Abstract
In present communication, we report an outbreak of Trypanosoma evansi in equine herd n = 30 (horse and mules) which, were reared in fly proof stables as well as in open paddock maintained under semi-intensive system of management, and its effective control using trypanocidal drug. The infection was monitored by antibody ELISA up to 180 days post-treatment (PT). A total of 8 out of 14 equines (57.14 %) which were maintained only in open paddocks were found positive with T. evansi infection parasitologically. The infected animals were treated with quinapyramine methyl sulphate and chloride combination administered at the prescribed dose rate on 3rd day of screening. The parasite could not be detected from any treated animals from day-3 PT up to 6 month. Further, we also could not observe relapse of infection, neither in treated group nor in equine herd maintained at the farm. Sero-conversion was observed in all eight animals by 10th day of screening, indicating that immune response was due to recent infection as the animals became chronologically positive. The antibody titre reached at the peak by 10–14th day in all infected animals, and started declining by 17th day of screening, further reached to near cut off level by 180 days. Since, antibodies persisted up to 6 month PT and antibody detection assays are not able to differentiate between current and past infections in treated cases. The detection of circulating antigen assay and parasitological techniques in combination may be performed for effective diagnosis and management of T. evansi infection.
Keywords: Trypanosoma evansi, ELISA, Immunoblot diagnosis, Quinapyramine sulphate/chloride
Introduction
Trypanosomosis commonly known as ‘surra’ is caused by Trypanosoma evansi, a extracellular haemoflagellate, commonly prevalent in Africa, Latin America, Middle East and South East Asia in domestic and wild animals. The parasite is known to infect different host species and is mechanically transmitted by different biting flies such as Tabanids, Stomoxys and Lyperosia etc. in Indian sub-continent. Camels and horses are very susceptible to the infection and death may occur within weeks or months while infections of cattle and buffaloes usually lead to an immuno-suppression resulting in an increased susceptibility to other infectious diseases.
The disease among equines has already been reported from various parts of India (Chaudhri et al. 1985; Laha and Sasmal 2008; Yadav and Kumar 2011) In general, vector involved is Tabanus and high prevalence of these biting flies has been reported in monsoon season, associated with an increased prevalence of surra in camel, cattle, buffaloes and horses (Gill 1991). Apart from seasonal variations in the abundance of vectors, other factors that influence transmission is the degree of parasitaemia. Diagnosis of the disease in equines is either based on demonstration of parasites in blood or indirectly by detecting parasite antigens or PCR assay, or by the detection of specific T. evansi antibodies (Brun et al. 1998). Besides this, for mass screening of equine population antibody ELISA is being practiced (Kumar et al. 2010). Though, this test is more sensitive, yet it has limitation of false detection of treated cases due to persistence of antibodies in drug treated animals. In present communication, we report the early detection of trypanosomosis in horses at an organized farm and their treatment by quinapyramine methyl sulphate and chloride combination following the outbreak. The persistence of antibody levels were monitored following treatment.
Materials and methods
This study was carried out at an organized equine farm at Hisar, India with 30 equines (horses and mules), housed in concrete stables served with balanced diet and reared under semi intensive system of management. Out of 30 animals, 14 animals were reared in open paddocks and remaining kept in fly proof stables (Table 1). During Aug. 2009 (monsoon season) one horse (H-238), kept in open paddock died within 24 h after showing symptoms of progressive ataxia, head tilt, paddling of leg, frequent micturition, and severe neurological abnormalities. On parasitological examination of wet blood film (WEF) of this case, the horse was found positive for T. evansi. Thereafter blood/serum samples were immediately collected from all the equines kept at farm for subsequent clinical/immunological observations for 6 months PT. The sequential serum samples were also chronologically examined by ELISA with some modification (Wernery et al. 2001). Briefly, a series of checkerboard titrations were conducted to determine the optimum concentration of whole cell lysate antigen (WCL) and conjugates for use in ELISA assay. ELISA plates (Nunc) were coated with 50 μl of 1.0 μg/ml of antigen in 0.1 M carbonate/bicarbonate buffer (pH 9.6) per well. Blocking was done with 100 μl of 5 % skim milk in PBST (SM-PBST) for 1 h at 37 °C. Subsequently, 50 μl of test serum (1:100 diluted in 5 % SM-PBST) were added to each well and incubated for 1 h at 37 °C. Thereafter, 50 μl of 1:10,000 diluted IgG–peroxidase conjugate (Sigma) was added to each well and the plates incubated for 1 h at 37 °C. Finally, substrate (TMB) was added. The reaction was stopped by adding 0.5 N H2SO4 to each well. The absorbance was read at 450 nm on ELISA reader (Bio Tek, USA) and results were expressed as mean OD of duplicate samples. The cut off values were determined using mean OD ± 3SD of uninfected serum samples from the herd. Prior to treatment of animals with drug all the ELISA positive animals were subjected to immunoblot (Towbin et al. 1979). Based on the results of parasitological and antibody ELISA/immunoblot assays, all the infected animals were treated with combination of quinapyramine methyl sulphate (2.5 mg/kg b.wt.) and quinapyramine methyl chloride (1.7 mg/kg b.wt.) drug (Triquin™, Wockhrdt Ltd.) subcutaneously along with supportive therapy. The blood samples were collected at regular intervals up to 180 days PT to evaluate parasitaemia and antibody titre by ELISA for persistence of antibody level post treatment. Cut off values were determined using mean OD ± 3SD of uninfected serum samples collected from non-endemic areas.
Table 1.
Chronological examination of equine herd for T. evansi infection (parasitological and ELISA)
| S.No. | Screening date | Parasitological examination (nos +) | ELISA |
|---|---|---|---|
| 1. | 8.08.09 (Died) | H-238 | Not done |
| 2. | 12.08.09 | P-7, F-218 | All negative |
| 3. | 13.08.09 | P-7, M-204, M-232, F-216, F-218, F-222, C-217 | F-218 |
| 4. | 14.08.09 | P-7, M-204, M-232, F-216, F-218, F-222, C-217, C-222 (all positive) | F-218, M-204, C-217 |
| Treatment of with quinapyramine sulphate/chloride | |||
| 5. | 18.08.09 | Nil | F-218, M-204, C-217, C-222 |
| 6. | 21.08.09 | Nil | All positive P-7, M-204, M-232, F-216, F-218, F-222, C-217, C-222 |
| 7. | 25.08.09 | Nil | |
| 8. | 28.08.09 | Nil | F-218, M-204, C-217, F-216, F-222, P-7, F-222 |
| 9. | 14.09.09 | Nil | F-218, M-204, C-217, F-222 |
| 10. | 8.10.09 | Nil | F-218, M-204, C-217 |
| 11. | 14.12.09 | Nil | F-218, C-217 |
| 12. | 12.02.10 | Nil | M-204 |
H Horse, M Mule, C Colt, F Filly
Results and discussion
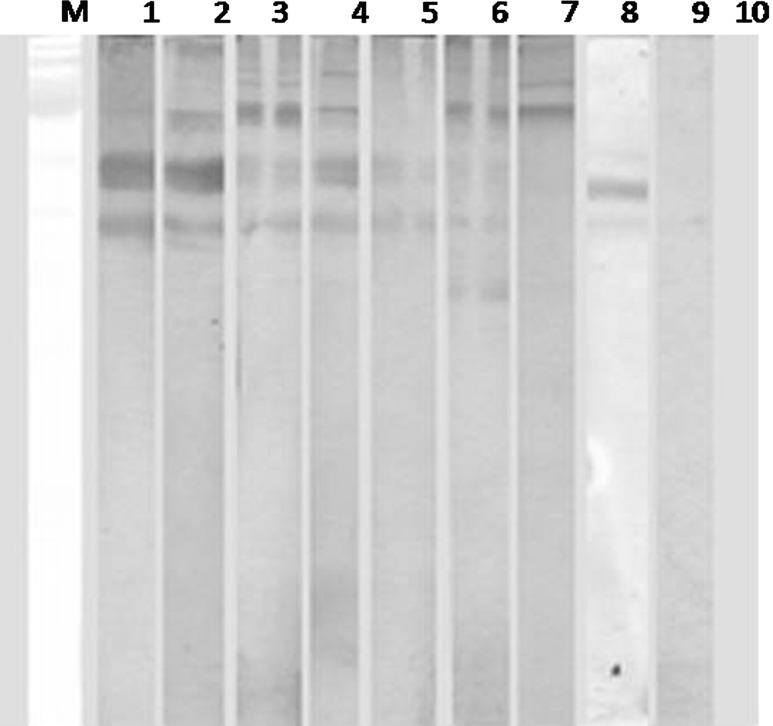
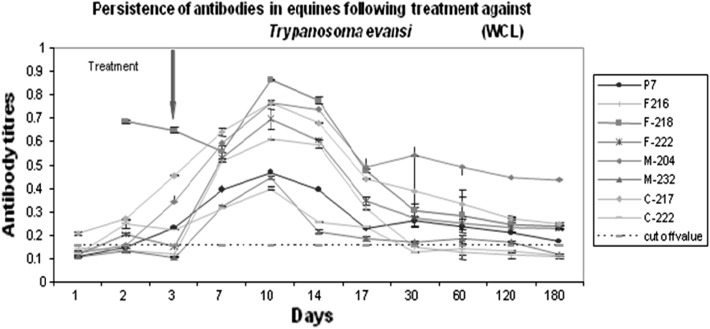
None of the animals were found positive for infection by wet blood film examination or demonstrated antibodies by ELISA maintained in fly proof stable during the period of this study. Though, out of 14 animals, eight animals (57.14 %) reared in open paddock were found positive for T. evansi infection as evidenced from the parasitological examination. On first day of screening, only two animals (P-7 & F-218) were found positive but subsequently within 3 days, the parasites were detected in six other animals (Table 1). Moderate (six animals) to high parasitaemia (two animals) was recorded in the infected cases which were reconfirmed by immunoblot (Fig. 1). It is pertinent to mention here that all these infected animals were housed in open paddocks. The tabanids are reported to visit and feed on horses within 5–50 m range (Barros and Foil 2007), it is assumed that this infection might have also spread from the infected animal (H-238) which died just before an outbreak and animal (F-218) showing very high antibody titre at the time of initial screening by the tabanids prevalent at farm during monsoon season (Fig. 2).
Fig. 1.
Immunoblot using serum samples of equines infected (pre-treated) with T. evansi. Lane M contains pre-stained molecular weight marker; lane 1–9 immunoblot with infected pre-treated equine serum samples; lane 10 immunoblot with un-infected equine serum sample
Fig. 2.
Persistence of antibodies using ELISA in equine following treatment against Trypanosoma evansi
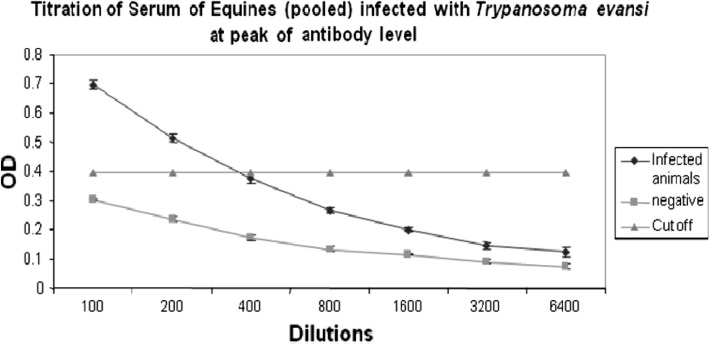
Quick transmission of infection in herd could have been due to the fact that all these animals were kept in open paddock with easy access to flies. On day-3 PT with the drug, parasites were not detected from any treated animals up to 6 months (Table 1). Further, we could not observe relapse of infection, either in treated group or in equine herd maintained in fly proof stables. It is evident from Table 1 that initially one animal was found parasitologically positive, all the animals were negative by antibody ELISA. On day-3 all animals demonstrated parasite in their blood, thereafter one by one animals showed rising trend of antibodies. Moreover, peak sero-conversion was observed in all eight animals by 10–14th day of screening, indicating that immune response was due to recent infection. The results as evaluated by ELISA indicated that in all the eight animals except F-218, antibody titres were as low as negative controls at first screening, later on increased slowly and reached at peak in all the animals by day 14 (10 days PT). The antibody titre of animal no. F-218, was as high as OD 0.7 before treatment which indicated that it must have been infected much earlier than other animals. The titre decreased slowly and continuously after 10-14 days PT, in all animals, further reaching to almost near cut off level by 180 days, except in animal M-204. The parasites were cleared from circulation following treatment at early stage of infection, even though antibody titre at peak was recorded low (1:400) in pooled serum samples (Fig. 3). Monzon et al. (2003) reported that the antibody titres decline sharply following treatment of T. evansi infected horses. They detected antibodies in 3 out of 10 horses, 6 months PT and further used ELISA to determine persistence of antibody levels following treatment with quinapyramine sulphate. They further observed that antibody titre fell progressively post treatment in majority of horses, while two horses remained positive for longer duration i.e. 22.6 and 12.8 months, respectively. In the present investigation, majority of animals (seven) showed sharp antibody titres decline after treatment and persisted up to 180 days PT. Since the IgG antibodies are not able to differentiate between current and previous infections, the employment of IgG ELISA for post-treatment follow up for trypanosome detection is of little value. Therefore, it is concluded that a combination of assays for detection of circulating antigens, DNA may be performed for effective diagnosis and management of T. evansi infection.
Fig. 3.
Titration of pooled serum samples of infected equines using ELISA
Acknowledgments
Facilities for research work and encouragement received from Director, National Research Centre on Equines are thankfully acknowledged. The authors are also thankful to R. K. Dayal and Neeraj Kumar Yadav for providing technical assistance during the study.
References
- Barros ATM, Foil LD. The influence of distance on movement of tabanids (Diptera: Tabanidae) between horses. Vet Parasitol. 2007;144:380–384. doi: 10.1016/j.vetpar.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Brun R, Hecker H, Lum ZR. Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review) Vet Parasitol. 1998;79:95–107. doi: 10.1016/S0304-4017(98)00146-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri SS, Yadav CL, Gupta RP, Ruprah N. Parasitic infection of equines in Haryana. Indian J Anim Sci. 1985;55:766–769. [Google Scholar]
- Gill BS. Trypanosomes and trypanosomiases of Indian livestock. Pusa: Publication and Information Division, ICAR; 1991. [Google Scholar]
- Kumar R, Yadav SC, Kumar S, Khurana SK (2010) Sero-prevalence of Trypanosoma evansi in equids of northern region of India using antibody-ELISA. In: Proceedings of national congress of veterinary parasitology on “Parasitology today-ecology to molecular biology”. Department of Veterinary Parasitology, CCS Haryana Agricultural University, Hisar, 18–20 Feb 2010, pp 19–20
- Laha R, Sasmal NK. Endemic status of Trypanosoma evansi infection in a horse stable of eastern region of India—a field investigation. Trop Anim Health Prod. 2008;40:357–361. doi: 10.1007/s11250-007-9107-0. [DOI] [PubMed] [Google Scholar]
- Monzon CM, Mancebo OA, Russo AM. Antibody levels by indirect ELISA test in Trypanosoma evansi infected horses following treatment with quinapyramine sulphate. Vet Parasitol. 2003;111:59–63. doi: 10.1016/S0304-4017(02)00331-X. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery U, Zachariah R, Mumford JA, Luckins T. Preliminary evaluation of diagnostic tests using horses experimentally infected with Trypanosoma evansi. Vet J. 2001;161:287–300. doi: 10.1053/tvjl.2000.0560. [DOI] [PubMed] [Google Scholar]
- Yadav SC, Kumar R (2011) Prevalence of T. evansi infection in equines in India for 2009–2010. In: Annual meeting of NTTAT Group, Paris, France 22 May 2011