mouse was allowed to explore the entire arena for another 10 min with both doors opened. Test mice were re-confined in the central chamber while an empty wire cup-cage (subject), and a cup cage containing a novel stranger mouse (stranger 1) were put into either end of the arena. Each test mouse then explored the testing arena, novel cage and stranger for 10 min of testing after the doors were re-opened. The social novelty test was conducted after the social interaction test. Test mice were re-confined to the central chamber and a second ‘new’ stranger mouse (stranger 2) was put under the second empty cup cage. Stranger 1 (the novel mouse from the social interaction test) remained at the same place as in social interaction test. A new testing session lasted 10 min after the doors were opened. A digital camcorder mounted on a tripod was used to record mouse behavior during test sessions. Chamber entries and time spent by stranger mice was recorded by a genotype-blind observer.
2.5. Statistical analysis
Results are expressed as mean ± SEM. Behavioral data were analyzed using Student t-test (2-tail). Differences were considered as statistically significant when p < 0.05.
3. Results
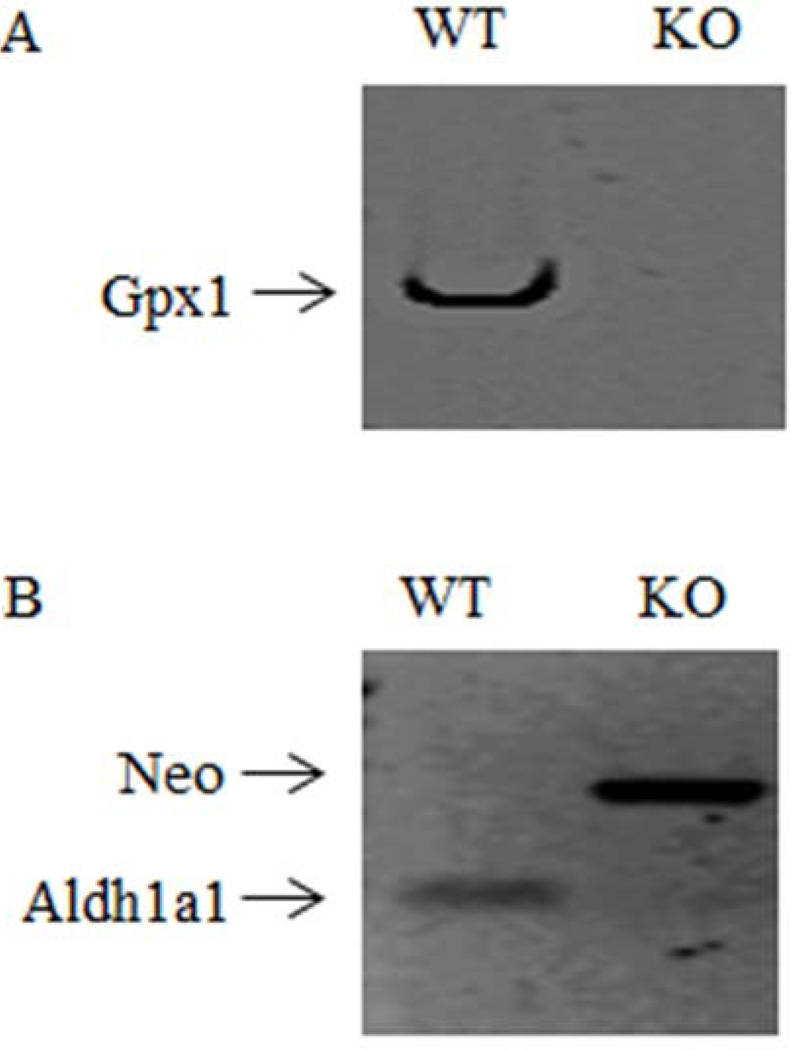
All mice used were genotyped by PCR to separately confirm the absence of Gpx1 gene (Figure. 2A) and the presence of the Neo cassette which deletes the expression of Aldh1a1gene (Figure. 2B) in the Aldh1a1/Gpx1 KO mice.
Figure 2.
Expression of Aldh1a1 and Gpx1 mRNA in Gpx1/Aldh1a1 KO and WT mice. Representative genotyping gel. Gpx1/Aldh1a1 KO animals did not contain a functional copy of the Gpx1 gene (2A) or Aldh1a1 gene (2B), but were positive for the Neo insert used to disrupt gene expression (2B).
3.1. Effects of Aldh1a1 and Gpx1 gene deletion on body weight
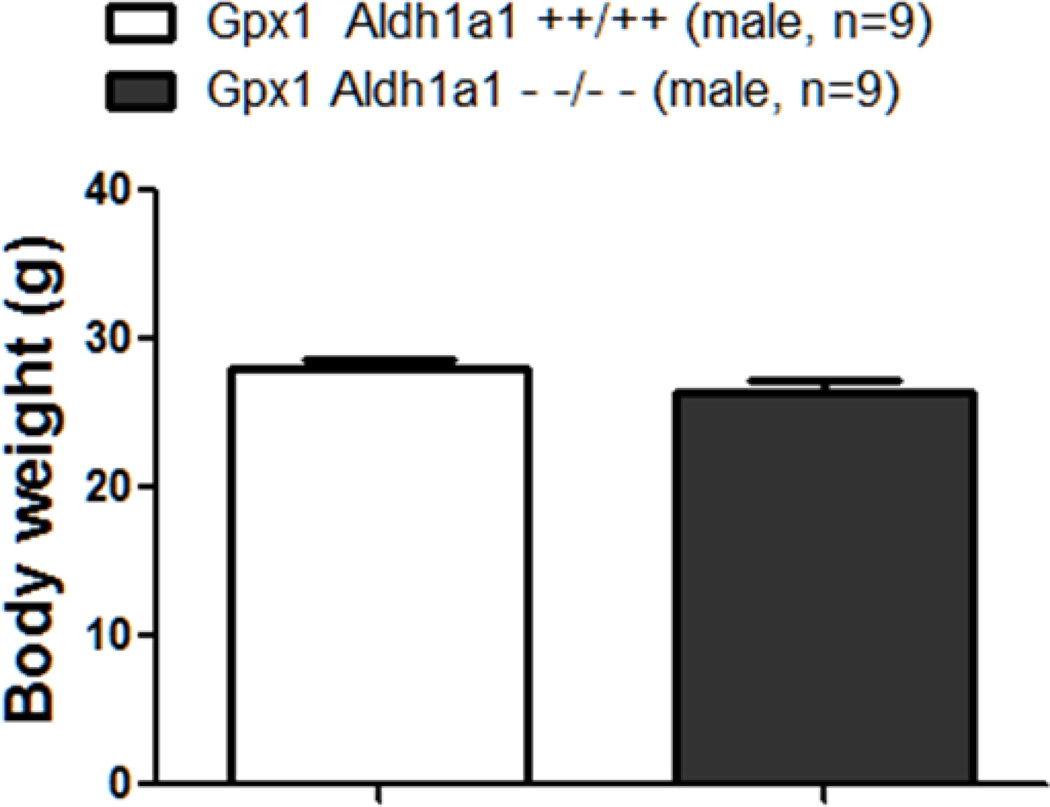
As shown in Figure 3, Aldh1a1/Gpx1 KO and WT mice did not have difference in body weight (26.36 ±0.87 g Vs. 27.99 ± 0.63 g, p=0.418).
Figure 3.
Effect of deletion of Aldh1a1 and Gpx1 on body weight.
3.2. Effect of Aldh1a1 and Gpx1 gene deletion on performance in Y-maze test
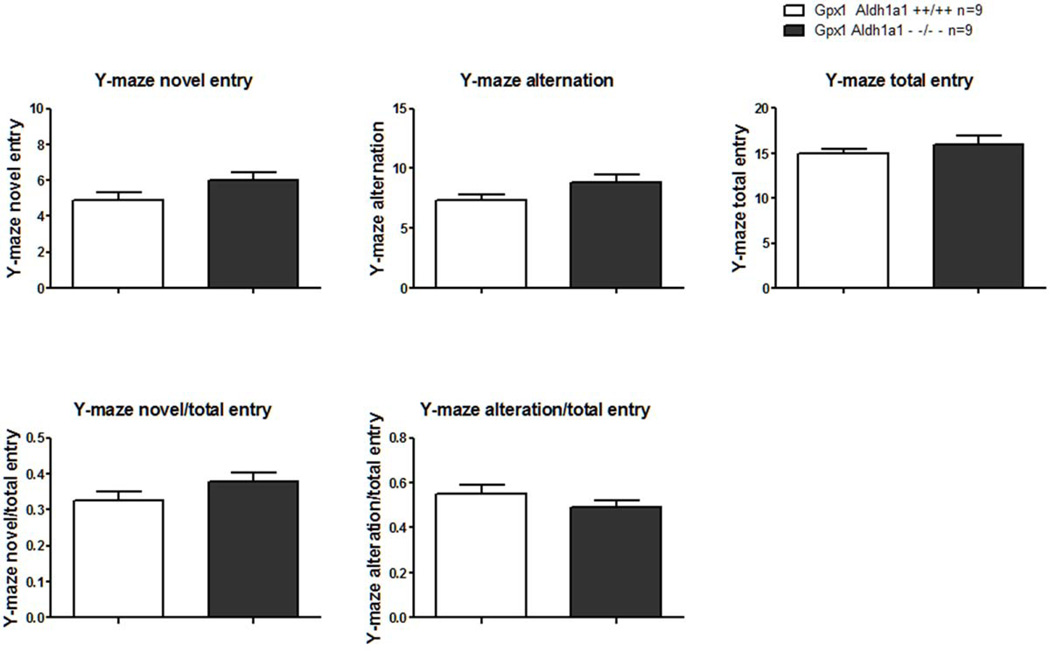
Three-arm Y-maze test was conducted first to evaluate general locomotor activity, cognition and memory in Aldh1a1/Gpx1 KO and WT mice. The number of novel arm entry for Aldh1a1/Gpx1 KO was close to that of WT mice (6.00±0.44 Vs. 4.89±0.45, p=0.098, Figure 4A). The number of alternation between Aldh1a1/Gpx1 KO and WT mice were similar (8.78±0.72 Vs. 7.33±0.47, p=0.113, Figure 4B). The number of total arm entry for Aldh1a1/Gpx1 KO mice was not significantly different from that of WT mice (16.00±1.01 Vs. 15.00±0.47, p=0.384, Figure 4C). Furthermore, the ratio of the number of novel arm entry to the number of total arm entry showed in Figure 4D was comparable between Aldh1a1/Gpx1 KO and WT mice (novel/total entry, 0.38±0.02 Vs.0.33±0.02, p=0.128). Additionally, the ratio of the number of alteration to the number of total arm entry showed in Figure 4E paralleled in Aldh1a1/Gpx1 KO and WT mice (alternation/total entry, 0.49±0.03Vs. 0.55±0.04, p=0.217).
Figure 4.
Effect of deletion of Aldh1a1 and Gpx1 on performance in three-arm Y-maze test. 4A, the absolute number of novel arm entry; 4B, the absolute number of alteration; 4C, the absolute number of total arm entry; 4D, the relative number of novel arm entry expressed as the ratio of the absolute number of novel arm entry to the absolute number of total arm entry; 4E, the relative number of alteration expressed as the ratio of the absolute number of alteration to the absolute number of total arm entry.
3.3. Effect of Aldh1a1 and Gpx1 gene deletion on performance in social interaction and social novelty test
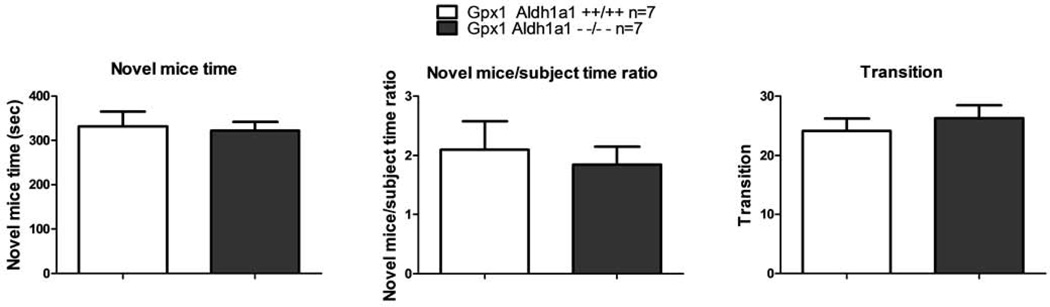
In social interaction test, the amount of time Aldh1a1/Gpx1 KO mice spent with the novel mice did not differ from WT mice as shown in Figure 6A (Novel mice time, p=0.810). The mean novel mice time was 321.9±20.06 seconds Vs. 331.5±33.63 seconds for Aldh1a1/Gpx1 KO mice and WT mice, respectively. The ratio of the time spent with the novel mice to the time spent with the novel subject for Aldh1a1/Gpx1 KO mice was also not different from the ratio for the WT mice (Novel mice/subject time ratio, 1.85±0.30 Vs. 2.10±0.48, p=0.663, Figure 6B). Additionally, the Aldh1a1/Gpx1 KO mice and the WT mice performed comparable number of transitions among the three chambers (26.29 ± 2.22 Vs. 24.14 ± 2.12, p=0.499, Figure 6C).
Figure 6.
Effect of deletion of Aldh1a1 and Gpx1 on performance in social interaction test. 6A, the absolute amount of time spent with novel mice; 6B, the relative amount of time spent with novel mice expressed as the ratio of the absolute amount of time spent with novel mice to the absolute amount of time spent with the subject; 6C, the absolute number of transition
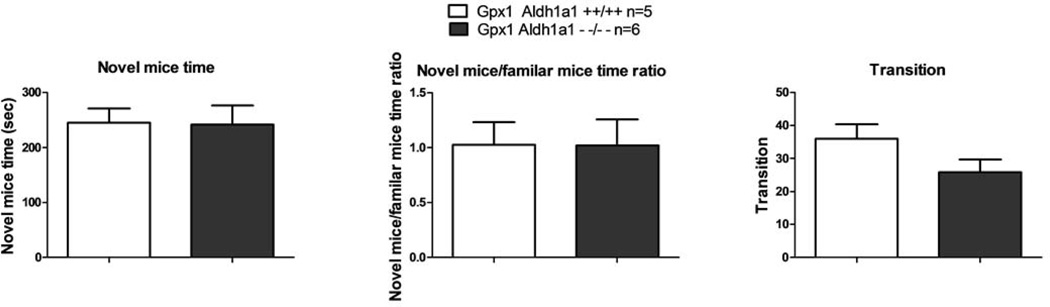
In social novelty test, the Aldh1a1/Gpx1 KO mice and the WT mice spent similar amount of time with the novel mice as shown in Figure 7A (novel mice time, 242.2 ±34.28 seconds Vs. 245.2 ± 25.58 seconds, p=0.948). Likewise, the ratio of the time spent with the novel mice to the time spent with the familiar mice for Aldh1a1/Gpx1 KO mice was almost the same as the ratio for the WT mice as shown in Figure 7B (novel mice/familiar mice time ratio, p=0.991). The mean value of the novel mice/familiar mice time ratio was 1.02±0.24 Vs. 1.02±0.21 for Aldh1a1/Gpx1 KO mice Vs. WT mice. Besides, the number of transitions among the three chambers the Aldh1a1/Gpx1 KO mice and the WT mice performed was alike (25.83 ± 3.79 Vs. 36.00 ± 4.41, p=0.112, Figure 7C).
Figure 7.
Effect of deletion of Aldh1a1 and Gpx1 on performance in social novelty test. 7A, the absolute amount of time spent with novel mice; 7B, the relative amount of time spent with novel mice expressed as the ratio of the absolute amount of time spent with novel mice to the absolute amount of time spent with familiar mice; 7C, the absolute number of transition.
4. Discussion
Schizophrenia is a highly prevalent mental disease affecting quality of life of millions of patients worldwide. Cardinal symptoms of schizophrenia include positive and negative symptoms as well as cognitive and social dysfunction. In spite of the high prevalence of schizophrenia, its mechanisms of pathogenesis are still under investigation. Much evidence has suggested that oxidative stress is important for pathogenesis of schizophrenia [1, 8, 9]. Hydrogen peroxide and biogenic aldehydes are major sources of oxidative stress in vivo which are cleared mainly by GPX and ALDH, respectively. GPX1 and ALDH1 are richly expressed in the brain [14, 28]. To shed light on whether GPX1 and ALDH1 play a role in pathogenesis of schizophrenia, we used a new mouse model with deficiency of Aldh1a1 and Gpx1 genes. We conducted a battery of behavioral tests to evaluate whether Aldh1a1/Gpx1 KO mice have schizophrenia-like behavioral phenotypes.
Overweight or underweight has been linked to schizophrenia. For instance, a study with 15,171 subjects reported that underweight as well as obesity is a characteristic in Japanese schizophrenia inpatients [32]. Another study enrolled 896 patients and showed that obesity is associated with reduced cognitive function in Chinese patients with schizophrenia [33]. To make sure that body weight does not confound the behavioral tests we were using, body weight was measured before the mice were chosen for behavioral tests. We found that the Aldh1a1/Gpx1 KO and WT mice had similar body weight, which thus excluded the confounding effect of body weight on the following behavioral tests.
To evaluate whether Aldh1a1/Gpx1 KO mice have schizophrenia-like cognitive deficit or altered locomotor activity, we did the three-arm Y-maze test. We measured novel arm entry to evaluate spatial memory and alternation to measure working memory of the mice. As shown in Figure 4A and 3B, we did not detect difference in the absolute number of novel arm entry or the absolute number of alternation between Aldh1a1/Gpx1 KO and WT mice. We also measured total arm entry to evaluate general locomotor activity, which was similar between Aldh1a1/Gpx1 KO and WT mice (Figure 4C). We further calculated the relative novel arm entry and alteration calibrated by the number of total arm entry as shown in Figure 4D and Figure 4E, both novel/total entry and alternation/total entry ratios were comparable between Aldh1a1/Gpx1 KO and WT mice. These data indicate that deletion of Aldh1a1 and Gpx1 genes does not lead to dysfunction in locomotion, spatial memory or working memory.
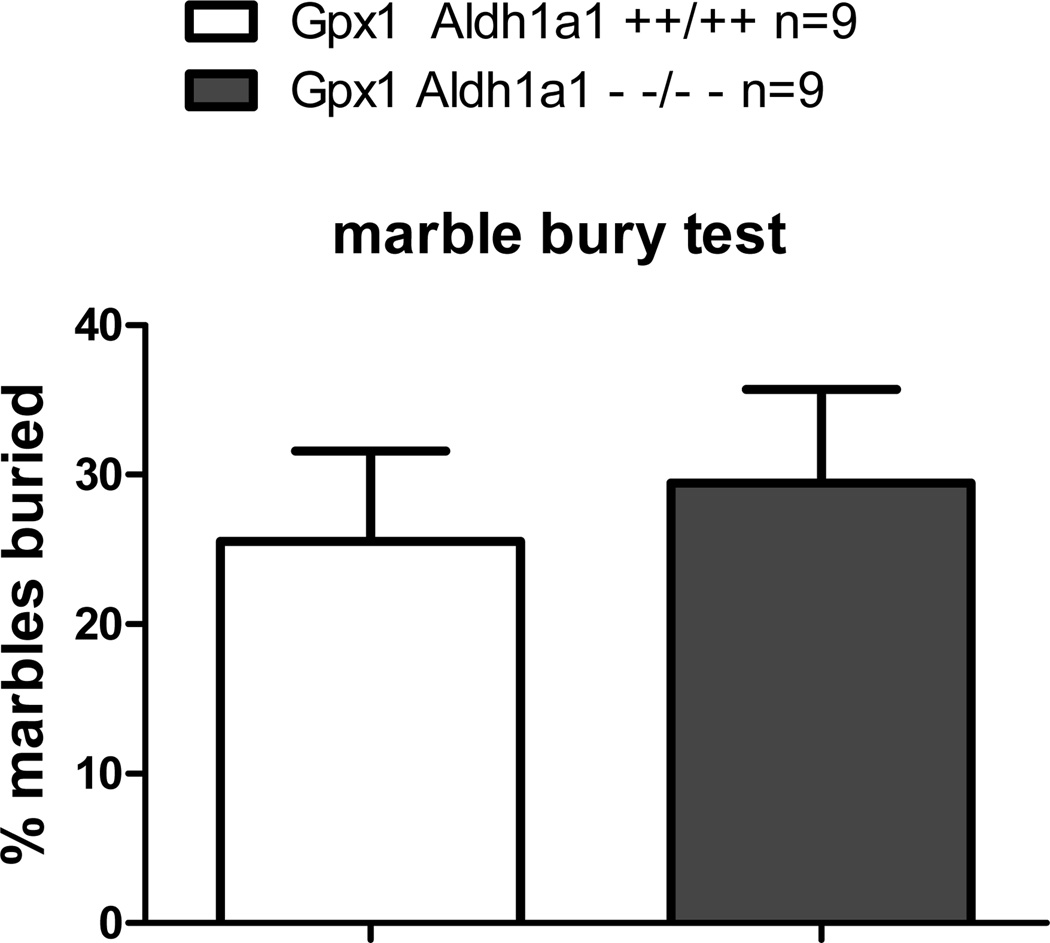
To evaluate whether Aldh1a1/Gpx1 KO mice have schizophrenia-like negative symptoms such as anxiety, we conducted marble bury test. Marble bury test has been widely used to measure anxiety in rodents. The number of marbles buried is an index of anxiety-related behavior. Mice with anxiety bury more marbles compared to mice without anxiety and marble bury test is also sensitive to anxiolytic treatment [34]. As shown in Figure 5, Aldh1a1/Gpx1 KO did not bury different number of marbles compared to WT mice, indicating absence of anxiety-like behavior in mice when Aldh1a1 and Gpx1 genes are deleted.
Figure 5.
Effect of deletion of Aldh1a1 and Gpx1 on performance in marble bury test.
To evaluate whether Aldh1a1/Gpx1 KO mice have schizophrenia-like social dysfunction, social interaction test and social novelty tests were conducted. The amount of time spent with novel mice was not altered by deletion of Aldh1a1 and Gpx1genes in both tests, indicated by either similar absolute amount of time or similar relative amount of time calibrated by the time spent with subject cage in social interaction test and by the time spent with familiar mice in social novelty test. The absolute number of transitions in both social interaction test and social novelty tests were also comparable between Aldh1a1/Gpx1 KO and WT mice. These data imply that deficiency of Aldh1a1 and Gpx1genes does not lead to social dysfunction.
Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations [35]. Because we did not see cognitive and social dysfunction as well anxiety-like behavior based on the above data we obtained, we did not further treat Aldh1a1/Gpx1 KO mice with amphetamine to see if amphetamine-induced dopamine release and locomotor activity is altered or not as a measure of positive symptoms. However, it would be interesting to test whether Aldh1a1/Gpx1 KO mice are vulnerable to ketamine or amphetamine in adolescent mice, as both chemicals have been widely used to induce schizophrenia like phenotype.
Oxidative stress plays an important role in pathogenesis of Schizophrenia [1, 8, 9]. Higher plasma levels of MDA [23], lower plasma GPX levels [22], lower GPX activity [21], as well as higher plasma GSH level [23] were detected in schizophrenia patients compared to normal control. Additionally, loss of Aldh1a1 gene significantly increased basal extracellular dopamine level like in schizophrenia [29]. However, we did not find that deficiency in 2 anti-oxidative stress genes Aldh1a1 and Gpx1 is associated with Schizophrenia-like behavior including anxiety, hyperactivity, cognitive deficit or social dysfunction as we originally hypothesized. There are three possible reasons to explain the lack of schizophrenia-like behavior in Aldh1a1/Gpx1 KO mice. Firstly, deficiency of these two important oxidative stress response genes is not enough to give rise to oxidative stress to cause schizophrenia. There are many forms of Aldh and Gpx which might compensate the effect of Aldh1a1 and Gpx1 gene loss. For example, there are 22 families of ALDH (http://www.aldh.org/) and more than 5 isoforms of GPX [36]. Future studies may include oxidative challenges such as controlled introduction of free radicals during juvenile development by the oxidative stress-inducing agent paraquat or hydrogen peroxide. Secondly, although deletion of Aldh1a1 might cause accumulation of DOPAL (the aldehyde metabolite of dopamine) leading to oxidative damage to dopaminergic neurons, nondopaminergic neurons like cholinergic [37] and glutamatergic [38] neurons have also been reported to play an important role in pathogenesis of schizophrenia. Thirdly, Aldh1a1 is most richly expressed in mesencephalon dopaminergic neurons [28] and not richly expressed in other brain regions that have been suggested to be intensively involved in schizophrenia such as hippocampus [39,40], nucleus accumbens [39], prefrontal cortex [41] and white matter [42]. Furthermore, several studies failed to reveal association of the genetic polymorphisms of GPX1 or ALDH1A1 with schizophrenia. For example, no association between the Pro197Leu [18] or Pro200Leu [43] polymorphisms in GPX1 with schizophrenia was observed in schizophrenia patients. Case-control analyses also failed to detect association of five single nucleotide polymorphisms of ALDH1A1 in Chinese Han schizophrenia subjects [26]. Therefore, homozygous deletion of GPX1 and ALDH1A1 genes might not be enough to give rise to schizophrenia behavioral phenotype.
In addition, it has been reported that neuroinflammation plays an important role in schizophrenia [11]. GPx1 is predominately localized to microglia [24, 25], so another original rationale for us to test schizophrenia behavior phenotypes in Aldh1a1/Gpx1 KO mice is that loss of GPx1 gene leads to microglia activation, a marker of neuroinflammation, to cause schizophrenia–like behavior. Since in this study mice were not presented with any form of an oxidative challenge, and we saw no evidence that KO alone produced schizophrenia-like behaviors, we did not test any neuroinflammation marker in Aldh1a1/Gpx1 KO mice. In future studies a controlled oxidative challenge may better reveal the contribution of Gpx1 to pathogenesis of schizophrenia from the angle of neuroinflammation.
In summary, homozygous deletion of Gpx1 and Aldh1a1 genes alone was not associated with schizophrenia–like behavior including anxiety, hyperactivity, cognitive or social dysfunction in mice. Our study is innovative in that we used mice with deletion of both Gpx1 and Aldh1a1 genes to study contribution of oxidative stress to pathogenesis of schizophrenia. Establishment of this baseline is an important first step before undertaking further studies of oxidative challenge in these mice to examine the function of the two specific oxidative response genes Gpx1 and Aldh1a1 as a risk factor for idiopathic pathogenesis of schizophrenia. As such our Aldh1a1/Gpx1 KO mice are a valuable model to study the role of oxidative stress in the pathogenesis of schizophrenia, especially under stress conditions in adolescent mice induced by ketamine or amphetamine to study whether Aldh1a1 and Gpx1 deletion predispose the mice to develop schizophrenia. Also, future analysis of Parkinson-like behavior in Aldh1a1/Gpx1 KO mice might be interesting since mRNA expression of ALDH1 [27] and Gpx1 [44] reduced in substantia nigra of the brains derived postmortemly from patients with Parkinson’s disease.
Acknowledgements
We are grateful to Dr. G. Duester (Sanford-Burnham Medical Research Institute, La Jolla, CA) for mice lacking the Aldh1a1 gene and Dr. H. Van Remmen for mice lacking the Gpx1 gene. We are also grateful to Dr. P. Hasty for the SR mice and Dr. V. Galvan for the three-arm Y-maze. We also thank Ms. Vivian Diaz at the Nathan Shock Aging Animal and Longevity Assessment Core (San Antonio) for taking good care of the mice. This work was supported by a grant from the VA Office of Research & Development VA 1 I01 BX001641 (RS) and a VA VISN-17 grant (EF); PHS grants: AG013319 (RS), MH086708 (GG); and a Translational Science Pre-doctoral Training Grant from the Clinical and Translational Science Award Program (XB) at the University of Texas Health Science Center at San Antonio.
Footnotes
Conflict of interest: None declared.
References
- 1.Boskovic M, Vovk T, Kores Plesnicar B, Grabnar I. Oxidative stress in schizophrenia. Curr.Neuropharmacol. 2011;9:301–312. doi: 10.2174/157015911795596595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 3.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch.Gen.Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch.Gen.Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 5.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q.J. Nucl.Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 6.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int.Rev.Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 7.Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr.Res. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Neri I, Ramirez-Bermudez J, Montes S, Rios C. Possible mechanisms of neurodegeneration in schizophrenia. Neurochem.Res. 2006;31:1279–1294. doi: 10.1007/s11064-006-9162-3. [DOI] [PubMed] [Google Scholar]
- 9.Fendri C, Mechri A, Khiari G, Othman A, Kerkeni A, Gaha L. Oxidative stress involvement in schizophrenia pathophysiology: a review. Encephale. 2006;32:244–252. doi: 10.1016/s0013-7006(06)76151-6. [DOI] [PubMed] [Google Scholar]
- 10.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav.Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J.Neuroinflammation. 2013;10 doi: 10.1186/1742-2094-10-43. 43,2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11 doi: 10.1186/1741-7015-11-200. 200,7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwell B. Reactive oxygen species and the central nervous system. J.Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 14.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, et al. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J.Biol.Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 15.Cheng WH, Ho YS, Ross DA, Valentine BA, Combs GF, Lei XG. Cellular glutathione peroxidase knockout mice express normal levels of selenium-dependent plasma and phospholipid hydroperoxide glutathione peroxidases in various tissues. J.Nutr. 1997;127:1445–1450. doi: 10.1093/jn/127.8.1445. [DOI] [PubMed] [Google Scholar]
- 16.de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, et al. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J.Biol.Chem. 1998;273:22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 17.Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic.Biol.Med. 2000;28:754–766. doi: 10.1016/s0891-5849(00)00161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinkai T, De Luca V, Zai G, Shaikh S, Matsumoto C, Arnold PD, et al. No association between the Pro197Leu polymorphism in the glutathione peroxidase (GPX1) gene and schizophrenia. Psychiatr.Genet. 2004;14:177–180. doi: 10.1097/00041444-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa D, Hashimoto K, Hashimoto T, Shimizu E, Watanabe H, Fujita Y, et al. Association study between the genetic polymorphisms of glutathione-related enzymes and schizophrenia in a Japanese population. Am. J.Med.Genet.B.Neuropsychiatr.Genet. 2009;150B:86–94. doi: 10.1002/ajmg.b.30776. [DOI] [PubMed] [Google Scholar]
- 20.Souza RP, Tampakeras M, Basile V, Shinkai T, Rosa DV, Potkin S, et al. Lack of association of GPX1 and MnSOD genes with symptom severity and response to clozapine treatment in schizophrenia subjects. Hum.Psychopharmacol. 2009;24:676–679. doi: 10.1002/hup.1076. [DOI] [PubMed] [Google Scholar]
- 21.Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2008;32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 23.Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, alpha-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian.J.Clin.Biochem. 2006;21:34–38. doi: 10.1007/BF02912908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenau J, Noack H, Asayama K, Wolf G. Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia. 1998;24:252–256. [PubMed] [Google Scholar]
- 25.Power JH, Blumbergs PC. Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:63–73. doi: 10.1007/s00401-008-0438-3. [DOI] [PubMed] [Google Scholar]
- 26.Wan C, Shi Y, Zhao X, Tang W, Zhang M, Ji B, et al. Positive association between ALDH1A2 and schizophrenia in the Chinese population. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2009;33:1491–1495. doi: 10.1016/j.pnpbp.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Galter D, Buervenich S, Carmine A, Anvret M, Olson L. ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson's disease and in the ventral tegmental area in schizophrenia. Neurobiol.Dis. 2003;14:637–647. doi: 10.1016/j.nbd.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Westerlund M, Galter D, Carmine A, Olson L. Tissue- and species-specific expression patterns of class I, III, and IV Adh and Aldh 1 mRNAs in rodent embryos. Cell Tissue Res. 2005;322:227–236. doi: 10.1007/s00441-005-0038-7. [DOI] [PubMed] [Google Scholar]
- 29.Anderson DW, Schray RC, Duester G, Schneider JS. Functional significance of aldehyde dehydrogenase ALDH1A1 to the nigrostriatal dopamine system. Brain Res. 2011;1408:81–87. doi: 10.1016/j.brainres.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, et al. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol.Cell.Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J.Neurochem. 2011;116:291–303. [Google Scholar]
- 32.Inamura Y, Sagae T, Nakamachi K, Murayama N. Body mass index of inpatients with schizophrenia in Japan. Int.J.Psychiatry Med. 2012;44:171–181. doi: 10.2190/PM.44.2.h. [DOI] [PubMed] [Google Scholar]
- 33.Guo X, Zhang Z, Wei Q, Lv H, Wu R, Zhao J. The relationship between obesity and neurocognitive function in Chinese patients with schizophrenia. BMC Psychiatry. 2013;13 doi: 10.1186/1471-244X-13-109. 109,244X-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol.Biochem.Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 35.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc.Natl.Acad.Sci.U.S.A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn H, Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic.Biol.Med. 2002;33:154–172. doi: 10.1016/s0891-5849(02)00855-9. [DOI] [PubMed] [Google Scholar]
- 37.Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov.Med. 2012;14:413–420. [PMC free article] [PubMed] [Google Scholar]
- 38.Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb.Exp.Pharmacol. 2012;(213):267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact.Funct.Neurosurg. 2009;87:256–265. doi: 10.1159/000225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J.Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eack SM, Wojtalik JA, Newhill CE, Keshavan MS, Phillips ML. Prefrontal cortical dysfunction during visual perspective-taking in schizophrenia. Schizophr.Res. 2013;150:491–497. doi: 10.1016/j.schres.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, Sasamoto A, et al. Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog. Neuropsychopharmacol.Biol.Psychiatry. 2013;48C:117–123. doi: 10.1016/j.pnpbp.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Boskovic M, Vovk T, Saje M, Goricar K, Dolzan V, Kores Plesnicar B, et al. Association of SOD2, GPX1, CAT, and TNF genetic polymorphisms with oxidative stress, neurochemistry, psychopathology, and extrapyramidal symptoms in schizophrenia. Neurochem.Res. 2013;38:433–442. doi: 10.1007/s11064-012-0937-4. [DOI] [PubMed] [Google Scholar]
- 44.Duke DC, Moran LB, Pearce RK, Graeber MB. The medial and lateral substantia nigra in Parkinson's disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics. 2007;8:83–94. doi: 10.1007/s10048-006-0077-6. [DOI] [PubMed] [Google Scholar]