Abstract
Background
in 1962, during the Algerian war, approximately one and a half million French people living in Algeria were repatriated to France in very poor and often life-threatening conditions. These subjects constitute a cohort for the study of the long term impact of gene-environment interaction on depression.
Aims
To examine the interaction between a highly stressful life event and subsequent depression, and its modulation by the serotonin transporter gene (5-HTTLPR).
Method
A community sample of elderly persons aged 65 years and over residing in the Montpellier region of the South of France was randomly recruited from electoral rolls. Genotyping was performed on 248 repatriated persons and 632 controls. Current and lifetime major and minor depressions were assessed according to DSM-IV criteria.
Results
A significant relationship was observed between exposure to repatriation and subsequent depression (p<0.002), but there was no significant effect of gene alone (p=0.62). After controlling for age, gender, education, disability, recent life events and cognitive function, the gene-environment interaction (Repatriation X 5-HTTLPR) was globally significant (p<0.002; OR= 3.21 [2.48–5.12]). Subjects carrying the two short ( S ) alleles of 5-HTTLPR were observed to be at higher risk (p<0.005; OR=2.34 [1.24–4.32]) and particularly when the repatriation occurred before the age of 35 (p<0.002; OR=2.91 [1.44–5.88]) but did not reach significance in subjects who were older at the time of the event (p=0.067).
Conclusion
The association between depression and war repatriation is significantly modulated by 5-HTTLPR genotype but this appears to occur only in persons who were younger at the time of exposure.
Keywords: Age Factors; Aged; Aged, 80 and over; Algeria; Alleles; Depressive Disorder; epidemiology; genetics; Diagnostic and Statistical Manual of Mental Disorders; Environment; Female; France; epidemiology; Genetic Predisposition to Disease; Genotype; Humans; Life Change Events; Logistic Models; Male; Prospective Studies; Risk Factors; Serotonin Plasma Membrane Transport Proteins; genetics; Time Factors; War
Introduction
Stressful life events and adverse life conditions have been shown to play an important role in the aetiology of depression[1,2]. However, only a minority of persons subjected to severe life stressors develop psychopathology, suggesting the existence of moderating factors [3]. Previous research has suggested that genetic susceptibility may influence the association through gene-environment interaction (G × E); notably the locus 5-HTTLPR of the serotonin transporter gene (5-HTT). In children [4], adolescents [5,6] and young adults [7–12] it has been observed that subjects with one or two short ( S ) alleles of the 5-HTTLPR polymorphism, are more likely to develop depression and suicidal ideation after exposure to stress than individuals with one or two long (L) alleles [13]. Not all studies have, however, found this interactive effect, and a recent meta-analysis conducted by Risch et al [14] of 14 studies did not find a significant interaction between the serotonin transporter gene, life events and depression. Critics of the meta-analysis have pointed to possible problems of mismeasurement and non-standardization of the environmental stressor [15] and the heterogeneity of the cohorts. In previous reports we did not find an association with the S allele in an elderly population in relation to either recent life events [16] or childhood trauma[17], although it has been found in an elderly Asian population [18]. A number of factors might contribute to the conflicting results in elderly populations, notably reliance on recall of distant stressful events which may be subject to recall bias, particularly in subjects with high neuroticism scores [19,20] who tend to over-report stressors. Jacobs et al (2006)[10] have suggested that the trait of neuroticism may itself interact with 5-HTTLPR genotype and thus constitute a confounding factor being in itself largely genetically determined.
Before concluding the absence of a 5-HTTLPR interaction effect it is perhaps worthwhile considering whether this may only be evidenced by more precise and objective measures of environmental impact including war and major catastrophe[21], or may be specific to a restricted exposure window such as age at time of event. A further limiting factor in previous research has been the length of follow-up following the stressful event; this commonly being limited to less than 5 years although the pathogenic effects of traumatic life events have been shown to influence depression incidence throughout life [2].
In the present study we examined the impact of a common stressor on the incidence of depression between early adulthood and old age (37 to 40 years after the traumatic event), and the modulating effect of the 5-HTTLPR genotype. Our assumption is that while individuals are exposed to a common traumatic event, there will be individual differences in stress reactivity. The stressful event is repatriation of French citizens living in Algeria in 1962 as a result of the Algerian war (decolonization conflict). As France was loosening its colonial hold in Africa during the 1960s, entire populations were affected. Over one and a half million persons have experienced profound dislocations. They were uprooted from their homes in Algeria, lost almost all their possessions and confronted poor living conditions on arrival. Many of these repatriated persons came to Montpellier in the south of France, where the present study was conducted. This cohort of repatriated individuals have thus been exposed to a common event known to be associated with exceptionally high rates of subsequent depression [22] and occurring at a specific point in time. The environmental component of the gene-environment interaction is thus more rigorously defined than in previous studies and the study design allows us to examine the effect of age at the time of the event and long term mental health.
Method
Study design and sample
The present study is part of the ESPRIT Project (Enquête de Santé Psychologique–Risques, Incidence et Traitement), a prospective general population study of life-time psychiatric disorder in persons over 65 in the Montpellier region of the South of France. The methodology of the study is described in detail elsewhere [23]. Briefly, a random sample of 1863 community-dwelling persons over 65 was drawn from the 15 electoral rolls of the Montpellier district between March 1999 and February 2001. In 1962, 80 000 French repatriates arrived in Montpellier from Algeria constituting at the time 20.7% of the population. In our cohort 13% of recruited subjects were repatriated from the Algerian war. Subjects were examined in a clinical research centre at the Gui de Chauliac Neurology Hospital in Montpellier. Subjects unable to come to the centre were examined in their homes. Participants were administered a number of standardized questionnaires by trained staff and underwent clinical examinations. Ethics approval for the ESPRIT study was granted by the Ethics Committee of the University Hospital of Kremlin-Bicêtre (France) and all procedures were carried out with the written consent of the participants.
Measurement of exposure
The present study was conducted on the 880 subjects from the ESPRIT study (1863 subjects) for whom 5-HTTLPR genotyping was available, all psychiatric and repatriation questionnaires were completed and with no missing data on adjustment variables. Two hundred and forty eight Caucasian repatriates from the Algerian war were identified by a structured questionnaire relating to their experience of repatriation. Amongst the repatriated group we distinguished two level of stress. Those who experienced only a repatriation back to France (Repat) implicating loss of possessions, unemployment and social disorganization, and a second level in which subjects experienced not only repatriation but also at least one war-related traumatic event such as experiencing or witnessing torture, severe injury or death (RepatT). We used the initial screening item for Post Traumatic Stress Disorder from DSM-IV and also required subjects to recount the traumatic event in order to identify this sub-group. This question identified 62 repatriated subjects who had also suffered war-related trauma. These groups have been differentiated in the analysis as being exposed to repatriation alone (Repat) and repatriation plus war trauma (RepatT). A control group was also constituted of 632 subjects from the ESPRIT study who were not repatriated and had not experienced any other form of trauma.
Measurement of depression
Life-time and current depression were assessed by the Mini International Neuropsychiatric Interview (MINI) (French version 5.00) validated in the general population setting [24] which provides DSM IV diagnoses. For this study depression included both minor and major depressive episodes occurring after 1962, the former having a much higher prevalence in older populations. All cases were validated by a panel of psychiatrists blind to repatriation status. Cases of depression occurring before 1962 were eliminated from the analyses.
Blood samples for DNA collection for 5-HTTLPR genotyping were taken after the baseline clinical interview on subjects. 5-HTTLPR insertion/deletion polymorphisms were assayed in two stages. Genomic DNA was extracted from white blood cells harvested from 15 ml EDTA blood samples using DNA extraction kits from Amersham-Pharmacia Biotech. Subsequently, amplification of 5-HTTLPR was carried out using the primers HTTLPRF (GGCGTTGCCGCTCTGAATTGC) and HTTLPRR (GAGGGACTGAGCTGGACAACCCAC) in reaction mixtures with a total volume of 25 μl, with 200 ng of genomic DNA, 10 pM of the primers, 120 nM dNTP, containing 7-deaza-dGTP instead of dGTP (Roche), 5% DMSO (Sigma), 1.5 mM MgCl2, and 1.25 U Taq polymerase (Eurobio, Brunschwig). Temperatures were 60°C for 30 s for annealing and 72°C for 1 mn for extension. PCR products (8 μl) were subjected to 45 mn electrophoresis at 120 V in a PCR CheckIT gel (Elchrom Scientific AG, Switzerland) before being viewed under UV to assess the 5-HTTLPR genotypes. An adenosine/guanine (A/G) single nucleotide polymorphism (SNP; rs25531), located in the close vicinity of 5-HTTLPR, has recently been reported to modify transcriptional activity. On the basis of these in vitro functional data it has been proposed to recode the 5-HTTLPR/rs25531 allele as S or “low expressing allele (SA, SG and LG) and L or “high expressing allele” (LA) [25]. As this new allelic dichotomy still awaits replication in in vivo studies, we initially examined both allelic systems (S/L and S/L) within the present study. Recoding did not significantly alter results so we report here only analyses relating to S and L.
Other measures
Due to the age of the subjects (over 65), cognitive impairment and disability were included as adjustment factors in statistical analyses. Cognitive functions were evaluated using the Benton Visual Retention test (BVRT)[26], the Trail Making Test (TMTB)[27], the Isaacs’ Set Test [28] and a word recall test with both delayed free recall and recall with semantic prompts[29]. These tests covered declarative verbal and spatial memory, central executive and semantic retrieval abilities. Cognitive impairment was defined as having a score in the lowest quartile range in relation to the relevant age and education matched comparison group [30]. Impairment in the performance of everyday activities was assessed with the Lawton instrumental Activities Daily Living Scale [31] and Katz Activities Daily Living Scale [32], disability being defined as increased difficulty in at least one IADL or ADL. As recent negative life events may confound the relationship with past events, the occurrence of negative events occurring in the past year (notably bereavement, rupture in significant relationships, financial and legal problems, dismissal, severe illness, loss of a highly valued object) was ascertained using the 12-item Gospel Oak Questionnaire[33].
Statistical analyses
Associations between SLE, 5-HTTTLPR genotype and depression were initially measured by univariate analyses (Chi-square tests). Multivariate logistic regression was used to investigate the presence of G × E for depression occurring since repatriation. Models were fitted for the main effect of SLC6A4 (5-HTTLPR) genotype (included as L/L; L/S and S/S,) assuming a multiplicative effect for the S allele (LL = 0, SL/LS = 1, SS = 2) and environmental effect was classified as 0 (Control group), 1 (Repat), 2 (RepatT). The main effects of stress, genotype and interaction between genotype and stressor were investigated entering these variables as ordinal variables with Wald statistics analysed against 1 degree of freedom. All models were adjusted by age, gender, education, disability, cognitive function and recent life-events. Statistical analyses were carried out with SPSS 15.0 software (Chicago, Illinois).
Results
The mean (SD) age of the 880 participant was 72.5 years (SD=5.1) at baseline in the study with 62 % women. There was no significant age differences between the repatriated group (73.2 years; SD=5.6) and the control group (72.9 years; SD=5.5). Education levels were similar to those for the general elderly population (23.4 % low; 31.5 % medium-low; 23.1 % medium-high; 21.7 % high). Life-time prevalence of major or minor depression was 54 % in the whole sample (51.3% in the control group; 54.8% in the Repat group and 74.2 % in the RepatT group). Life-time prevalence of major depression alone was 23 % in the whole sample (21.4 % in the control group; 23.3 in the Repat group and 37.3 in the RepatT group). At baseline, repatriates show a significantly higher rate of current depression and/or antidepressant use (p<0.01) (23.1 % in the control group; 28.7 % in the Repat group; 42.2 % in the RepatT group).
The frequencies of the 3 5-HTTLPR genotypes were: LL= 27.2 % (239), SL=50.4 % (444) and SS= 22.4 % (197) which is consistent with those found in other European populations with no deviation from Hardy-Weinberg equilibrium (x2=1.00, df=1, p<0.41). As has been commonly observed previously, depression was associated with repatriation (p<0.001), but was not associated with genotype (p=0.62) (table 1). Kendall s non-parametric test showed no correlation between 5-HTTLPR genotype and exposure to repatriation (p=0.955)
Table 1.
Baseline DSM-IV life time depression and genotypes for the two repatriated groups (Repat and RepatT) and controls.
| Source | Total sample n=880 |
No Depression n=408 (46%) |
Depression n=472 (54%) |
X2 (linear) | p |
|---|---|---|---|---|---|
| Stressor : War events | |||||
| -Controls | 632 (71.8) | 308 (75.5) | 324 (68.6) | 12.07 | <0.002 |
| -Repat | 186 (21.1) | 84 (20.6) | 102 (21.6) | ||
| -RepatT | 62 (7.1) | 16 (3.9) | 46 (9.8) | ||
| 5-HTTLPR (%) | |||||
| -S/S | 239 (27.2) | 105 (26) | 135 (28.6) | 0.96 | 0.62 |
| -S/L | 444 (50.4) | 209 (51) | 234 (49.6) | ||
| -L/L | 197 (22.4) | 94 (23) | 103 (21.8) |
Control group: not repatriated and no trauma
Repat group: exposed to repatriation alone
RepatT group: exposed to repatriation plus war trauma
Logistic regression was used to examine the effect of the main covariates and interaction, with depression as the dependant variable. All factors were entered as ordinal variables with Wald statistics and models adjusted for age, gender, education, disability, cognitive function and recent life-events. War exposure (Wald: 12.71; p<0.001) and War exposure X 5-HTTLPR (Wald: 13.22; p<0.002) were statistically significant, but there were no significant effect of gene alone (Wald: 1.43; p=0.232). Stratified analyses showed that the association between war exposure score and depression was significant for s/s homozygotes (p<0.005, OR=2.34 [1.24–4.32]) and for heterozygotes S/L (p<0.004, OR=1.64 [1.16–2.31]) but not for L/L homozygotes (p=0.514)( table 2, figure 1). Sensitivity analysis conducted by grouping the Repat and RepatT groups also showed a significant War exposure X 5-HTTLPR (Wald: 5.5 p=0.01).
Table 2.
Logistic regression analysis of the interaction between War events and 5-HTTLPR genotype, in predicting life time depression.
| Models | Wald | p | OR (95 %CI) |
|---|---|---|---|
| War events | 12.71 | 0.001 | 2.58 (1.49–4.38) |
| 5-HTTLPR | 1.43 | 0.232 | 1.23 (0.92–2.14) |
| War events×5-HTTLPR | 13.22 | 0.002 | 3.21 (2.48–5.12) |
| Association between war events and depression Stratified by genotype : |
|||
| SS | 7.31 | 0.005 | 2.34 (1.24–4.32) |
| SL | 7.91 | 0.004 | 1.64 (1.16–2.31) |
| LL | 0.42 | 0.514 | 1.18 (0.72–1.96) |
All models were adjusted by age, gender, education, disability, cognitive functions and recent life-events.
Figure 1.
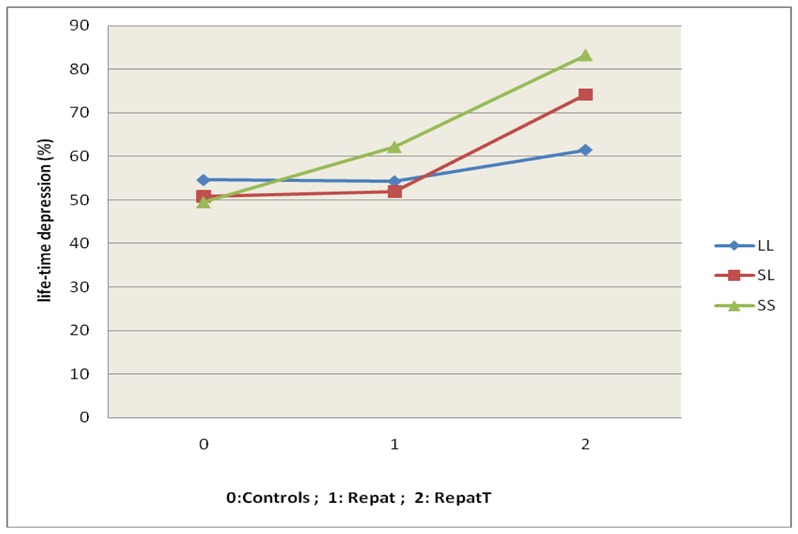
Interaction between the genotypes (S/S, S/L, L/L) and the number of war events with regard to depression.
Control group: not repatriated and no trauma
Repat group: exposed to repatriation alone
RepatT group: exposed to repatriation plus war trauma
We observed a significant third order interaction term: War exposure X 5-HTTLPR X Age (p<0.002). Gene-by-environment interaction was tested taking into account age by using stratified analyses with a 35 year cut-off (the mean age of the repatriated group in 1962). In this model, adjusted for covariates, war exposure X 5-HTTLPR was statistically significant for repatriation occurring before the age of 35 (p<0.001, OR=1.29 [1.11–1.5]) but not after 35 (p=0.067). The association between exposure and depression was significant for S/S homozygotes (p<0.002, OR=2.91 [1.44–5.88]) and heterozygotes (p<0.047, OR=1.57 [1.04–2.45]) but not for L/L homozygotes (p=0.267) (table 3).
Table 3.
Logistic regression analysis of the interaction between War events and 5-HTTLPR genotype, stratified by age group, in predicting life time depression.
| Models | Wald | p | OR ( 95 % IC) |
|---|---|---|---|
| War Events×5-HTTLPR×Age | 10.01 | 0.002 | 1.2 (1.1–1.4) |
| War Events | |||
| <35 years* | 10.66 | 0.001 | 1.73 (1.25–2.4) |
| ≥ 35 years** | 2.3 | 0.129 | 1.33 (0.92–1.91) |
| 5-HTTLPR | |||
| <35 years* | 1.81 | 0.178 | 0.86 (0.69–1.07) |
| ≥ 35 years** | 0.69 | 0.405 | 0.91 (0.73–1.13) |
| War Events ×5-HTTLPR | |||
| <35 years* | 10.44 | 0.001 | 1.29 (1.11–1.5) |
| ≥ 35 years** | 3.34 | 0.067 | 1.17 (0.98–1.39) |
| Association between war events and depression Stratified by genotype : |
|||
| SS | |||
| <35 years† | 8.87 | 0.002 | 2.91 (1.44–5.88) |
| ≥ 35 years†† | 2.88 | 0.089 | 2.02 (0.9–4.44) |
| SL | |||
| <35 years‡ | 3.92 | 0.047 | 1.57 (1.04–2.45) |
| ≥ 35 years‡‡ | 0.62 | 0.430 | 1.22 (0.74–2.03) |
| LL | |||
| <35 years§ | 1.23 | 0.267 | 1.55 (0.72–3.36) |
| ≥ 35 years§§ | 0.07 | 0.785 | 0.89 (0.4–2.01) |
All models were adjusted by age, gender, education, disability, cognitive functions and recent life-events.
n=442,
n=438;
n=120,
n=119;
n=224,
n=220;
n=99,
n=98
Discussion
To our knowledge this is the first study of gene-environment interaction which has used a stressful historical event as a common exposure as well as life-time follow-up of incident depression. We observed a significant relationship between exposure to this highly stressful event and depression onset [4–11,13,18]. As with previous research we found no significant relationship between depression and genotype, however, genotype was found to modulate the relationship between the stressful event and depression, with the presence of an S allele conferring higher risk. This relationship is further observed to be dose-related, with S homozygotes being at higher risk than heterozygotes, and being non-significant in LL subjects. LL homozygotes thus appear in our study to be resilient to stress-related depression. Most previous studies have combined heterozygotes and homozygotes in order to maximize statistical power; the few studies which have differentiated these have reported a similar intermediary position [10,13,34] or alternatively a dominant S effect [18,35,36] although two studies also report a recessive S [11,37].
We also examined the possibility that age at time of exposure may have had an effect on vulnerability to depression. Given that our source population was adult at the time of exposure, we were not able to examine an extensive age-range, but only a dichotomous classification of under and over 35 years at the time of repatriation. We observed exposure X gene effects to be stronger when the exposure occurred at the younger age, and to lose significance above 35 years. Our findings raise the possibility that younger adults may be more vulnerable to depression following exposure to repatriation, and that furthermore there may be a gene environment effect which diminishes with age. These observations should be considered as preliminary. Unfortunately from our data it was not possible to determine the exact age range at which subjects are most vulnerable or at what age the interaction effect begins to diminish, if indeed it does. Kim et al (2007)[18] found the presence of an S allele to increase risk of major and minor depression in elderly persons after stressful life events, whereas the British EPIC study [38] with a broader age range (41–80 years) found no increased risk linked to genotype. Within our own cohort, previous analyses suggest that the S allele has no interactive effect in relation to recent life events in relation to late-onset depression with a tendency rather for the L allele to increase vulnerability[16]. In a further study we observed that the association between late-onset depression and adverse childhood environment was also increased in the presence of an L allele[17], however, data was not available to determine whether this was still the case for depression occurring at younger ages closer to the event.
Our study does not resolve the on-going debate as to whether 5-HTTLPR genotype has a modulating effect on the relationship between life events and depression, however, it does suggest that, should this be the case, the relationship may be more complex than previous study designs have anticipated, depending on the age of exposure, the type of exposure (severity of the life event and also its chronicity) and depression sub-type (late or early onset). The role of the L allele in relation to late-onset depression also remains unclear. This study does not enable us to conclude with regard to these factors, but does indicate the importance of further exploring the effects of age and exposure type in relation to genetic vulnerability in future studies.
This study has a number of strengths: (i) subjects were exposed to a common event of civil war repatriation at a given time point thus reducing recall bias (ii) the temporal relationship between exposure to the stressful event and major depression onset is known and limited to hypotheses of one-way causality (iii) the diagnosis of depression is made with a standardized structured interview and not a self-administered questionnaire as has been the case with many previous studies (iv) life-time depression onset is known thus permitting us to assess impact of the stressor over a period of 40 years; studies with short-term follow-up risk type II errors due to delayed depression onset (v) life-time prevalence of depression is slightly higher (54 %) in our sample than in other European countries [23] giving sufficient statistical power to examine interaction effects which require a sample size four times greater than that necessary to detect the principal effect (environment) [39].
It is important to note, however, that our study has an important limitation in that past episodes of depression have been evaluated retrospectively, so that time of onset can only be estimated by reference to concurrent events. While we required validation of cases from a panel of psychiatrists, this was of course increasingly difficult with early onset cases. Retrospective diagnosis, even when based on DSM criteria, has been shown elsewhere to lead to an underestimation of prevalence [40] which in the case of our study would reduce the true significance of the association between exposure and depression and the genetic interaction. While reporting of depressive symptoms is open to recall bias, everyday clinical practice depends largely on this method.
Closing remarks
Our findings support the very large body of existing literature that shows repatriation, immigration and refugee status to be very significant risk factors for depression. In accordance with previous studies we found no direct relationship between depression and 5-HTTLPR genotype, however, persons in the study who were younger at the time of the exposure (under 35 years) were observed to more frequently report depression in their lifetime, and this risk further increased in the presence of an S allele. This relationship appears moreover to be dose-dependent, increasing with the number of S alleles. In cases where populations are exposed to this type of social trauma, our findings also suggest that younger adults with an S allele may be particularly vulnerable, and therefore possibly a priority group for intervention.
Acknowledgments
Funding/support. The ESPRIT Project is financed by the regional government of Languedoc-Roussillon, the Agence Nationale de la Recherche (ANR) and an unconditional grant from Novartis.
Role of the sponsors: None of the funding organizations or sponsors played a role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data or in the preparation, review or approval of the manuscript.
Footnotes
Authors Contributions: Dr Sylvaine Artero had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: Artero, Ritchie
Analysis and interpretation of data: Artero, Ritchie, Malafosse
Drafting of the manuscript: Artero, Ritchie, Malafosse
Critical revision of the manuscript for important intellectual content: Malafosse, Touchon, Ritchie, Dupuy
Financial disclosures. None reported
Declaration of interest: None
References
- 1.Mollica RF, Sarajlic N, Chernoff M, Lavelle J, Vukovic IS, Massagli MP. Longitudinal study of psychiatric symptoms, disability, mortality, and emigration among Bosnian refugees. Jama. 2001;286:546–54. doi: 10.1001/jama.286.5.546. [DOI] [PubMed] [Google Scholar]
- 2.Steel Z, Silove D, Phan T, Bauman A. Long-term effect of psychological trauma on the mental health of Vietnamese refugees resettled in Australia: a population-based study. Lancet. 2002;360:1056–62. doi: 10.1016/S0140-6736(02)11142-1. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg EL, Comstock GW. Epidemiology of life events: frequency in general populations. Am J Epidemiol. 1980;111:736–52. doi: 10.1093/oxfordjournals.aje.a112952. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 6.Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–9. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 7.Cervilla JA, Molina E, Rivera M, Torres-Gonzalez F, Bellon JA, Moreno B, Luna JD, Lorente JA, Mayoral F, King M, Nazareth I, Group PSC, Gutierrez B. The risk for depression conferred by stressful life events is modified by variation at the serotonin transporter 5HTTLPR genotype: evidence from the Spanish PREDICT-Gene cohort. Mol Psychiatry. 2007;12:748–55. doi: 10.1038/sj.mp.4001981. [DOI] [PubMed] [Google Scholar]
- 8.Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 9.Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–4. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–96. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 12.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 14.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenen KC, Galea S. Gene-environment interactions and depression. Jama. 2009;302:1859. doi: 10.1001/jama.2009.1575. author reply 61–2. [DOI] [PubMed] [Google Scholar]
- 16.Power T, Stewart R, Ancelin ML, Jaussent I, Malafosse A, Ritchie K. 5-HTTLPR genotype, stressful life events and late-life depression: No evidence of interaction in a French population. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie K, Jaussent I, Stewart R, Dupuy AM, Courtet P, Ancelin ML, Malafosse A. Association of adverse childhood environment and 5-HTTLPR Genotype with late-life depression. J Clin Psychiatry. 2009;70:1281–8. doi: 10.4088/JCP.08m04510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62:423–8. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychol Med. 1993;23:679–90. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- 20.Van Os J, Jones PB. Early risk factors and adult person--environment relationships in affective disorder. Psychol Med. 1999;29:1055–67. doi: 10.1017/s0033291799001026. [DOI] [PubMed] [Google Scholar]
- 21.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 22.Lindert J, Ehrenstein OS, Priebe S, Mielck A, Brahler E. Depression and anxiety in labor migrants and refugees--a systematic review and meta-analysis. Soc Sci Med. 2009;69:246–57. doi: 10.1016/j.socscimed.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie K, Artero S, Beluche I, Ancelin ML, Mann A, Dupuy AM, Malafosse A, Boulenger JP. Prevalence of DSM-IV psychiatric disorder in the French elderly population. Br J Psychiatry. 2004;184:147–52. doi: 10.1192/bjp.184.2.147. [DOI] [PubMed] [Google Scholar]
- 24.Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonara I, Sheehan K, Janavs J, Dunbar G. The Mini International Neuropsychiatric Interview (MINI), a short diagnostic interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:232–41. [Google Scholar]
- 25.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 26.Benton A. Manuel pour l application du test de retention visuelle. Applications cliniques et experimentales. Paris: Centre de psychologie appliqué; 1965. [Google Scholar]
- 27.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1965;8:271–6. [Google Scholar]
- 28.Isaac B, kennie AT. The Set test as an aid to the detection of dementia in old people. British Journal of Psychiatry. 1973;123:467–70. doi: 10.1192/bjp.123.4.467. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, Touchon J, Portet F, Ousset PJ, Vellas B, Michel B. “The 5 words”: a simple and sensitive test for the diagnosis of Alzheimer’s disease. Presse Med. 2002;31:1696–9. [PubMed] [Google Scholar]
- 30.Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–84. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 31.Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988 [PubMed] [Google Scholar]
- 32.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. Jama. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 33.Harwood RH, Prince MJ, Mann AH, Ebrahim S. The prevalence of diagnoses, impairments, disabilities and handicaps in a population of elderly people living in a defined geographical area: the Gospel Oak project. Age Ageing. 1998;27:707–14. doi: 10.1093/ageing/27.6.707. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, Blair IP, Parker G, Schofield PR. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–5. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- 35.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 36.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–6. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–9. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Cooper RS. Gene-environment interactions and the etiology of common complex disease. Ann Intern Med. 2003;139:437–40. doi: 10.7326/0003-4819-139-5_part_2-200309021-00011. [DOI] [PubMed] [Google Scholar]
- 40.Kruijshaar ME, Barendregt J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. 2005;20:103–11. doi: 10.1007/s10654-004-1009-0. [DOI] [PubMed] [Google Scholar]


