Abstract
Ellagic acid is a polyphenolic phytochemical present in many fruits and nuts with anti-cancer properties demonstrated in experimental tumor studies. Embelin is a benzoquinone phytochemical isolated from the Japanese herb Ardisiae Japonicae and has been shown to induce apoptosis in cancer cells. We found that ellagic acid and embelin each dose-dependently increased apoptosis and inhibited proliferation in human pancreatic cancer cells, MIA PaCa-2 and HPAF-II cells, and in pancreatic stellate cells (PaSCs) which are progenitors of pancreatic cancer desmoplasia. In each of these cell types, combinations of ellagic acid and embelin at low micromolar concentrations (0.5–3 μM) induced synergistic increases in apoptosis and decreases in proliferation. Ellagic acid decreased NF-κB transcriptional activity, whereas embelin decreased STAT-3 phosphorylation and protein expression of its downstream target survivin, in cancer cells. In vivo dietary ellagic acid alone or in combination with embelin decreased tumor size and tumor cellularity in a subcutaneous (s.c.) xenograft mouse model of pancreatic cancer. These results show that ellagic acid and embelin interact with divergent intracellular signaling pathways resulting in augmentation of apoptosis and inhibition of proliferation at low micromolar concentrations for the key cellular components of pancreatic adenocarcinoma.
Keywords: Ellagic acid, Embelin, pancreatic cancer, apoptosis, proliferation
Introduction
Pancreatic cancer is the fourth most common cause of death in Western countries with almost the same rate of incidence and mortality per year (1, 2). Pancreatic cancer is very resistant to radio- and chemo- therapies. The cancer is uniquely characterized by an abundant and dense desmoplastic reaction. The desmoplasia contains myofibroblastic pancreatic stellate cells (PaSCs) that produce large amounts of extracellular matrix (ECM) as well as cytokines, chemokines and growth factors that likely provide a microenvironment that promotes cancer cell growth (3–5). Evidence is emerging that there is a symbiotic relationship between pancreatic cancer cells and PaSCs of the tumor that results in an overall increase in the rate of growth of the tumor and possibly metastasis (6, 7). Therefore, the development of therapies targeting both cancer cells and stroma components such as the PaSCs has emerged as a necessary strategy.
Cancer cells protect themselves from cell death by up-regulating pro-survival mechanisms. One survival mechanism utilized by cancer cells consists of activation of the Jak2/STAT3 signaling pathway leading to up-regulation of the caspase inhibitors, inhibitor of apoptosis proteins (IAPs) (8). Activation of the transcription factor NF-κB is another major key pro-survival and anti-apoptotic mechanism in cancer cells (9, 10). NF-κB is constitutively active in pancreatic cancer cells, and its inhibition leads to pancreatic cancer cell death and inhibition of tumor growth (11).
Traditional herbal medicine is a rich resource for molecular target-specific drug discovery. Many herbal products (phytochemicals) have demonstrated anticancer activities in experimental models of human cancer, although their precise molecular mechanisms of action are still unclear. Ellagic acid (2,3,7,8-tetrahydroxy[1]benzopyrano[5,4,3,–cde][1] benzopyran-5,10-dione) is a polyphenol phytochemical present in a variety of fruits and nuts such as pomegranates, strawberries, raspberries, blackberries and walnuts. Accumulative evidence indicates that ellagic acid has anti-carcinogenic, antioxidant and anti-fibrosis properties in several cell types (12–15).
The anti-carcinogenic effects of ellagic acid have been reported in experimental models of skin, esophageal, and colon cancers (15, 16). However, the effects of ellagic acid on pancreatic cancer have not been studied. Furthermore, the mechanisms mediating the anti-cancer effects of ellagic acid, in general, remain unknown.
Embelin is a benzoquinone phytochemical derived from the Japanese herb Ardisiae Japonicae. Embelin has traditionally been used in Chinese medicine as anticancer agent as well as a contraceptive (17–19). Recent studies have demonstrated that embelin forms complexes with X-linked inhibitor of apoptosis protein (XIAP), a key member of the IAP family (20). Binding of embelin with XIAP prevents XIAP interaction with and inhibition of caspase-9. Embelin induced apoptosis in prostate cancer cells that display high levels of XIAP, but had minimal effect on normal prostate cells with low levels of XIAP, supporting a key role for XIAP in embelin mediated anticancer activities (20).
In the present study we investigated the effects of ellagic acid and embelin alone and in combination on human pancreatic cancer cells and mouse PaSCs. In addition, we investigated whether dietary ellagic acid and embelin reduced tumor growth in a xenograft mouse model of pancreatic cancer. Our data indicates that ellagic acid and embelin induce apoptosis and decrease proliferation in cultured pancreatic cancer cells and stellate cells. We found that ellagic acid inhibited proliferation and stimulated apoptosis in pancreatic cancer cells. Embelin alone inhibited STAT-3 activity as measured by its phosphorylation; whereas, combination of embelin and ellagic acid inhibited NF-κB transcriptional activity. Finally, we found that mice fed diets supplemented with ellagic acid and embelin have reduced xenograft tumor growth compared to mice fed control diets.
Material and Methods
Reagents
Antibodies against Bcl-xL, phospho-STAT3, survivin, and GAPDH were from Cell Signaling (Beverly, MA); survivin from Santa Cruz Biotechnology (Santa Cruz, CA). Ellagic acid and embelin were from chromadex (Irvine, CA). Ellagic acid for mouse diet was from Sigma-Aldrich (St. Louis, MO). All solvents used were HPLC grade (Fisher Scientific, Fairlawn, NJ). β-glucuronidase/sulfatase (type H-5 from Helix Pomatia) was purchased from Sigma-Aldrich. Internal standard 3, 3′, 4′-trihydroxyflavone was purchased from Indofine (Hillsborough, NJ). All other chemicals were from Sigma-Aldrich.
Cell culture
Human pancreatic ductal adenocarcinoma cells, the poorly differentiated MIA PaCa-2 (CRL-1420) and moderately differentiated HPAF-II cell (CRL-1997) lines, were obtained from the American Type Culture Collection (Manassas, VA). MIA PaCa-2 cells were grown in 1/1 DMEM/F-12 medium (Life Technologies, Grand Island, NY) supplemented with 15% FBS, 4 mM L-glutamine, and 1% antibiotic/antimycotic solution (25 μg/mL Amphotericin B, 10,000 U/ml of Penicillin G, 10,000 μg/mL of Streptomycin) from Omega Scientific (Tarzana, CA). HPAF-II cells were grown in DMEM supplemented with 20% FBS.
Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2 and used between passages 3 and 9. For the experiments, MIA PaCa-2 and HPAF-II cells were cultured for 72 h.
Pancreatic stellate cells were isolated from two month-old C57BL/6 mice as previously described (21). Briefly, pooled pancreata from 2–3 mice were digested with a mixture of collagenase P and pronase and separated by density gradient centrifugation (12% Nycodenz). The stellate cell type was confirmed by morphology (stellate-like or spindle-shaped cells) and positive staining for <-SMA and desmin. Cells were grown in 1/1 DMEM/F-12 medium supplemented with 15% FBS, 4 mM L-glutamine, and antibiotic/antimycotic solution. For the assays, cultured-activated primary cells or passage 1–2 cells were incubated in 1% FBS cultured medium.
Transfections
Transient transfections of MIA PaCa-2 cells were performed using the electroporation Amaxa System NucleofectorTM (Amaxa Inc, Gaithersburg, MD) according to the manufacturer protocol. To knockdown survivin, 400nM of survivin siRNA (Santa Cruz Biotechnology) was applied. Control cells were transfected with the Silencer Negative Control siRNA #1 (Ambion, Foster City, CA).
In vitro Proliferation Studies
Proliferation was assessed by measuring the level of 3Hthymidine incorporation into DNA and with MTT assay using Thiazolyl Blue Tetrazolium Bromide as substrate (Sigma-Aldrich, St. Louis, MO).
Measurement of apoptosis
Internucleosomal DNA fragmentation in cultured cells was measured by using Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals, Manheim, Germany) according to the manufacturer’s instructions (22–24).
Western blot analysis
Cells were re-suspended in RIPA phosphorylation buffer (50 mM NaCl, 50 mM Tris/HCl pH 7.2, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 10 mM Na2HPO4 + NaH2PO4, 100 mM NaF, 2 mM Na3VO4, 80 μM glycerophosphate, 20% glycerol, 1 mM PMSF, 5 μg/ml each of pepstain, leupeptin, chymostatin, antipain, and aprotinin), sonicated and centrifuged for 15 min at 16,000 × g at 4 °C. Protein extracts were resolved by SDS-PAGE for immunoblot analysis as previously described (22–24). The following primary antibodies were used: Bcl-xL, phospho-STAT3, survivin and GAPDH. Primary antibodies were recognized using specific horseradish peroxidase conjugated secondary antibodies (Biorad; Hercules, CA). Immuno-reactive bands were visualized by chemiluminescence (Pierce, Rockford, IL) in the FluorChem-HD2 imager, and densitometrically quantified using FluorChem software (Alpha Innotech; Santa Clara, CA).
NF-κB-Transcriptional activity measurement
NF-κB transcriptional activity was assessed with the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison WI). Briefly, MIA PaCa-2 cells were simultaneously co-transfected with the pGL3-4κB-Luc and pRL-TK plasmids by using the Nucleofector™ II System (Amaxa, Inc) according to the manufacturer’s protocol. pGL3-4κB-Luc contains the reporter gene encoding firefly luciferase driven by a promoter region containing 4 copies of NF-κB responsive element. pRL-TK is a Renilla luciferase driven by a basic thymidine kinase promoter thereby playing the role of a reference plasmid.
Diets and in vivo tumor growth inhibition studies
Six week-old male athymic nude mice (Nu/Nu; Charles River Laboratories, Wilmington, MA) were used for the subcutaneous (s.c.) xenograft model of pancreatic cancer (25). One week before tumor implantation, mice were randomly assigned to the following dietary groups (6 mice per group): control group, standard diet (AIN-76A, Dyets Inc); EA group, standard diet supplemented with ellagic acid (150 mg/kg diet; equivalent to a daily dose of 25 mg/kg body weight); EM group, standard diet supplemented with embelin (450 mg/kg diet equivalent to a daily dose of 75 mg/kg body weight), and EA+EM group, standard diet supplemented with ellagic acid and embelin (150 and 450 mg/kg diet, respectively). Based in allometric calculations of body surface area, if the daily dose for mice is 25 mg/kg of body weight for ellagic acid and 75 mg/kg of body weight for embelin, then the estimated daily dosage for human intake is 5.3 mg/kg body weight and 16 mg/kg body weight, respectively.
Stability of the phytochemicals in the diets was evaluated by HPLC in day 0 (as control), 1, 2 and 3 by HPLC following vigorous extraction with ethyl acetate containing BHT as an antioxidant (embelin) or with 70% aqueous methanol containing 1% of HCl (ellagic acid). Percentage of recovery of embelin in freshly prepared diet was 100%, and 88% for ellagic acid. After 3 days at room temperature, the percentage of recovery was reduced to 90% for embelin and 85% for ellagic acid. Therefore, freshly prepared diet containing the phytochemicals was provided to the mice every other day.
After one week on diets, each mouse was inoculated in the right flank region by subcutaneous injection with the human pancreatic tumor cell line HPAF-II (ATCC # CRL-1997; 2 × 106 HPAF-II cells suspended in 0.2 ml of culture medium). After tumor implantation, mice continued on diets for 5 weeks more. Mice were monitored daily for general health status, food consumption and measurements of external tumor size. Body weight was evaluated two times per week. The tumor volume was evaluated by caliper measurements of the perpendicular diameters using the following formula: [½length*½width*½depth]*3.14*4/3. After 5 weeks on diets, mice were sacrifice and tumors measured, weighted and excised for analysis (snap-frozen for measurements of tissue levels of phytochemicals, and fixed in formalin for histological analysis). In addition, blood samples, collected by heart puncture, and liver tissues were harvested for the determination of levels of the phytochemicals and toxicity.
All experimental procedures were conducted according to animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Los Angeles, and in compliance with guidelines of the American Association of Laboratory Animal Care.
Measurement of embelin and ellagic acid content in diets, blood and tumors
HPLC analysis of the embelin was performed with a Inertsil ODS-4 column (150 × 4.6 mm, 3 μm, GL Sciences, Torrance, CA) on an Agilent 1100 HPLC system (Santa Clara, CA) comprised of an auto-sampler and quaternary pump coupled to a photodiode array detector. The mobile phase consisted of a binary gradient of 0.1% (v/v) ortho-phosphoric acid in water (eluent A) and acetonitrile (eluent B), used with a flow rate of 0.6 mL/min in the following conditions: 20% B (0–3 min); 20–55% B (3–10 min); 55–98% B (10–18 min); 98%B (18–25 min), and 98–20% B (25–32 min). For the analysis of the ellagic acid, the mobile phase consisted of a binary gradient of 0.2% (v/v) ortho-phosphoric acid in water (eluent A) and methanol (eluent B), used with a flow rate of 0.5 mL/min in the following conditions: 20% B (0–5 min); 20–85% B (5–20 min); 85% B (20–21.5 min) and 85–20%B (21–28 min). The column temperature was held at 30° C. The chromatograms were recorded at 286 nm for embelin and 254 nm for ellagic acid. Data were analyzed with the Hewlett Packard Chemstation® software. Concentration and stability of embelin and ellagic acid in animal diets were determined by HPLC using external calibration. Animal plasma and tissue concentrations were determined by internal calibration. Calibration standards were prepared from the stock solutions by serial dilution. For all calibration curves, there was a linear relationship between peak area and concentration in the range of 0.4 – 32 and 0.2–11.5 μg/mL for embelin and ellagic acid, respectively.
Histological Analysis
Histological analysis of tumor and liver sections was performed at the Pathology core of the UCLA Center for Excellence in Pancreatic Diseases. Formalin-fixed tissue sections from at least 4 animals per group were embedded in paraffin. Tumor cellularity was assessed in hematoxylin and eosin (H&E) stained tissue sections as the percentage of tumor area that was occupied by epithelial cancer cells. Necrosis within the tumor area was determined by measuring the extent of areas with loss of cellularity. Cellularity and necrosis were evaluated by morphometric analysis using MetaMorph imaging system (Universal Imaging Corporation, PA) in digitized pictures obtained from multiple random, non-overlapping sections under a high power field (x 400-magnification, 10–12 random fields per section). Images were captured with all exposures manually set at equal times with a Nikon Eclipse E600 microscope equipped with a digital camera using the SPOT imaging software (Diagnostic Instruments, MI).
Embelin extraction from mouse plasma and tissue
To the mouse plasma samples (100 μL) was added 10 μL Internal standard 3, 3′, 4′-trihydroxyflavone (100 μM). The sample was then extracted vigorously with 200 μl of acetone twice. Combined supernatant was dried with a SpeedVac, re-dissolved in acetone/H2O (80:20), vortexed, and then sonicated. A 50 μL aliquot of the mixture was injected into the HPLC. For analysis of mouse tissue, frozen tissue (0.1–0.2 g) was weighed and homogenized in isotonic buffer containing using a tissue grinder, and internal standard was added. The mixture was then extracted with acetone similarly as plasma samples.
Ellagic acid extraction from mouse plasma and tissue
Extraction of ellagic acid from mouse plasma and tissue samples was performed similarly as described previously (26). We found enzymatic hydrolysis released more ellagic acid compared with direct extraction or with acid hydrolysis from plasma samples but not from tissue samples. Frozen tissue (0.2–0.3 g) was weighed and homogenized 1 ml of 70% aqueous methanol containing 1% HCl solution. Another 1 ml was used to rinse the homogenizer. The resulting mixture was centrifuged and the pellet re-extracted twice. Supernatants were combined and dried. The residue was reconstituted in 100 μL of MeOH/H2O 80:20 and injected into the HPLC. Ellagic acid and its metabolites urolithins were not detected in nude mouse plasma or tissues.
Other analyses
To determine in vivo toxicity of ellagic acid and embelin in mice carrying xenograft pancreatic tumors, liver and kidney function were assessed by blood chemistry analysis (Division of Laboratory Animal Medicine, University of California, Los Angeles).
Statistical analysis
Data from at least 3 independent experiments or 6 mice per group (for studies using xenograft tumors) are presented by bar charts and expressed as means ± SEM (standard error of the mean). One-sided two-sample t-tests (Fig. 3C, 6A, 6B, 7B and 7C) and one-sided one-sample t-tests (others) were used to compare ellagic acid, embelin and the combination of the two phytochemicals with controls. The synergistic effects of the combination of ellagic acid and embelin were analyzed by using linear regression models. We considered there is a synergistic effect when the effect of the combined treatment is significantly greater than the sum of the individual treatments (embelin or ellagic acid). The rate of tumor volume growth was analyzed using a mixed effects linear regression model with random intercept and random slope (27).
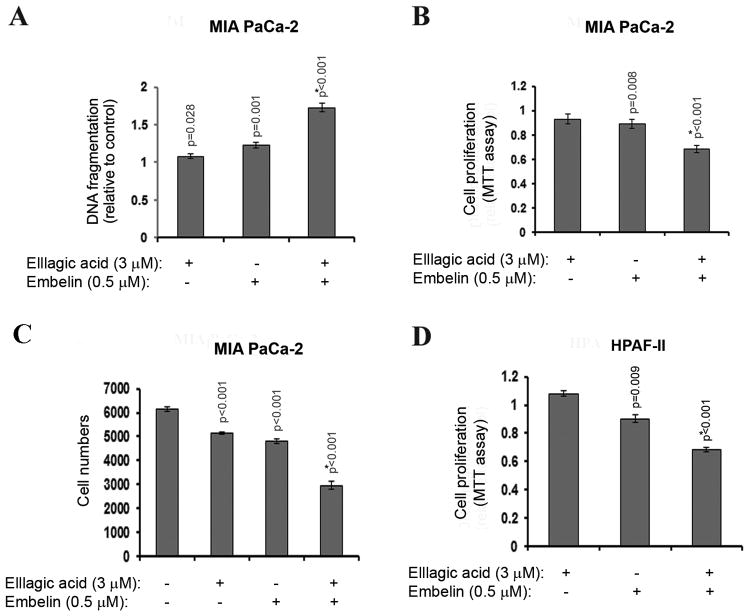
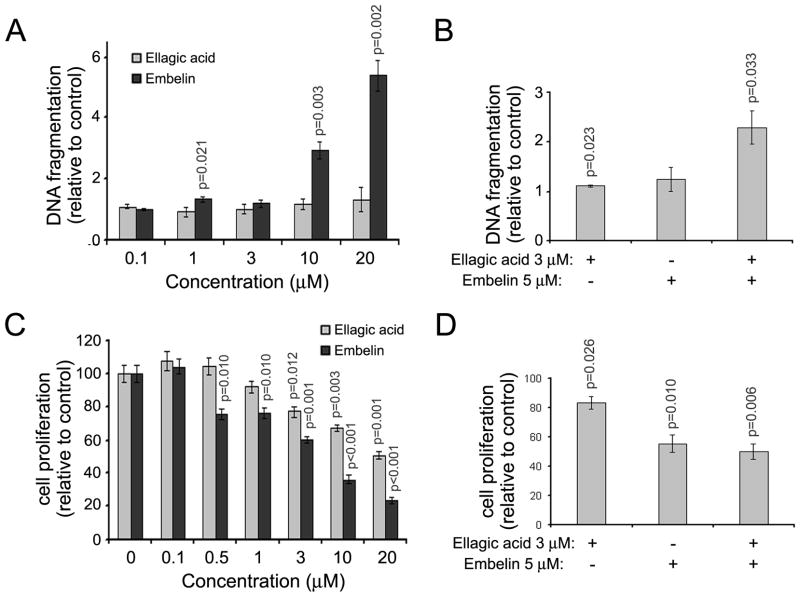
Figure 3. The effects of embelin and ellagic acid on apoptosis and proliferation are synergistic.
MIA PaCa-2 (A–C) and HPAF-II (D) pancreatic cancer cells were cultured for 72h in the presence of indicated doses of embelin or ellagic acid or combination of both compounds. (A) Apoptosis was assessed by measuring internucleosomal DNA fragmentation by ELISA. Proliferation was assessed by MTT assay (B, D) or cell counting (B). For A, B and D, the measures are relative to control. P-values are for one-sided one-sample (for A, B and D) and two-sample (for C) t-tests comparing with controls (1 for A, B and D). P* is the p-value for testing the synergistic effect. A synergistic effect is defined as that the mean difference between controls and the combination group is greater than the sum of the mean differences between controls and embelin group and between controls and ellagic acid group. With Bonferroni’s adjustment, p<0.0125 are considered as significant.
Figure 6. Dietary ellagic acid or embelin had minor effects on body weight gain or food consumption in a xenograft mouse model of pancreatic cancer.
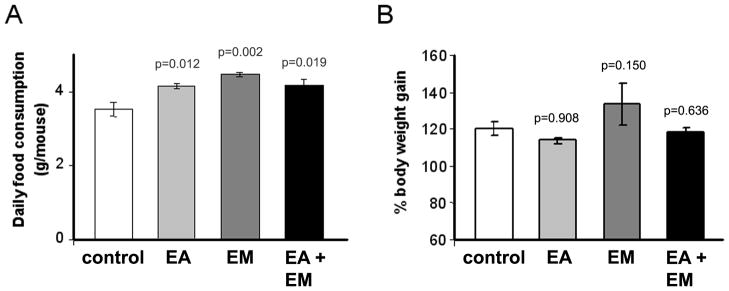
Nude male mice were randomly distributed in 4 diet groups: standard diet (control group); standard diet supplemented with ellagic acid (150 mg/kg diet; EA group), embelin (450 mg/kg diet; EM group) or with ellagic acid plus embelin (EA+EM group). After one week on diets, 2×106 HPAF-II cells were injected s.c. into the right flank of the mice. Mice continued on diets for 5 weeks more and then sacrificed. (A) Graph shows average daily food consumption during the 6-week feeding period. Bars represent mean ± SEM of 6 mice per group. (B) Graph shows percentage of body weight gain at the end of the feeding period compared to initial body weight values (mean ± SEM; 4–6 mice per group).). P-values are for one-sided two-sample t-tests comparing with controls. The synergistic effects (defined similar to Fig. 3) were tested. With Bonferroni’s adjustment, p<0.0125 is considered statistically significant. As indicated by the p-values, statistical analysis revealed a significant increase in food consumption in mice on ellagic acid or embelin diets (6A). Not significant changes were found in body weight gain in any of the dietary groups.
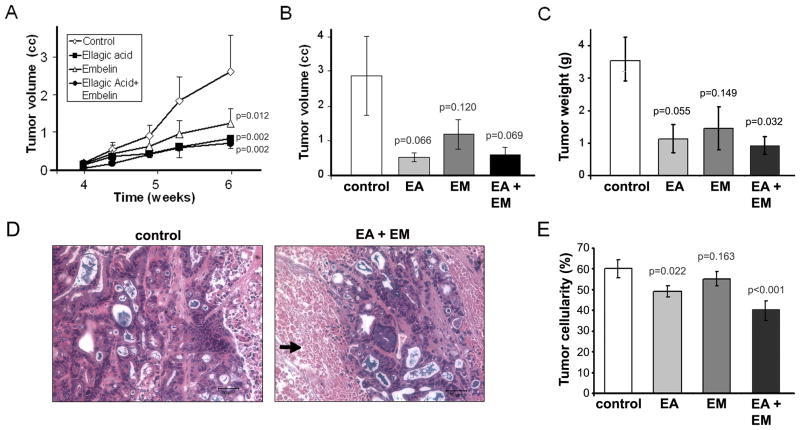
Figure 7. Ellagic acid alone or in combination with embelin significantly reduces pancreatic tumor volume in a mouse xenograft model.
Inhibition of HAPF-II s.c. tumor growth was measured in mice fed diets supplemented with ellagic acid (EA), embelin (EM), or a combination of ellagic acid and embelin (EA+EM). Mice fed standard diet without phytochemical supplementation were used as controls. Diets started one week before implantation of tumor cells and continued for 5 weeks more. Mice were sacrificed at the end of the 6-week period due to large tumors in most control mice. Values in graphs (panels A, B, C, and E) represent mean ± SEM; n= 4–6. For A, p-values are for one-sided tests comparing the slopes of the treatment groups to the slope of the controls. For B, C and E, p values are for one-sided two-sample t-tests comparing with controls. The synergistic effects (defined similar to Fig. 3) were tested. With Bonferroni’s adjustment, p<0.0125 is considered statistically significant. (A) Graph illustrates tumor growth during the last two weeks. Values show external tumor volume measured two times per week (mean ± SEM; n= 6). Compared to controls, tumor growth was significantly reduced in mice fed diets supplemented with ellagic acid (p=0.002), embelin (p=0.012) or a combination of ellagic acid and embelin (p=0.002). No synergistic effects were found between ellagic acid and embelin (p=0.959). (B) Graph shows internal tumor volume at sacrifice (6 weeks on diets). Tumor volume at sacrifice was reduced in mice on ellagic and/or embelin diets but the differences compared to controls did not reach statistical significance (P<.05). No synergistic effects were found between ellagic acid and embelin (p=0.955). (C) Tumor weight at sacrifice was reduced in mice on ellagic and/or embelin diets but the differences compared to controls only reach statistical significance (P<.05) in mice fed a combination of ellagic acid and embelin (P=0.03). No synergistic effects were found between ellagic acid and embelin (p=0.546). (D) Representative H&E staining in tumor tissue sections from mice fed for 6 weeks control diet or diet supplemented with ellagic acid and embelin. Pictures were taken from the center of the tumor. Compared to controls, tumors in mice fed EA+EM displayed extensive areas with low cellularity (black arrow). Bars, 50 μm. (D) Tumor cellularity was measured in H&E stained tumor tissue sections by morphometric analysis of the total area occupied by tumor cells. Graphs show mean ± SEM of values obtained in at least 10 randomly selected histological sections per mouse; data obtained from 3 mice per group were analyzed. As indicated in the figure, cellularity within the tumor significantly decreased in mice fed diets supplemented with ellagic acid (p=0.022) alone or in combination with embelin (p<0.001). No synergistic effects were found between ellagic acid and embelin (p=0.319).
Bonferroni’s adjustment was made to control the overall Type I error rate at 0.05. Thus, p-values smaller than 0.05/k are considered as statistically significant, where k is the total number of statistical tests performed for the experiment. All statistical analyses were performed using SAS software (Version 9.2; SAS Institute Inc., Cary, NC).
Results
Ellagic acid and embelin dose-dependently stimulate apoptosis in pancreatic cancer cells
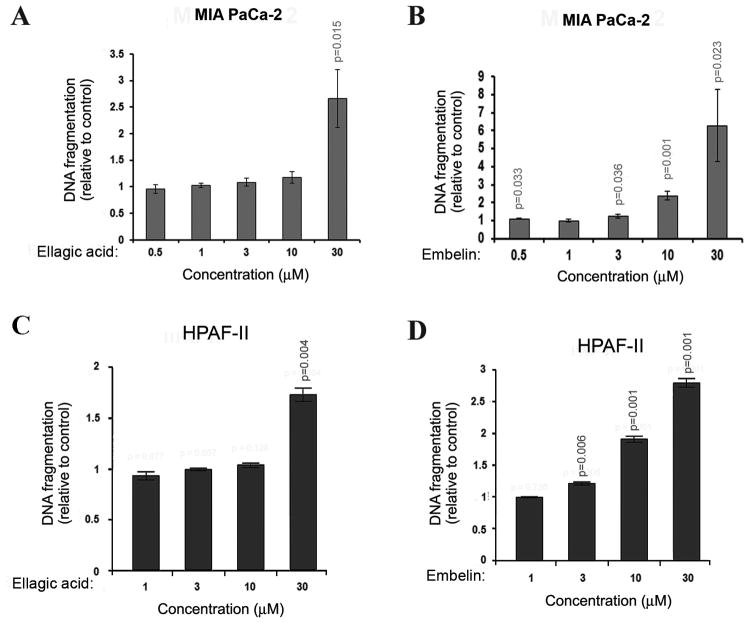
To determine the effect of ellagic acid and embelin on apoptosis in pancreatic cancer cells we cultured undifferentiated MIA PaCa-2 and moderately differentiated HPAF-II cells in the presence of different concentrations of ellagic acid or embelin for 72h and measured the level of DNA fragmentation. Ellagic acid dose-dependently increased the level of DNA fragmentation in both cell lines. Ellagic acid significantly stimulated apoptosis in MIA PaCa-2 cells at the concentration of 10μM compared to 30μM in HPAF-II cells (Fig. 1A, C). Embelin induced a dose dependent increase in apoptosis (Fig. 1B, D) and was more potent than ellagic acid in inducing DNA fragmentation in both cell lines. Embelin significantly stimulated DNA fragmentation at concentrations as low as 0.5 μM in MIA PaCa-2 and 3μM in HPAF-II cells (Fig. 1B, D).
Figure 1. Ellagic acid and embelin dose-dependently stimulate apoptosis in pancreatic cancer cells.
MIA PaCa-2 (A, B) and HPAF-II (C, D) pancreatic cancer cells were cultured for 72h in the presence of indicated doses of embelin or ellagic acid. Apoptosis was assessed by measuring internucleosomal DNA fragmentation by ELISA. P-values are for one-sided one sample t-tests comparing with 1 (controls). With Bonferroni’s adjustment, p<0.01 are considered as significant for A and B, p<0.0125 are considered as significant for C and D.
Ellagic acid and embelin dose-dependently inhibit proliferation in pancreatic cancer cells
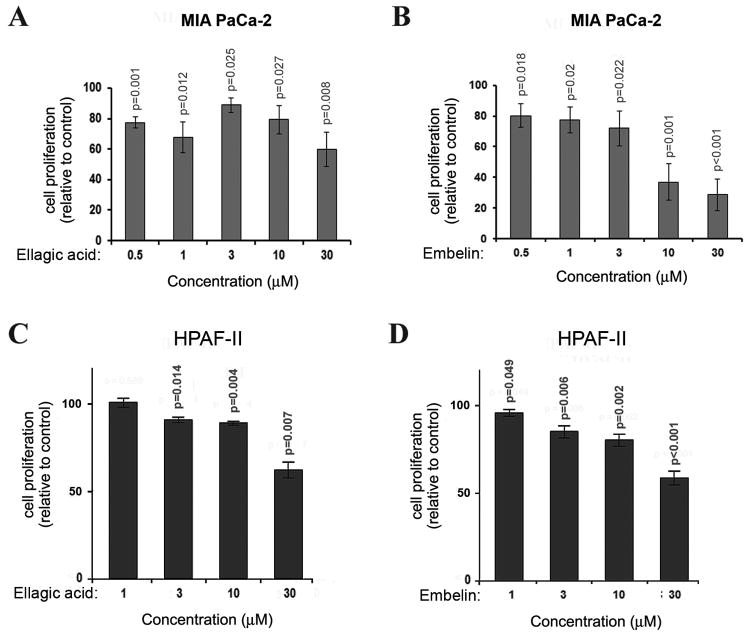
To determine the effect of ellagic acid and embelin on proliferation in pancreatic cancer cells we cultured MIA PaCa-2 and HPAF-II cells in the presence of ellagic acid or embelin for 72h and measured the level of 3H-thymidine incorporation into DNA. Both phytochemicals dose dependently inhibited proliferation in the two cell lines under study (Fig. 2). The phytochemicals induced a significant decrease in proliferation at the concentration of 0.5 μM in MIA PaCa-2 cells (Fig. 2A, B). The effect on HPAF-II was reached at 1.0 μM for embelin (Fig. 2D) and 3.0 μM for ellagic acid (Fig. 2C).
Figure 2. Ellagic acid and embelin dose-dependently inhibit proliferation in pancreatic cancer cells.
MIA PaCa-2 (A, B) and HPAF-II (C, D) pancreatic cancer cells were cultured for 72h in the presence of indicated doses of embelin or ellagic acid. Proliferation was assessed by measuring 3H-thymidine incorporation into DNA. P-values are for one-sided one-sample t-tests comparing with controls (100 for A and B, 1 for C and D). With Bonferroni’s adjustment, p<0.01 are considered as significant for A and B, p<0.0125 are considered as significant for C and D.
Data in figures 1 and 2 indicated that ellagic acid and embelin dose-dependently stimulated apoptosis and inhibited proliferation in pancreatic cancer cells. The effects were at observed at low micromolar concentrations, especially in MIA PaCa-2 cells. We then checked whether the combination of the two phytochemicals would have synergistic effects on apoptosis and proliferation.
Combination of ellagic acid and embelin induce a synergistic effect on apoptosis and proliferation in pancreatic cancer cells
To determine the effect of treatment with a combination of ellagic acid and embelin on apoptosis and proliferation of pancreatic cancer cells we cultured MIA PaCa-2 and HPAF-II cells in the presence of different concentrations of ellagic acid and embelin and measured the effect on apoptosis and proliferation. Combinations of ellagic acid at 3μM and embelin at 0.5μM induced synergistic effects on apoptosis as measured by DNA fragmentation level in MIA PaCa-2 cells (Fig. 3A) and on proliferation as measured by MTT assay (Fig. 3B). Counting of cell numbers confirmed these data by showing that combination of ellagic acid and embelin decreases MIA PaCa-2 cell number to a level significantly lower from the level induced by each compound alone (Fig. 3C). Similarly to MIA PaCa-2 cells, combination of ellagic acid (3 μM) and embelin (0.5μM) induced a synergistic effect on proliferation of HPAF-II cells (Fig. 3D).
Ellagic acid and embelin down-regulate NF-κB and STAT3 pro-survival pathways
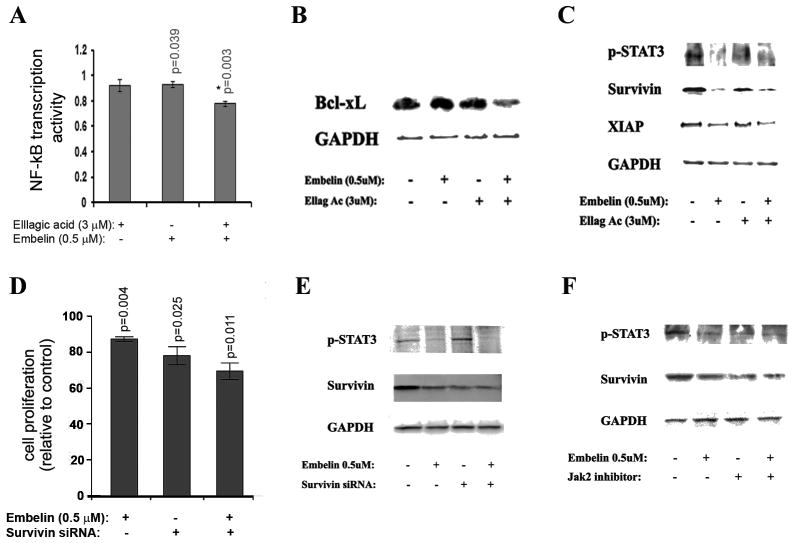
We have previously published that ellagic acid at high concentrations (more than 10μM) induced apoptosis in pancreatic cancer cells through a mechanism that involves regulation of transcription factor NF-κB (28). Therefore; we measured the effect of ellagic acid and embelin on NF-κB activity in MIA PaCa-2 cells. We found that low concentrations of ellagic acid (3 μM) and embelin (0.5 μM) alone do not affect NF-κB transcription activity. However, the combination of the two compounds significantly decreased NF-κB transcription activity (Fig. 4A) indicating a synergistic effect of the two compounds leading to a decrease in NF-κB activity. Furthermore, we found that ellagic acid and embelin alone did not affect the protein level of antiapoptotic Bcl-xL and that the combination of the two compounds did cause a decrease in the protein level of Bcl-xL (Fig. 4B). Published data indicate that embelin prevents the antiapoptotic effect of XIAP by blocking the interaction of XIAP with caspases (29). Our data indicate that embelin down-regulated protein expression of survivin (Fig. 4C).
Figure 4. Embelin and ellagic acid inhibit NF-kB and STAT3 pathways.
(A–C) MIA PaCa-2 cells were cultured for 72h in the presence of indicated doses of embelin or ellagic acid or Jak inhibitor AG490 (50 μM). (A) NFκB binding activity was measured by dual luciferase reporter assay (Promega). (D) Proliferation was assessed by measuring 3H-thymidine incorporation into DNA. (D, E) Cells were transfected with survivin or control siRNA using the electroporation Amaxa System Nucleofector. P-values in A and D are for one-sided one-sample t-tests comparing with 1 (controls). P* is the p-value for testing the synergistic effect (defined similar to Fig. 3). With Bonferroni’s adjustment, p<0.0125 are considered as significant.
The regulation of survivin protein level is most likely through down-regulation of the phosphorylation level of STAT3 (Fig. 4C). To confirm that the regulation of survivin level by embelin is mediated by STAT3 phosphorylation we cultured cells in the presence of the Jak/STAT inhibitor AG490 and found that the Jak inhibitor decreased the level of survivin and that combination of AG490 and embelin did not further decrease the protein level of survivin (Fig. 4F). Embelin slightly further reduced proliferation in cells transfected with survivin siRNA compared to control siRNA (Fig. 4D). The anti-proliferation effect of combined embelin and survivin siRNA was smaller than the additive effect of each one alone suggesting that embelin regulates proliferation in pancreatic cancer cells, at least in part, through the survivin pathway.
Ellagic acid and embelin induce apoptosis and decrease proliferation in cultured mouse pancreatic stellate cells
To determine the effect of ellagic acid and embelin on PaSCs, cultured-activated primary mouse stellate cells were treated for up to 72 h with ellagic acid and embelin alone or in combination. Embelin induced a dose- (Figure 5A) and time-dependent (not shown) increase in stellate cell apoptosis as determined by DNA fragmentation assay. As shown in Figure 5A, embelin at 10 and 20 μM concentrations significantly augmented apoptosis by 3 and 6-fold, respectively, compared to control. Ellagic acid, at concentrations ranging from 0.1 to 20 μM had no effect on stellate cell apoptosis. However, the combination of ellagic acid and embelin at concentrations that by themselves had minor effects on apoptosis (5 μM embelin and 3 μM ellagic acid), synergistically increased DNA fragmentation by two-fold compared to individual treatment with the phytochemicals (Figure 5B).
Figure 5. Ellagic acid and embelin induce apoptosis and decrease proliferation in cultured mouse pancreatic stellate cells.
Culture-activated mouse pancreatic stellate cells were incubated for 72 h in 1% FBS-containing medium in the presence or absence of embelin and/or ellagic acid. (A and B) Apoptosis was assessed by measuring DNA fragmentation (Roche ELISA). (C and D) Cell proliferation was estimated by MTT assay. Graphs showed mean ± SEM of 3–4 independent studies. P-values are for one-sided one-sample t-tests comparing with controls (1 for A and B, 100 for C and D). For A and C, the effects of ellagic acid and embelin were tested separately. For B and D, the synergistic effects (defined similar to Fig. 3) were tested. With Bonferroni’s adjustment, p<0.01 is considered as significant in A, p<0.0071 are considered significant in C, and p<0.0125 are considered significant in B and D. P-values greater than 0.05 are indicated in the figures. At the indicated doses, Ellagic acid and embelin had a synergistic effect on apoptosis (5B; p=0.033) but not on cell proliferation (5D; p=0.999).
Stellate cell proliferation was significantly reduced after 72h -incubation with either embelin or ellagic acid at starting concentrations of 3 μM, although embelin was more potent than ellagic acid (Figure 5C). Differently from the effects observed on apoptosis, the combination of embelin (5 μM) and ellagic acid (3 μM) had no synergistic effects on stellate cell proliferation (Fig. 5D). Treatment of cultured-activated mouse PaSC with ellagic acid or embelin for 24 or 48h did not change protein expression levels of fibronectin or alpha-smooth muscle expression as measured by Western blotting and expressed relative to total proteins (Fig. 8). These data suggest that the phytochemicals do not alter pathways involved in synthesis/degradation of these proteins. Therefore, treatment with ellagic acid or embelin may reduce production of ECM proteins in tumors by decreasing the number of active PaSC but not to by directly decreasing protein production.
Figure 8. Ellagic acid and embelin had minor effects on fibronectin production in cultured mouse pancreatic stellate cells.
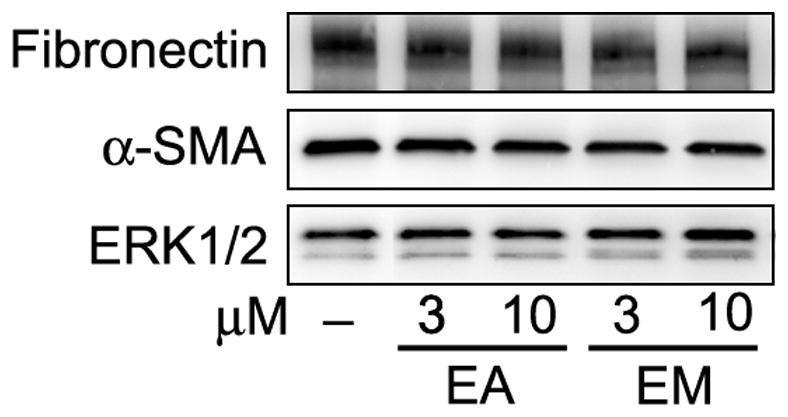
Culture-activated mouse pancreatic stellate cells were incubated for 48 h in 1% FBS-containing medium in the presence or absence of embelin and/or ellagic acid at the indicated concentrations. Protein levels of fibronectin and α-smooth muscle actin (a marker of the activated phenotype of the stellate cell) were determined by Western blotting analysis. Levels of ERK1/2 were used as loading control. Immunoblot is representative of 3 independent experiments.
Ellagic acid and embelin diets reduce tumor growth in mouse xenograft model of pancreatic cancer
To assess the in vivo effects of ellagic acid and embelin on pancreatic tumor growth we used the HPAF-II xenograft model as described in the Material and Methods section. Nude mice were fed standard diets enriched with embelin alone (450 mg/kg diet), ellagic acid alone (150 mg/kg diet) or a combination of ellagic acid and embelin. Based in daily food consumption, we estimated that the mice received an average of 2 mg/mouse/day embelin (EM dietary groups) and 0.7 mg/mouse/day ellagic acid (EA dietary groups). After one week on diets, human pancreatic HPAF-II cells were inoculated by subcutaneous injection in the right flank region of each mouse, and mice continued on diets for 5 weeks more. Five weeks post-inoculation, mice were sacrificed due to the large tumor size in mice in the control group. Average daily food consumption was slightly greater in mice fed diets supplemented with ellagic acid and/or embelin compared to mice fed control diet (Fig. 6A), however we did not find differences in body weight gain between mice in any dietary group (Fig. 6B). As illustrated in Fig. 6B, after 6 weeks on diets mice gained approximately 20% of their initial body weight irrespectively of the dietary group or the tumor size. Blood analyses at sacrifice indicated normal liver and kidney function in all animals in the dietary groups (Table 1), suggesting that the phytochemicals were well-tolerated and did not induce apparent toxicity.
Table 1.
Blood chemistry in mice fed diets supplemented with ellagic acid and/or embelin
| Control | EA | EM | EA+EM | ||
|---|---|---|---|---|---|
| Albumin | g/dL | 2.1±0.1 | 2.1±0.0 | 2.2±0.1 | 2.3±0.1 |
| T-bilirubin | mg/dL | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| Gamma glutamyl Transferase (GGT) | U/L | 10.3±2.1 | 7.5±1.1 | 9.5±1.8 | 9.8±1.2 |
| Protein-total | g/dL | 4.6±0.2 | 4.7±0.1 | 4.7±0.1 | 5.0±0.2 |
| Blood urea nitrogen (BUN) | mg/dL | 15.3±1.5 | 19.0±0.8 | 20.5±1.5 | 22.2±1.2 |
| Creatinine | mg/dL | 0.2±0.0 | 0.3±0.0 | 0.3±0.0 | 0.3±0.0 |
| Alanine aminotransferase (ALT) | U/L | 24.3±3.2 | 45.2±15.9 | 24.8±2.3 | 33.8±3.9 |
| Alkaline phosphatase (ALP) | U/L | 53.0±3.2 | 68.5±4.8 | 59.7±3.5 | 72.5±5.1 |
| Aspartate aminotransferase (AST) | U/L | 188.0±51.2 | 154.0±28.4 | 128.2±24.6 | 116.3±8.6 |
| Lactate dehydrogenase (LDH) | U/L | 714.3±235.5 | 473.8±129.2 | 465.7±141.1 | 347.7±79.3 |
Data: mean±SEM (n=6)
Nude male mice were fed for 6 weeks control diets (control) or diets supplemented with ellagic acid (EA), embelin (EM), or a combination of ellagic acid and embelin (EA+EM). Xenograft s.c. tumors were implanted in each mouse one week after diet initiation. Mice were kept on diets for 5 more weeks and then sacrificed. Table shows blood chemistry for the selected parameters at sacrifice. Values represent mean ± SEM of 6 animals in each group; there are no significant differences between groups (Bonferroni t test).
All blood values were within normal range, indicating normal liver and kidney function. These data suggest that the phytochemicals were well tolerated and did not induce apparent toxicity.
Dietary ellagic acid and embelin alone or in combination significantly reduced tumor growth in nude mice. As illustrated in Fig. 7A, compared to control mice, tumor growth in mice fed diets supplemented with ellagic acid alone or in combination with embelin was significantly reduced during the last 3-weeks on diets. At sacrifice, the average volume of the resected tumors was lower in the ellagic acid groups compared to the control group and embelin group, although the differences between groups did not reach statistical significance (P<0.05; Fig. 7B). Tumor weight was also lower in the ellagic acid groups, and, compared to controls, reached significantly differences in mice fed a combination of ellagic acid and embelin (P<0.032; Fig. 7C). Of note, although tumors in the control group were solid, some tumors in mice fed with the ellagic acid and embelin were soft and filled with fluid. Consistent with this, histological analysis of H&E stained tumor tissue sections revealed a significant reduction in tumor cellularity and increased necrosis in mice fed diets supplemented with ellagic acid or a combination of embelin and ellagic acid (Fig. 7C). Of note, we did not seen considerable change in the level of apoptosis in tumors most likely because of the death of cells through necrosis. In conclusion, administration of ellagic acid and embelin at the indicated doses was well-tolerated by the mice and showed an inhibitory effect on pancreatic tumor growth.
To determine tissue distribution of ellagic acid and embelin, we performed HPLC analysis of blood, liver and tumor tissue samples from mice fed for 6 weeks control diet or diets supplemented with the phytochemicals. After 6 weeks on diets, embelin was detected in plasma (0.21±0.05 μg/ml; mean±SEM; 5 mice per group) but not in liver or tumor tissues. Ellagic acid was undetectable in plasma or other tissues. In previous pilot studies, we found that plasma levels of embelin peaked at one hour after gavage administration of embelin at 70 mg/kg body weight to mice (3.55 ± 0.13 μg/ml; mean ± SEM, n=3) to greatly decline at 3 h after administration (0.26 ± 0.06 μg/ml). Similarly, we found that ellagic acid could be detected in rat plasma at low levels (40 ng/ml), but only within 1 hour of gavage administration of ellagic acid at doses much higher (400 mg/kg) than the ones used in the current study (25 mg/kg). These data suggest that rapid metabolism of ellagic acid and embelin after oral administration explains the low circulating levels of these phytochemicals found in mice on dietary ellagic acid and embelin as well as the inability to measure them in tissues.
Discussion
Our studies provide insights into the beneficial effects of two phytochemicals on key cellular participants in pancreatic adenocarcinoma, the cancer cells themselves and the PaSCs that are responsible for the desmoplasia of the cancer. Of importance, pancreatic cancer is characterized by a prominent dense desmoplastic (fibroblastic) reaction that surrounds the cancer cell glands of this tumor. Clinically-derived data suggest that there is a direct relationship between the extent of the fibroblastic reaction and poorer disease outcome (30–32).
Research studies over the past few years have shown the importance of the myofibroblastic PaSCs in formation of pancreatic cancer desmoplasia as well as the importance of the stellate cells in promoting the growth of pancreatic adenocarcinoma (3, 4, 33–37). PaSCs are normally located in the peri-acinar space of the exocrine pancreatic tissue in a “quiescent” state having long cytoplasmic projections that encircle the base of the pancreatic acinus. Quiescent PaSCs have a low rate of proliferation and production of extracellular matrix proteins, growth factors and cytokines. However, when recruited by a developing adenocarcinoma, they transform from their normal quiescent state into an “activated” state (also known as a “myofibroblastic” state) (3).
Activated PaSCs proliferate, migrate, and produce large amounts of extracellular matrix proteins, and cytokines, chemokines and growth factors which are all involved in the growth and proliferation of a pancreatic adenocarcinoma (37). Further, cancer cells promote the activation and proliferation of the stellate cells (35, 36). Although the complete network of mechanisms by which cancer cells and PaSCs in the microenvironment of the pancreatic adenocarcinoma promote each other growth is unknown, the present study was performed to determine if phytochemicals which affect pancreatic cancer cells may also have effects on PaSCs in order to consider a more comprehensive approach to finding agents with benefits for this cancer. For comparability to the activated state of the PaSC in human adenocarcinoma, in this study we maintained primary mouse PaSCs in culture conditions that promote their proliferative, pro-fibrotic activated phenotype (38).
One set of key findings of our study was that ellagic acid and embelin each dose-dependently inhibited proliferation and increased apoptosis in human pancreatic cancer cells, MIA PaCa-2 and HPAF-II cells, and the pancreatic stellate cells. Also, in each of these cell types, combinations of ellagic acid and embelin at low micromolar concentrations had synergistic effects on proliferation and apoptosis. Thus, ellagic acid and embelin are phytochemicals now shown to have beneficial effects on both key cell types in this cancer.
Another key finding of our study was that, at least for the cancer cells, the two phytochemicals blocked two different intracellular signaling systems. This data suggest that the synergistic effects of ellagic acid and embelin on proliferation and apoptosis were likely due to the blocking of different signaling systems known to promote cancer. For example, ellagic acid acted primarily by inhibiting the NF-κB pathway while embelin acted primarily by inhibiting activation of STAT-3 which led to decreased expression of XIAP and survivin, members of the inhibitors of apoptosis family. Considering this background, the findings presented here lead to three important conclusions: (1) Ellagic acid and embelin affect both key survival cell pathways, proliferation and apoptosis; (2) Both compounds have synergistic effect on inhibiting proliferation and promoting apoptosis in vitro in cancer cells; (3) both compounds have different targets. These results suggest that there is a rational approach to choosing combinations of phytochemicals for treatment strategies of this and possibly other cancers.
The final key finding from our study is that ellagic acid and a combination of embelin and ellagic acid significantly decreased tumor growth and tumor cellularity in a subcutaneous xenograft mouse model of pancreatic cancer using the HPAF-II cells. The time-dependent decrease in tumor volume was significantly decreased by each compound alone. We did not see a synergistic response in the in vivo studies that would reproduce the in vitro synergistic response of the cancer cells, likely because different tuning in tissue concentrations of the phytochemicals in our in vivo model. Nevertheless, the importance of the studies is that dietary administration of each of the agents had a significant effect on the tumors without having adverse effects on the animals. One limitation of the model is the lack of immune cells and activated stellate cells in the tumor microenvironment of subcutaneous tumors in nude mice. We plan to surmount this limitation by using genetic models of pancreatic cancer.
In sum, our in vivo studies indicate that ellagic acid and embelin have effects on tumor growth at doses that do not affect normal tissues and cause no apparent toxicity.
Acknowledgments
We acknowledge the support of the Animal and Morphology Cores of the UCLA Center of Excellence for Pancreatic Disease (P01AT003960) for their assistance with the xenograft model of pancreatic cancer.
This work was supported by the UCLA Center for Excellence in Pancreatic Diseases (NCCAM: 1 P01 AT003960), by the Department of Veterans Affairs, the NIAAA award (K01 AA019996 to ME) and the NIAAA (R01 AA019954 to AL).
References
- 1.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 2.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1997;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–7. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J Gastroenterol Hepatol. 2012;27 (Suppl 2):127–34. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 7.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya S, Ray RM, Johnson LR. STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J. 2005;392(Pt 2):335–44. doi: 10.1042/BJ20050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayala GE, Dai H, Ittmann M, Li R, Powell M, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;1:64(17):6082–90. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 10.Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;30:25(51):6800–16. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor- B and I B kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:10:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 12.Mukhtar H, Das M, Khan WA, Wang ZY, Bik DP, et al. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethylbenz(a)anthracene-, benzo(a)pyrene-, 3-methylcholanthrene-, and N-methyl-Nnitrosourea-induced skin tumorigenesis in mice. Cancer Res. 1998;1;48(9):2361–5. [PubMed] [Google Scholar]
- 13.Thresiamma KC, Kuttan R. Inhibition of liver fibrosis by ellagic acid. Indian J Physiol Pharmacol. 1996;40(4):363–6. [PubMed] [Google Scholar]
- 14.Osawa T, Ide A, Su JD, Namiki M. Inhibition of lipid peroxidation by ellagic acid. J Agric Food Chem. 1987;35:808–812. [Google Scholar]
- 15.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22(11):1737–46. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 16.Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17(9):611–25. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Sumino M, Sekine T, Ruangrungsi N, Igarashi K, Ikegami F. Ardisiphenols and other antioxidant principles from the fruits of Ardisia colorata. Chem Pharm Bull (Tokyo) 2002;50:1484–7. doi: 10.1248/cpb.50.1484. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Sanyal SN, Kanwar U. Antispermatogenic effect of embelin, a plant benzoquinone, on male albino rats in vivo and in vitro. Contraception. 1989;39:307–20. doi: 10.1016/0010-7824(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 19.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1995;40:109–13. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 20.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, et al. Discovery of embelin as a cellpermeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 21.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43(1):128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279(33):34643–54. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 23.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1137–47. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 24.Edderkaoui M, Hong P, Lee JK, Pandol SJ, Gukovskaya AS. Insulin-like growth factor-I receptor mediates the prosurvival effect of fibronectin. J Biol Chem. 2007;14;282(37):26646–55. doi: 10.1074/jbc.M702836200. [DOI] [PubMed] [Google Scholar]
- 25.Lu QY, Zhang L, Moro A, Chen MC, Harris DM, et al. Detection of Baicalin Metabolites Baicalein and Oroxylin-A in Mouse Pancreas and Pancreatic Xenografts. Pancreas. 2012;41(4):571–6. doi: 10.1097/MPA.0b013e318232e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Angst E, Park J, Moro A, Dawson DW, et al. Quercetin Aglycone is Bioavailable in Murine Pancreas and Pancreatic Xenografts. J Agric Food Chem. 2010;58 (12):7252–7257. doi: 10.1021/jf101192k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- 28.Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, et al. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14(23):3672–80. doi: 10.3748/wjg.14.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71(1):209–19. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe I, Hasebe T, Sasaki S, Konishi M, Inoue K, et al. Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin expression correlate with outcome. Pancreas. 2003;26:326–33. doi: 10.1097/00006676-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–8. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 32.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 33.Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–51. 1051 e1–8. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 34.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–21. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–87. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]