Abstract
In Iran, Cysticercus tenuicollis, the metacestode stage of Taenia hydatigena is endemic. The migration of this parasite causes traumatic hepatitis and death in young animals. The objective of this work was to evaluate hematological, biochemical and pathological findings in 50 goats infected with C. tenuicollis in comparison with 50 non-infected goats, as control group. This study was carried out as case–control. Blood and liver samples were taken from the goats, analyzed for hematology and biochemical parameters and liver samples were prepared for paraffin blocks, sectioning and staining for further microscopic study in pathology laboratory. Significant decrease in red blood cell count, hemoglobin, packed cell volume and total protein (P < 0.05) and significant increase in white blood cell count, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase were observed in infected goats (P < 0.05), compared with those in non-infected control group. The microscopic lesion in liver included large concentric foci of hemorrhage in migration stage and decreased number of hepatocytes, dilation of sinusoids, presence of inflammatory cells in portal areas and double layered parasitic cyst formation in chronic stages. In conclusion, various changes in parameters could have deleterious effect on morbidity and mortality of the herd.
Keywords: Cysticercus tenuicollis, Hematology, Biochemistry, Pathology, Goat
Introduction
The adult stage of Cysticercus tenuicollis (Taenia hydatigena) has been reported in the small intestine of a large number of hosts including dogs, cats, mice and wild carnivores, including wolf and the fox throughout the world (Payan-Carreira et al. 2008; Senlik 2008). The intermediate hosts, most commonly sheep and goats, become infected by ingesting proglottids or eggs, passed in the feces of the dog in pastures or feeding areas (Kaufmann 1996).
The prevalence of C. tenuicollis found in goats reported by authors, ranged from 16.3 to 46.6 %, globally (Radfar et al. 2005; Muktar 1988; Woynshet 2008).
The cysticerci of T. hydatigena are responsible for a high degree of morbidity and mortality in livestock (Abidi et al. 1989).
Heavy infections and traumatic hepatitis are lead to death in young animals (Soulsby 1986).
Diagnosis of infection in animals is based on detection of the cysts during meat inspection where the other methods such as serological (ELISA), biochemical and hematological tests can be useful in live animals (Berezhko 1989).
The purpose of this study was to compare hematological, biochemical parameters and pathological findings in liver among naturally infected goats with C. tenuicollis and non-infected control group. These findings can be useful for further studies of cysticercosis and diagnostic purposes in goats, especially, in areas where fascioliosis is not endemic. Since no previous work has already been carried out on the present parameters this work can be of high priority and prime importance.
Materials and methods
Animal
In this study two groups of 50 goats each, were randomly selected. The first group was consisted of goats infected with C. tenuoicollis. The presence of disease condition was confirmed by inspection of carcasses at Kerman slaughterhouse. Each organ was inspected for related parasitic and microbial infection macroscopically. Animals with infections were excluded. A second arm was included as non-infected control group. The goats were free of other parasites (helminthic and pentastomid) and also they were clinically normal.
Hematology
Blood samples were taken from the jugular vein into evacuated EDTA tubes, stored at +4 °C and analyzed within 8 h. Total white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb), hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and number of platelets (PLT) were analyzed using a fully automated hematology analyzer, Sysmex Kx-21 (Sysmex Corporation, Japan).
Biochemistry
Sera were separated from blood samples by centrifugation at 2,500 rpm. Serum samples were frozen in plastic tubes at −20 °C until use. Serum activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and concentration of glucose, creatinine, calcium, total protein and total bilirubin were measured according to standard procedures using an automatic analyzer, Hitachi 911 (Roche Company, Switzerland) with Pars-Azmoon Commercial Kits (Pars-Azmoon Laboratories LTD, Tehran, Iran).
Pathology
At slaughter, carcasses were carefully inspected and infected livers with C. tenuoicollis were transported to the pathology laboratory at Faculty of Veterinary Medicine in Shahid Bahonar University.
Parasite migration routes were identified and 1 × 1 × 1 cm dimensions slices were prepared from the parasites and surrounding tissue. Samples were fixed in 10 % formalin and embedded in paraffin. Four μm thick tissue sections were stained with the haematoxylin and eosin (H&E) for histopathological study.
Statistical analysis
Data were analyzed using SPSS software. Results are expressed as mean ± standard deviation (SD). Significance of difference between groups was determined by Student’s t test at P < 0.05.
Results
Hematology
Hematological parameters of infected and non-infected goats are summarized in Table 1. The number of RBCs, Hb concentration and hematocrit values were reduced in infected group, compared with control group (P < 0.05). In contrast, the WBC count was significantly higher than that in control group (P < 0.05). However, there were no significant changes in MCV, MCH and MCHC values.
Table 1.
Mean of hematological parameters in infected goats with Cysticercus tenuicollis compared with non-infected control group
| Mean of hematological parameters ± SD | Infected | Non-infected |
|---|---|---|
| WBC* (103 cells/μl) | 14328.5 ± 1304.1 | 8805 ± 2493.7 |
| RBC* (106 cells/μl) | 7/625 ± 1.07 | 9.955 ± 2.21 |
| Hb* (g/dl) | 7.659 ± 0.8 | 9.997 ± 1.2 |
| Hct* (%) | 23.345 ± 3.05 | 29.365 ± 4.1 |
| MCV (Fl) | 31.185 ± 7.11 | 30.077 ± 3.53 |
| MCH (Pg) | 10.229 ± 2.03 | 10.27 ± 1.15 |
| MCHC (g/dl) | 33.079 ± 1.89 | 34.287 ± 1.57 |
SD Standard deviation
* Significant differences among infected goats with Cysticercus tenuicollis and non-infected control group (P < 0.05)
Biochemistry
Biochemical results are presented in Table 2. AST, ALT, ALP and total bilirubin increased significantly (P < 0.05), while total proteins decreased significantly (P < 0.05) in infected goats sera in comparison with the non-infected animals. However, there were no significant changes in serum levels of glucose, creatinine and calcium.
Table 2.
Mean of biochemical parameters in infected goats with Cysticercus tenuicollis compared with non-infected control group
| Mean of biochemical parameters ± SD | Infected | Non-infected |
|---|---|---|
| Glucose (mg/dl) | 116 ± 16.6 | 71 ± 15.4 |
| Cre (mg/dl) | 1.5 ± 0./6 | 1.7 ± 0.6 |
| Ca (mg/dl) | 9.4 ± 1.4 | 9.5 ± 1.41 |
| AST* (IU/L) | 42.87 ± 30.1 | 24.72 ± 6.8 |
| ALT* (IU/L) | 41.16 ± 16.2 | 22.9 ± 4.5 |
| ALP* (IU/L) | 250.46 ± 150 | 133.42 ± 60 |
| Bil* (mg/dl) | 0.8 ± 0.5 | 0.52 ± 0.2 |
| TP* (g/dl) | 6.62 ± 1.3 | 8.07 ± 1.4 |
SD Standard deviation
* Significant differences among infected goats with Cysticercus tenuicollis and non-infected control group (P < 0.05)
Pathology
At necropsy of infected goats with C. tenuicollis hemorrhagic streaks (Fig. 1), large concentric foci of hemorrhage in the hepatic parenchyma in the migration stage were observed. In histopathological examination, decreased number of hepatocytes, dilution of sinusoids (Fig. 2), presence of inflammatory cells in portal areas and double layered parasitic cyst formation in chronic stages (Fig. 3) were evident.
Fig. 1.
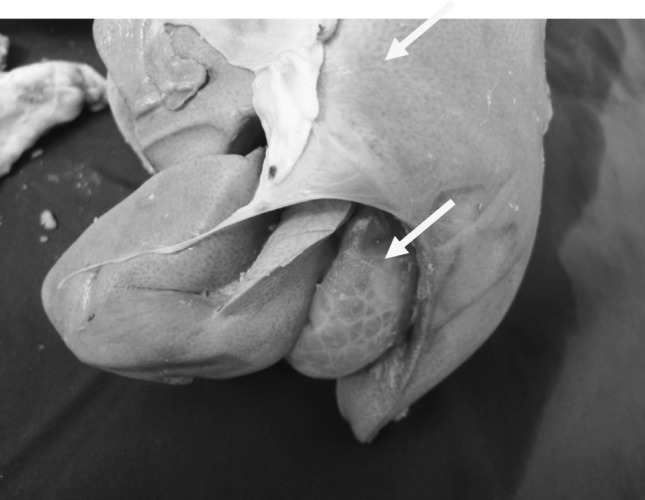
Hemorrhagic streaks caused by migration of Cysticercus tenuicollis
Fig. 2.
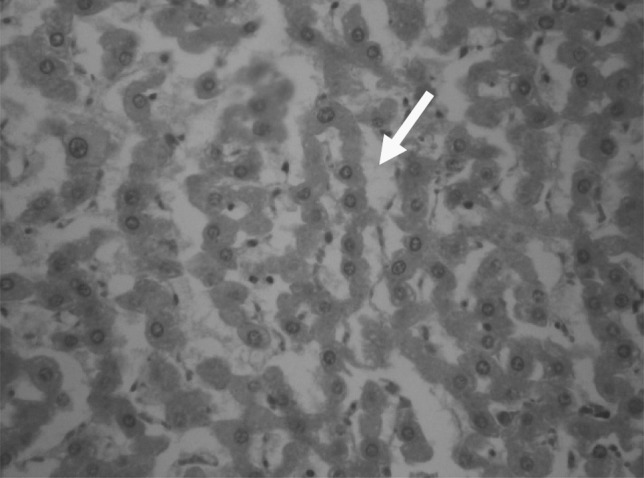
Decreased number of hepatocytes and dilation of sinusoids caused by migration of Cysticercus tenuicollis (H&E, ×1,000)
Fig. 3.
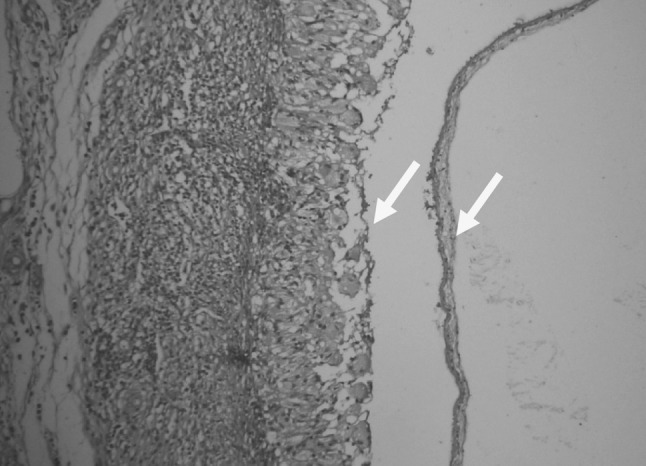
Double layers parasitic cyst formation in chronic stages caused by migration of Cysticercus tenuicollis (H&E, ×400)
Discussion
The migration of C. tenuicollis, cause traumatic hepatitis and death in young animals (Soulsby 1986). Similar to the larvae of Fasciola, migration of cysticerci in the liver may cause hemorrhagic and fibrotic tracts and serofibrinous peritonitis (Soulsby 1986; Balzek et al. 1985; Abdorahman et al. 1988). In addition, evaluation of haematological parameters in the present study showed that the mean number of WBCs in infected goats was approximately two times higher than that in non-infected goats, whereas the mean number of RBCs, Hb concentration and hematocrit values were reduced significantly (P < 0.05). Likewise, hematologic investigations in naturally infected pigs by T. hydatigena compared with non-infected pigs showed that the number of WBCs increased, while the number of RBCs and Hb concentration were decreased (Pathak et al. 1984).
The increased number of WBCs in the present study was similar to those previously reported by Sykes et al. (1980), Zhang et al. (2005) and Ahmed et al. (2006).
It seems that the increased number of WBCs may be due to liver failure, inflammation following larval migration and stimulation of immune responses. In other words, bleeding and congestions are caused by larval migration in the peritoneum and liver parenchyma due to reduction of RBCs, Hb and hematocrit concentration in infected goats.
Our study revealed that biochemical parameters including liver enzymes (AST, ALT.ALP) and total bilirubin were increased significantly (P < 0.05) and decreased significantly total protein (P < 0.05) in infected goats compared with control group. The present findings are consistent with those found by Pathak and Gaur (1981), who showed a significant increase in enzyme levels in infected goats with C. tenuicollis. Doaa et al. (2007) showed that levels of liver enzymes, total bilirubin, gammaglobulins and creatinine were increased in serum of infected sheep with Fasciola hepatica. Matanovic et al. (2007), reported similar results in infected cattle with Fasciola gigantica. Singh et al. (2004) reported an increase of AST levels 2 weeks post- infection synchronizing with the migratory phase of juvenile flukes (F. hepatica) in the liver parenchyma. They reported that liver damage is the most important cause of the increase in serum ALT activity in the infected sheep.
Larval migration to the liver, especially in young animals, leads to serious consequences. This migration causes hemorrhage and fibrosis in the hepatic parenchyma. Morbidity of infection in ruminants is high and mortality in lambs is reported (Soulsby 1986).
The condition of hepatitis cysticercosis, caused by C. tenuicollis in sucking pigs, was described by Bertullo (1943), Lloyds (1964) and Karasev et al. (1978). Pullin (1955) observed hemorrhagic wavy tracts on in the cortex of the liver of lambs that were experimentally infected with this parasite. Deorani (1967), described hepatitis cysticercosis in sheep and deer. However, these reports did not include description of the development of lesions, as the studies were largely made on natural cases, as well as Pathak et al. (1982) and Balzek et al. (1985) studied the pathology of C. tenuicollis in goats on successive days. Nourani et al. (2010) reported hepatic lesions of the cysticercosis caused by migrating cysticerci, most probably by the larvae of T. hydatigena.
In another study Darzi et al. (2002), on pathological investigation concluded that, the liver showed haemorrhagic, thickening of the Glisson’s capsule and inflammatory reaction in the vicinity of the cyst.
In the present study, the infected goats with C. tenuicollis, indicated large concentric foci of hemorrhage at the surface of liver at necropsy. In histopathological examination, decreased number of hepatocytes, dilution of sinusoids, presence of inflammatory cells in portal areas and double layered parasitic cyst formation in chronic stages were evident.
In conclusion the present findings indicated that C. tenuicollis hepatitis cysticercosa can cause a significant alteration of blood parameters, will damaged carcasses seriously and thus affecting the goats products.
Acknowledgments
The authors are grateful to Mr. Hassan Zadeh for his technical support.
References
- Abdorahman OM, Abdi BH, Ahmad MH. Pathological finding in the liver of sheep and goats in Somalia, Bollettion. Scientificodella Facolta di-zoote CINA e Veterinaria Universita nazonal. 1988;8:39–43. [Google Scholar]
- Abidi SM, Nizami WA, Khan P, Ahmad M, Irshadullah M. Biochemical characterization of Taeniahydatigena cysticerci from goat and pigs. J Helminthol. 1989;63:333–337. doi: 10.1017/S0022149X00009238. [DOI] [PubMed] [Google Scholar]
- Ahmed MI, Ambali AG, Baba SS. Hematological and biochemical responses of Balami sheep to experimental Fasciol agigantica infection. J Food Agric Environ. 2006;4(2):71–74. [Google Scholar]
- Balzek K, Schramlova J, Hulinsk D. Pathology of the migration phase of Taenia hydatigena larvae. Folia Parasitol. 1985;32:127–137. [PubMed] [Google Scholar]
- Berezhko VK. Comarative immunochemical characteristics and serological activity of the antigens of Cysticercus tenuicollis and Taenia hydatigena. Parazitologiia. 1989;5:399–406. [PubMed] [Google Scholar]
- Bertullo VH. Hepatitis cysticercosa de los lechones ocasionada per el Cysticercus tenuicollis de la Taenia marginata. Bul Min Direction Gana Montevideo. 1943;27:429–434. [Google Scholar]
- Darzi MM, et al. Pathology of Taenia hydatigena cysticercosis in a naturally infected Corriedale lamb. Vet Parasitol. 2002;16(2):173–174. [Google Scholar]
- Deorani VP. Histopathological studies on hepatitis cysticerocosa lesions in sheep and a deer. Indian Vet J. 1967;44(11):939–942. [PubMed] [Google Scholar]
- Doaa FT, Soliman EK, Abd El- khalek TMM (2007) Effect of Fascioliasis on hematological, serum biochemical and histopathological changes in sheep. Egypt J Sheep Goat Sci 2(2):15–34
- Karasev NF, et al. Distribution, clinical manifestation and pathology of Taenia hydatigena and Cysticercus tenuicollis in animals in Belorusia. Dostizh Vet Nulki Peredovog Zhivotnov. 1978;55:77–79. [Google Scholar]
- Kaufmann J. Parasitic infections of domestic animals. A diagnostic manual. Basel: Birkhauser; 1996. pp. 184–185. [Google Scholar]
- Lloyds TS. Hepatitis cysticercosa causing sudden death in a pig. Vet Rec. 1964;1076:1080. [Google Scholar]
- Matanović K, Severin K, Martinković F, Simpraga M, Janicki Z, Barisić J. Hematological and biochemical changes in organically farmed sheep naturally infected with Fasciolahepatica. Parasitol Res. 2007;101(6):1657–1661. doi: 10.1007/s00436-007-0709-2. [DOI] [PubMed] [Google Scholar]
- Muktar R (1988) Preliminary survey of gastro-intestinal helminthes in dogs, Cysticercus tenuicollis in sheep and goats, hydatidosis in sheep, goats and cattle, at Wolaita awraja. DVM thesis, AAU, FVM, Debrezeit, Ethiopia, pp 6–17
- Nourani H, Pirali Kheirabadi KH, Rajabi H, Banitalebi A. Research note an unusual migration of Taenia hydatigena larval in a lamb. Trop Biomed. 2010;27(3):651–656. [PubMed] [Google Scholar]
- Pathak KML, Gaur SNS. Serum levels of GOT, GPT and OCT enzyme in goats infected with Cysticecus tenuicollis. Vet Parasitol. 1981;8:95–97. doi: 10.1016/0304-4017(81)90022-4. [DOI] [Google Scholar]
- Pathak KML, Gaur SNS, Sharma SN. The pathology of Cysticercus tenuicollis infection in goats. Vet Parasitol. 1982;11:131–139. doi: 10.1016/0304-4017(82)90035-8. [DOI] [PubMed] [Google Scholar]
- Pathak KM, Kumar M, Gaur SN. Changes in blood cellular components, serum proteins and serum enzyme activities in pigs naturally infected with Cysticercus tenuicollis. Res Vet Sci. 1984;36(3):263–265. [PubMed] [Google Scholar]
- Payan-Carreira R, Silva F, Rodrigues M, Anjos Pires M. Cysticercus tenuicollis vesicle in fetal structures: report of a case. Reprod Domest Anim. 2008;43:764–766. doi: 10.1111/j.1439-0531.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- Pullin JW. Observat ion on the liver lesions in lambs experimentally, with cystecerci of Taenia hydatigena. Med Vet Sci. 1955;19:48–49. [PMC free article] [PubMed] [Google Scholar]
- Radfar MH, Tajalli S, Talalzadeh M. Prevalence and morphological characterization of Cysticercus tenuicollis (Taenia hydatigena cystcerci) from sheep and goats in Iran. Vet Arch. 2005;75:469–476. [Google Scholar]
- Senlik B. Influence of host breed, sex and age on the prevalence and intensity of Cysticercus tenuicollis in sheep. J Anim Vet Adv. 2008;7(5):548–551. [Google Scholar]
- Singh J, Bal M, Aradhana S, Gumber S. Efficacy of different flukicides against fascioliosis in sheep and goats. J Res. 2004;41(2):287–289. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: Baillier Tindall; 1986. pp. 113–115. [Google Scholar]
- Sykes AR, Coop RL, Rushton B. Chronic subclinical fascioliasis in sheep: effects on food intake, food utilisation and blood constituents. Res Vet Sci. 1980;28:63–70. [PubMed] [Google Scholar]
- Woynshet S (2008) Cross sectional study on the prevalence of Cysticercus tenuicollis in visceral organs of sheep and goats slaughtered at HELMEX export abattoirs. DVM thesis, FVM, and Addis Ababa University, Ethiopia, pp 8–13
- Zhang ZWY, Moreau E, Hope JC, Howard CJ, Huang WY, Chauvin A. Fasciola hepatica and Fasciola gigantica: comparison of cellular response to experimental infection in sheep. Exp Parasitol. 2005;111:154–159. doi: 10.1016/j.exppara.2005.06.005. [DOI] [PubMed] [Google Scholar]


