Abstract
Theileria annulata merozoite surface protein (Tams1) and native soluble piroplasm antigen of an Indian isolate of T. annulata were used to optimize an enzyme linked immunosorbent assay (ELISA) to detect anti-Theileria antibodies in cross-bred cattle experimentally infected with sporozoites or immunized with macroschizonts of the homologous strain of the parasite. The recombinant protein has showed similar antibody titre when compared with the sonicated merozoite/piroplasm antigen in an ELISA. IFAT was used as a reference test. In 56 sera of apparently healthy cattle collected from endemic area, ELISA showed positive antibody response in 29 samples with Tams1 whereas the soluble piroplasm antigen reacted positively with 34 samples and also with sera of cattle positive for Babesia bigemina. Microscopical detection of piroplasms was possible in the blood smears of 17 animals which were serologically positive in both native and recombinant antigens.
Keywords: Tams1 recombinant antigen, ELISA, Cross reaction, Babesia bigemina
Introduction
In India, bovine tropical theileriosis caused by Theileria annulata is an economically important disease in cross bred cattle (Minjauw and Mcleod 2003) and it is diagnosed by microscopical detection of schizonts and piroplasms during the acute phase of the disease. The indirect fluorescent antibody test (OIE 2000) has been recommended for serodiagnosis of the disease and PCR based assays with species specific primers (d’Oliveira et al. 1995) were performed to detect infection in carrier cattle harboring low level of parasitaemia. However, these tests are laborious and impractical for large-scale epidemiological surveys. Enzyme immunoassays based on soluble piroplasm antigen can be used for large scale detection of theileriosis in the field but it is difficult to standardize the test from crude parasite material, and there is always the requirement of experimental animals for parasite production (Manuja et al. 2000). To over come these problems, recombinant antigens of Theileria (Gubbels et al. 2000; Illhans et al. 1998; Williamson et al. 1989; Bakheit et al. 2004) were used with variable success. This paper communicates the use of recombinant merozoite surface antigen of an Indian isolate of T. annulata to determine the serological response in apparently healthy cattle in the field.
Materials and methods
Amplification and expression of Tams1 gene
An internal fragment of T. annulata merozoite surface antigen gene (Tams1) was PCR amplified from cDNA prepared from the total RNA isolated from the merozoites. The gene was cloned in prokaryotic expression vector, pPROExHT “b” (Invitrogen, USA) and expressed in Escherichia coli, DH5 α strain using 2 mM Isopropyl β-d-thio-galactopyranoside (IPTG) (Rajendran et al. 2008). The batches of culture aliquots were pelleted by centrifugation at 6,000 rpm for 10 min at room temperature (RT). The 6-histidine tagged fusion protein was purified using a single step nickel affinity chromatography using urea as a mild denaturant. The eluted recombinant Tams1 was dialyzed against PBS, pH 7.2 at 4 °C and protein concentration was determined Lowry et al. (1951). The purity of the protein was checked by 12 % sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis (Towbin et al. 1979).
Bovine sera
Positive sera
Two months old naïve crossbred calves were injected with 0.5 tick equivalent ground up tick sporozoites (GUTS) prepared from partially fed adult Hyalomma anatolicum ticks infected with T. annulata stock. Blood samples were collected from the calves between day 21 to day 30 post infection for the separation of serum and the sera were preserved as bovine positive control sera.
Immunized sera
Bovine male crossbred calves aged 6 months to 1 year, were inoculated subcutaneously near right prescapular lymphnodes with 2 × 106 bovine lymphoblasts infected with attenuated macroschizonts of T. annulata (Subramanian et al. 1987a) and challenged with 5 tick equivalent GUTS after 45 days of the inoculation. The post-challenge sera of the animals were preserved as immunized sera. The anti-Babesia bigemina bovine serum was procured from a heifer showing natural infection with the parasite.
Negative sera
A panel of ten serum samples of healthy neonatal crossbred bovine calves, negative for infection with any haemoparasites and anti-T. annulata antibodies in IFAT was marked as negative sera.
Field sera
Sera and blood smears were collected randomly from 56 apparently healthy cross-bred cattle reared in the T. annulata endemic zone of southern part of India during summer months when there is peak in tick infestation and preserved at −20 °C. Blood smears were stained with giemsa and examined microscopically to detect the presence of the stages of T. annulata or any other haemoprotozoa.
Enzyme linked immunosorbent assay
The purified soluble piroplasm antigen of T. annulata (Ray et al. 1998) was used in the concentration of 5 μg/ml of carbonate–bicarbonate buffer (pH 9.6) according to the standardized protocol for coating ELISA plates. Similarly four separate concentrations of 0.5, 1.0, 2.0 and 5.0 μg of the recombinant Tams1 per milliliter of buffer were used to coat the ELISA plates. All antigens were used in the volume of 100 μl per well and incubated at 37 °C for 1 h. The plates were blocked at 4 °C overnight with 5 % skimmed milk powder in phosphate buffered saline (PBS, pH 7.2). Positive and negative sera were diluted separately with PBS, pH 7.2 in the ratio of 1:50, 1:100 and 1:200 and incubated in the volume of 100 μl per well. The wells were washed three times with washing buffer containing PBS, pH 7.2, 0.05 % Tween 20 (PBS-T) after incubation with each reactant. The anti-bovine IgG (whole molecule) peroxidase conjugate (Sigma, USA) was used in 1:20,000 dilutions in PBS, pH 7.2 as per manufacturer’s instruction. The peroxidase mediated colour development was allowed for 30 min at room temperature with O-phenylene diamine dihydrochloride (Sigma, USA) in citrate buffer, pH 5.0. Further development of the color was stopped with 1 M phosphoric acid. The absorbance (OD) was read at 450 nm in an ELISA reader (Bio-rad, USA). The OD values were expressed as such taken from the ELISA reader. The ELISA cut-off value, which would serve as threshold between the positive and the negative sera samples was determined by calculating the mean OD values obtained from 10 negative sera plus two standard deviations.
Indirect fluorescent antibody test
The test (IFAT) was performed according to the protocol of OIE (2000) using piroplasm antigen. Serial two fold dilutions of positive and negative control serum samples starting from 1:20 to 1:640 were made with PBS, pH 7.2. Anti-bovine immunoglobulin fluorescein isothiocyanate conjugate (MS Genei, Bangalore, India) at a dilution of 1:80 with PBS was used as secondary antibody. Evans blue was incorporated into conjugate at a final dilution of 1:10,000 as a counter-stain. The slides were mounted with a cover slip in 50 per cent glycerol in PBS, pH 7.2 and examined under a fluorescent microscope (Nikon, Germany). The control positive serum showed fluorescence of piroplasm up to the dilution of 1:320. The sera showing the specific fluorescence to the highest dilution were considered as positive.
Results
Expression of Tams1
The amplified product size of the internal fragment of Tams1 gene (125–857) without N and C termini was 733 bp. The size of tams1 protein was 32 kDa and the concentration of the purified protein was 12 ng/μl.
Optimization of ELISA
Different concentrations of Tams1 and dilutions of sera were tried in order to get maximum differences in the OD values between the known positive and negative control sera. In the optimized protocol for ELISA, Tams1 was used at a concentration of 1 μg/ml in carbonate-bicarbonate buffer (pH 9.6) and all sera were diluted to 1:200 in PBS (pH 7.2). The mean of optical densities of ten negative bovine sera with Tams1 was 0.378 ± 0.064. Hence an optical density of 0.506 was considered as cut-off value for the assay. The mean of optical densities of ten negative bovine sera with soluble piroplasm antigen was 0.416 ± 0.096. Hence an optical density of 0.608 was considered as cut-off value for the assay. Two positive sera and ten immunized sera were tested in both IFAT and in optimized ELISA. The sera yielded identical positive reaction in both tests (Table 1).
Table 1.
Antibody response of bovine serum samples to T. annulata as measured by IFAT and ELISA
| Serum sample | Status of infection | IFAT titrea | ELISA Tams1/soluble piroplasm antigen |
|---|---|---|---|
| 1 | Negative | 20 | − |
| 2 | Infectedb | 160 | + |
| 3 | Infectedb | 160 | + |
| 4 | Immunizedc and challengedd | 160 | + |
| 5 | Immunizedc and challengedd | 320 | + |
| 6 | Immunizedc and challengedd | 320 | + |
| 7 | Immunizedc and challengedd | 160 | + |
| 8 | Immunizedc and challengedd | 160 | + |
| 9 | Immunizedc and challengedd | 160 | + |
| 10 | Immunizedc and challengedd | 320 | + |
| 11 | Immunizedc and challengedd | 160 | + |
| 12 | Immunizedc and challengedd | 320 | + |
| 13 | Immunizedc and challengedd | 320 | + |
aValues are expressed as reciprocal of end-point titre
b2-tick equivalent GUTS
c8 × 105 macroschizonts
d5-tick equivalent GUTS
Field sera sample
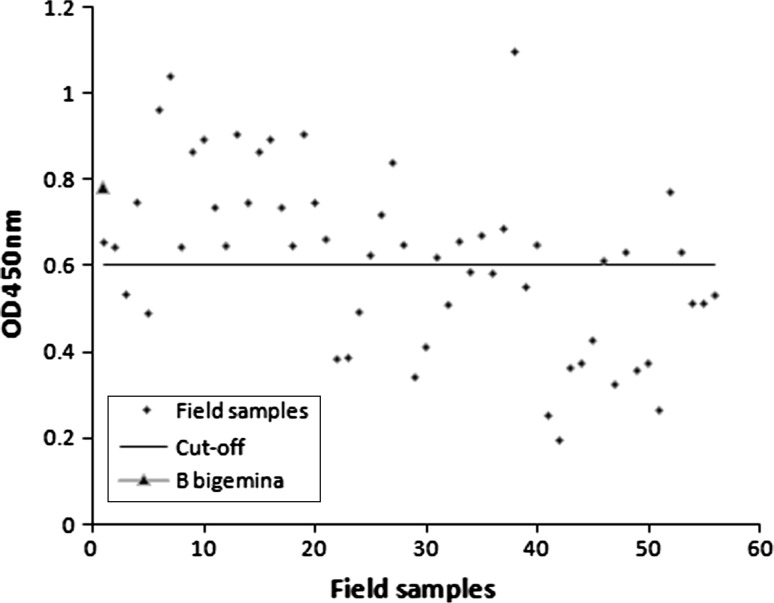
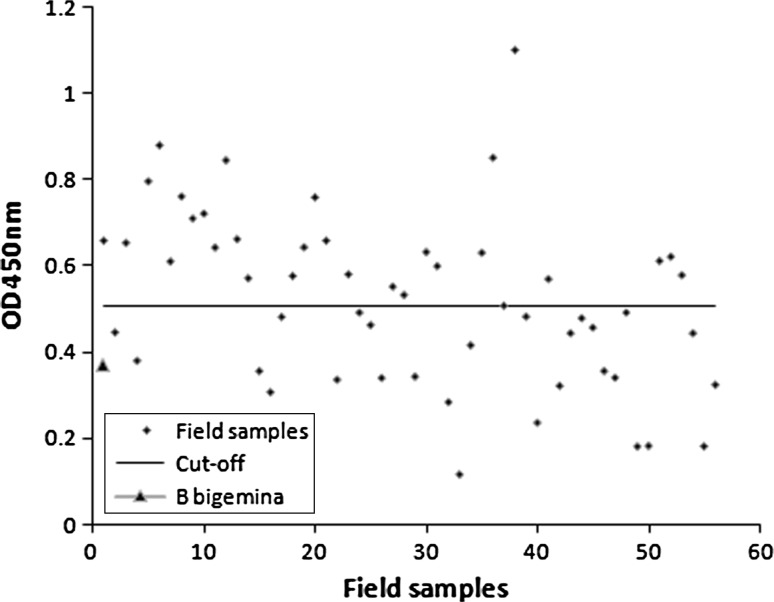
The distribution of the OD values of 56 sera samples is presented in the Figs. 1 and 2. As per the cutoff determined, 29 sera were positive to the Tams1 antigen and 34 sera were positive to the soluble piroplasm antigen. Out of of these cattle, rare intraerythocytic piroplasms (more than one in fifty microscopic fields) were detected in 17 animals. The study was not correlated with the IFAT. The anti-B. bigemina sera showed an OD value (0.355) well below the cut-off (0.508) level when tested with Tams1 antigen but an OD value (0.783) above the cut-off (0.608) level when tested with native piroplasm antigen.
Fig. 1.
ELISA profile of field sera samples collected from randomly selected crossbred cattle with soluble piroplasm antigen
Fig. 2.
ELISA profile of field sera samples collected from crossbred cattle with recombinant Tams1 being used as antigen
Discussion
The initial standardization and validation of an indirect ELISA based on the truncated form of recombinant Tams1 of an Indian strain of T. annulata is described in this paper. As the signal peptide (N terminus) is functional only in parasite and not involved in the development of immune response and the membrane anchor domain (C terminus) is very hydrophobic and probably contain no B cell epitopes (Gubbels et al. 2000), the Tams1 was expressed without both of these domains as 733 bp internal part of the gene (Rajendran et al. 2008). Two alleic forms of Tams1 namely Tams1–1 and Tams1–2 having molecular masses of 30 and 32 kDa respectively were detected either in pairs or singly in merozoite protein of T. annulata (Glascodine et al. 1990). These protein molecules were antigenically divergent and occurred in genotypically district parasites (Dickson and Shiels 1993). Analysis of merozoite protein of T. annulata (Parbhani isolate) exhibited the presence of a single peptide of 32 kDa molecular size (Rajendran et al. 2006) and partial sequence homology showed that it is having more homology to Tams1–2 (unpublished). The recombinant Tams1 was validated under controlled condition with known positive sera in which identical responses were recorded in IFAT with piroplasm antigen and in ELISA with soluble piroplasm antigen.
The optimized ELISA protocol in our laboratory was utilized for both the recombinant protein as well as native piroplasm antigen. To minimize the false-positive reactions, the cut-off was determined by calculating the mean OD values obtained from 10 negative sera plus two standard deviations. The sera (both experimentally infected and the field sera) have not been incubated with purified E. coli protein and also not decomplemented at 56 °C as these two test procedures were not found suitable to reduce the number of false-positive reactions in the test conducted by other workers (Gubbels et al. 2000). The cut-off determined for the threshold between positive and negative sera with recombinant Tams1 was 0.506 whereas for the soluble piroplasm antigen, it was 0.608. Although the determined cut-off level is slightly higher than the usual, most of the field samples showed moderately higher OD titre for both native as well as recombinant antigen in optimized ELISA (Figs. 1, 2).
The Tams1 ELISA revealed positive antibody response in 29 sera collected from the field, in which 17 animals were found positive microscopically for the presence of intraerythrocytic piroplasm and in rest of the 12 animals (Tams1 tested), the piroplasms could not be detected. In case of ELISA with soluble piroplasm antigen, 34 animals have shown antibody titers well above the determined cut-off level in which piroplasms were detected in 17 animals. Detection of higher seroreactivity by soluble piroplasm antigen could be due to the existence of serological cross reactions with other heamoprotozoan parasites in the field (Kachani et al. 1992). This was evident in the known antisera of bovine infected with B. bigemina which showed cross reaction with soluble piroplasm antigen of T. annulata. Cross reactions between T. annulata and B. bigemina has been reported in the range of molecular weights from 71 to 73 kDa proteins (Kachani et al. 1992). Some cross reaction of Tams1 with antiserum to T. parva was also reported by Gubbels et al. (2000). But in the absence of any other Theileria species infecting cattle in India, recombinant Tams1 could be a promising antigen for the detection of carrier status of T. annulata infection.
In India T. annulata infection is considered as an important malady in the cross bred bovine. However with the increase in genetic inheritance of Zebu cattle in the cross bred population, the incidence of bovine theileriosis has been drastically reduced in India and due to the existence of an endemic stability in the field (Das and Ray 2003), a large number of infected cattle remain symptomless carriers of the parasite. Although isolates of T. annulata with variable virulence has been reported in the country (Subramanian et al. 1987b), existence of any serological difference in the isolates was not indicated (Bansal and Ray 1994). Thus it may be assumed that the cattle all over the country acquire infection with a single serotype of T. annulata and Tams1 of a particular isolate could be suitable for serological detection of the carriers in which the piroplasm stage persists for a long period.
Acknowledgments
The author is highly thankful to the Director, IVRI for providing the facilities to carry out this research work, Dr. S. Sivamoorthy, Veterinary Assistant Surgeon, Piler, Andhra Pradesh for his help to collect field sera from crossbred animals and to Dr. Reji Gopalakrishnan for making the figures.
Declaration
All the experiments conducted on the bovine calves were as per the guidelines laid down by the Animal Ethics Committee of Indian Veterinary Research Institute, Izatnagar, Bareilly, India.
Footnotes
A part of Doctoral thesis submitted to the Indian Veterinary Research Institute, Izatnagar-243 122, Bareilly, Uttar Pradesh, India.
References
- Bakheit MA, Schnittger I, Salin DA, Boguslawski K, Beyer D, Fadi M, Ahmed JS. Application of the recombinant T. annulata surface protein in an indirect ELISA for the diagnosis of tropical Theileriosis. Parasitol Res. 2004;92:299–302. doi: 10.1007/s00436-003-1055-7. [DOI] [PubMed] [Google Scholar]
- Bansal GC, Ray D. Acquired resistance against Theileria annulata infection in cattle. J Vet Parasitol. 1994;8(1):35–37. [Google Scholar]
- d’Oliveira C, Van Der Weide M, Habela P, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995;13:2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Ray D. PCR based evaluation of Theileria annulata in ticks collected from fields in West Bengal. J Vet Parasitol. 2003;17(1):11–14. [Google Scholar]
- Dickson J, Shiels BR. Antigenic diversity of a major merozoite surface molecule in Theileria annulata. Mol Biochem Parasitol. 1993;57:55–64. doi: 10.1016/0166-6851(93)90243-Q. [DOI] [PubMed] [Google Scholar]
- Glascodine J, Tetley I, Tait A, Brown D, Sheils BR. Developmental expression of a Theileria annulata merozoite surface antigen. Mol Biochem Parasitol. 1990;40:105–112. doi: 10.1016/0166-6851(90)90084-Y. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, d’Oliveira C, Jongejan F. Development of an indirect Tams1 enzyme linked immunosorbent assay for diagnosis of T. annulata infection in cattle. Clin Diagn Lab Immunol. 2000;7(3):404–411. doi: 10.1128/cdli.7.3.404-411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illhans T, Williamson S, Kirvir E, Sheils B, Brown CGD. Theileira annulata: carrier state and immunity. Ann NY Acad Sci. 1998;849:109–125. doi: 10.1111/j.1749-6632.1998.tb11040.x. [DOI] [PubMed] [Google Scholar]
- Kachani M, Oliver RA, Brown CGD, Ouhelli H, Spooner RL. Common and stage specific antigens of T. annulata. Vet Immunol Immunopathol. 1992;34:221–234. doi: 10.1016/0165-2427(92)90166-N. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:266–275. [PubMed] [Google Scholar]
- Manuja A, Nichani AK, Kumar R, Rakha NK, Kumar B, Sharma KD. Comparison of cellular schizont, soluble schizont and soluble piroplasm antigens in ELISA for detecting antibodies against Theileria annulata. Vet Parasitol. 2000;87:93–101. doi: 10.1016/S0304-4017(99)00166-1. [DOI] [PubMed] [Google Scholar]
- Minjauw B, McLeod A (2003) Tick borne disease and poverty. The impact of ticks and tick-borne disease on the livelihoods of small scale and marginal livestock owners in India and eastern and Southern Africa. Research report, DFID, Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh
- OIE (2000) Manual of standards for diagnostic tests and vaccines, vol 11, Chap. 2.3, 4th edn Theileriosis, p 1–14
- Rajendran C, Ray DD, Bansal GC. Theileria annulata (Parbhani Strain) exhibits single genotype of 32 kDa merozoite surface antigen. J Vet Parasitol. 2006;21:133–135. [Google Scholar]
- Rajendran C, Ray DD, Bansal GC. Expression of gene encoding immunodominant merozoite surface protein of Theileria annulata in Escherichia coli. Indian J Biotechnol. 2008;7:200–203. [Google Scholar]
- Ray D, Bansal GC, Datta B. Purification of intraerythrocytic piroplasms of Theileria annulata from bovine blood. Indian J Anim Sci. 1998;68:1167–1168. [Google Scholar]
- Subramanian G, Naithani RC, Ray D. Host responses to and characterization of some isolates of Theileria annulata (Dachunkosky and Luhs, 1984) Vet Parasitol. 1987;25:75–77. doi: 10.1016/0304-4017(87)90067-7. [DOI] [PubMed] [Google Scholar]
- Subramanian G, Ray D, Bansal GC, Srivastava RVN. A field trial with a live schizont vaccine (Theileria annulata) in adolescent crossbred cattle in India. Indian J Anim Sci. 1987;58:635–639. [Google Scholar]
- Towbin H, Stachelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Tait A, Brown D, Walker A, Beck P, Sheils B, Fletcher J, Hall R. Theileria annulata sporozoite surface antigen expressed in Escherichia coli elicits neutralizing antibody. Proc Natl Acad Sci USA. 1989;86:4639–4643. doi: 10.1073/pnas.86.12.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]