Abstract
One hundred and sixty patients having clinical features of severe malaria reported during monsoon season-August–October 2010 at this tertiary care center of north India. Of these 110 (68.75 %) had Plasmodium vivax infection, 30 (18.75 %) were infected with P. falciparum and 20 (12.5 %) had co-infection due to P. vivax and P. falciparum. The diagnosis was made using Rapid Card Test and was confirmed by peripheral smear examination of thick and thin films. Several complications such as acute kidney injury, jaundice, severe anemia, metabolic acidosis, shock, hyperpyrexia, hypoglycemia, generalized tonic–clonic convulsions etc. were found to be more prevalent in patients with P. vivax infection. These symptoms were until recently known to be associated with falciparum malaria.
Keywords: Plasmodium vivax, Plasmodium falciparum, Severe malaria, Co-infection
Introduction
The burden of malaria mortality is double than estimated in the World Malaria Report (2011). India is only second to Africa where the largest number of deaths occur due to malaria (Murray Christopher et al. 2012). Dhingra et al. (2010) estimated that about 200,000 people die of malaria in India each year. Until recently Plasmodium falciparum was considered to be the main cause of complicated malaria, but now severe clinical consequences resulting from vivax malaria have been reported (Kochar et al. 2005; Diamond-Smith et al. 2009). In the recent years a large number of cases of complicated malaria caused by Plasmodium vivax have been reported. The objective of the present work is to highlight the prevalence of severe malaria caused by P. vivax and to emphasize the need to recognize the clinical symptoms and aggressively treat the patients to avert the consequent mortality. Although the presentation of these patients has been like those infected with P. falciparum but on investigation the causative agent found was P. vivax. What needs to be emphasized is the need to be watchful with such patients and to widen our sphere of differentials and consider that P. vivax can lead to severe malaria. Such patients if managed aggressively and treated accordingly can have a better outcome. In the present work an effort has been made to delineate the cause of various clinical manifestations of severe malaria, which until now were thought to be a hallmark of falciparum malaria.
Materials and methods
This is a single center, retrospective, observational study in which 160 patients admitted to a tertiary care center of western Uttar Pradesh from 1st August 2010 to 31st October 2010 with complaints of fever with chills and having clinical features of severe malaria were included. These patients were screened for a possible malaria infection using a Rapid Card Test and then confirmed by examination of thick and thin films in the peripheral smear by two different pathologists. Those patients not infected with P.vivax or P. falciparum, in whom IgM was positive for leptospira by ELISA, who were IgM and IgG positive for typhoid by ELISA and those who were IgM and IgG positive for dengue were excluded from the study.
The cases found to be infected with P. vivax, P. falciparum or a co-infection were managed accordingly with artesunate based combination therapy, doxycycline and clindamycin. Patients with acute kidney injury (serum creatinine >3), jaundice (serum bilirubin >3 mg/dl), severe anemia (hemoglobin <5 g/dl), metabolic acidosis, shock, hyperpyrexia, hypoglycemia, generalized tonic–clonic convulsions were found out in each category (P. vivax, P. falciparum and co-infection). The percentage involvement in each category was calculated. Application of one-way ANOVA-F test showed a high-significant difference among different groups at 5 % level of significance (p value <0.05). Mean and standard deviation of variables in all the three categories were calculated.
Results
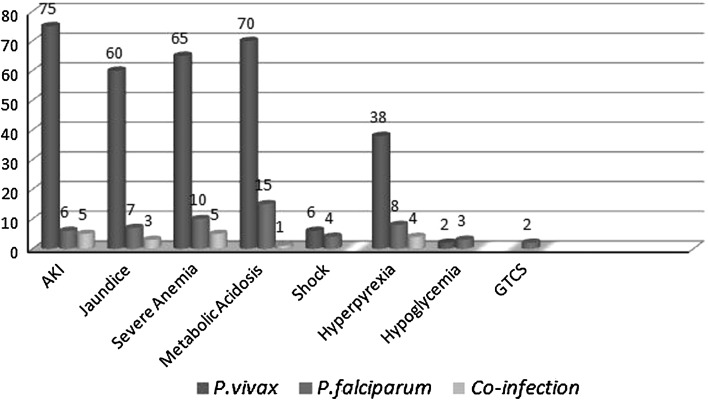
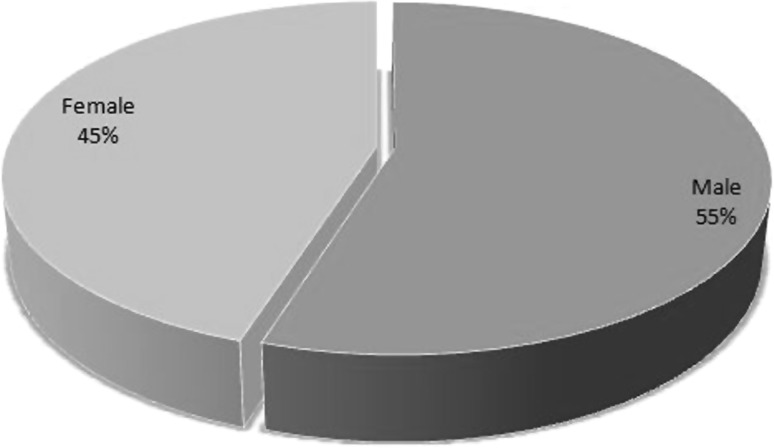
In the present pool of 160 patients, 110 (68.75 %) had P. vivax, 30 (18.75 %) had P. falciparum and 20 (12.5 %) had co-infection due to P. vivax and P. falciparum. The mean age of involvement of patients with P. vivax was 34.67 years (SD = 14.55). Clinical features of complicated malaria in patients of all the three groups at presentation are shown in Fig. 1. The sex distribution of the study population is shown in Fig. 2. Symptoms such as acute respiratory distress syndrome, disseminated intravascular coagulation (DIC) and coma, known to be features of severe malaria were not observed in any of the patients in the present study. The baseline characteristics of all the study patients are shown in Table 1. These characteristics in each of the three groups have been shown in Table 2. There was no mortality in any of the three groups. Application of one-way ANOVA-F test shows a high-significant difference among different groups for infection with P. vivax, P. falciparum and co-infection at 5 % level of significance (p < 0.05). A comparison of the characteristic features of patients of P. falciparum and P. vivax is shown in Table 3. Thirteen patients with P. falciparum and 9 patients with P. vivax having acute kidney injury were given renal replacement therapy in the form of hemodialysis. All the patients who were dialyzed had an uneventful recovery. Patients with severe anemia (hemoglobin <5 mg/dl) and thrombocytopenia (platelet count <150,000/mm3), who were hemodynamically unstable were given packed red blood cells and platelets transfusions respectively. On discharge, patients who were infected with P. vivax alone and those who had P. falciparum co-infection as well, were given a treatment of primaquine in a dosage of 15 mg/day in two divided doses for 14 days. A prior blood G6PD level was obtained before starting primaquine.
Fig. 1.
Clinical features of complicated malaria in patients of all the three groups at presentation
Fig. 2.
Pie chart showing the distribution of males and females in the study population
Table 1.
The baseline characteristics of all the patients in study group
| Mean | Standard deviation | |
|---|---|---|
| Age (years) | 34 | 14 |
| Haemoglobin (g/dl) | 6.31 | 3.2 |
| Platelets (per mm3) | 124352 | 76100.8 |
| Blood urea (mg/dl) | 87 | 54.6 |
| Serum creatinine (mg/dl) | 3.2 | 1.91 |
| Total bilirubin (mg/dl) | 3.31 | 2.4 |
| Direct bilirubin (mg/dl) | 2.3 | 1.69 |
| Indirect bilirubin (mg/dl) | 0.98 | 0.87 |
| SGOT (U/l) | 70.56 | 65 |
| SGPT (U/l) | 56.89 | 46 |
| Serum ALP (U/l) | 99.98 | 38.3 |
| pH | 7.23 | 0.14 |
| Temperature (°F) | 100.91 | 1.22 |
Table 2.
The baseline characteristics of patients in three groups
| P. vivax | P. falciparum | Co-infection | |
|---|---|---|---|
| Acute kidney injury | 75 | 6 | 5 |
| Jaundice | 60 | 7 | 3 |
| Severe anemia | 65 | 10 | 5 |
| Metabolic acidosis | 70 | 15 | 1 |
| Shock | 6 | 4 | – |
| Hyperpyrexia | 38 | 8 | 4 |
| Hypoglycemia | 2 | 3 | – |
| Generalized tonic–clonic convulsions | – | 2 | – |
Table 3.
Mean (SD) of patients infected with P. falciparum and P. vivax and p value of comparison between the two groups
| P. falciparum | P. vivax | p value | |
|---|---|---|---|
| Age (years) | 34.16 (13.3) | 34.85 (14.5) | 0.806 |
| Hemoglobin (g/dl) | 7.18 (3.69) | 5.84 (2.9) | 0.073 |
| Platelets (per mm3) | 149833.3 (102188.2) | 119323.6 (70498.4) | 0.132 |
| Blood urea (mg/dl) | 101.54 (58.78) | 83.16 (52.09) | 0.127 |
| Serum creatinine (mg/dl) | 2.48 (2.16) | 3.72 (1.70) | 0.005 |
| Total serum bilirubin (mg/dl) | 2.86 (1.73) | 3.64 (2.58) | 0.054 |
| Direct bilirubin (mg/dl) | 2.05 (1.27) | 2.53 (1.83) | 0.100 |
| Indirect bilirubin (mg/dl) | 0.78 (0.62) | 1.11 (0.95) | 0.028 |
| SGOT (U/l) | 77.5 (57.48) | 63.87 (65.10) | 0.269 |
| SGPT (U/l) | 50.23 (24.42) | 57.41 (52.58) | 0.286 |
| Serum alkaline phosphatase (U/l) | 98 (57) | 101.54 (33.4) | 0.747 |
| pH | 7.34 (0.10) | 7.24 (0.13) | 0.000 |
| Temperature (°F) | 101 (1.34) | 101.59 (1.20) | 0.692 |
Discussion
Malaria is one of the major public health problems of India. Around 1.5 million confirmed cases are reported annually by the National Vector Borne Disease Control Programme, of which about 50 % are due to P. falciparum (NMRI, New Delhi 2012). Malaria is completely curable provided effective treatment is started early. Delay in treatment may lead to serious consequences including death. Fever is the cardinal symptom of malaria. It is often associated with chills, rigors, headache, myalgia, arthralgia, anorexia, nausea and vomiting. Symptoms of malaria can often be non-specific and mimic other diseases like viral infections, enteric fever etc. These observations are largely based on a number of case reports worldwide and two recently reported large case series from Papua New Guinea (Genton et al. 2008) and Indonesia (Tjitra et al. 2008).
Malaria should be suspected in patients residing in endemic areas and in those patients who have recently visited an endemic area. All clinically suspected malaria cases should be investigated immediately by microscopy and/or Rapid Diagnostic Test. Complications in severe malaria are either sequestration related, such as cerebral malaria, renal dysfunction, hepatic dysfunction, and ARDS, or non-sequestration related, such as anemia and thrombocytopenia. Non-sequestration-related complications are found frequently in P. vivax infection. However, for sequestration-related complications, it was always presumed that coexisting P. falciparum infection may evade appearance in blood film because of heavy sequestration. The most common complications observed in the present study were jaundice and hepatic dysfunction, which are similar to the reported observations in severe P. falciparum malaria and P. vivax malaria in this region (Kochar et al. 2003a, b, 2005, 2006; Nautiyal et al. 2005). Acute kidney injury, which was the second most common complication, has also been reported frequently in the Indian subcontinent (Mehta et al. 2001; Mohapatra et al. 2002; Kochar et al. 2005). In our study acute kidney injury was observed in 75 patients in the P. vivax group, 6 patients in P. falciparum group and in 5 patients in the co-infected group. Cerebral malaria was observed in five patients; this has also been reported in one patient in Pakistan (Beg et al. 2002) and in three patients in India (Kochar et al. 2005) in malaria caused by P. vivax. Status epilepticus has also been reported in Turkey (Ozen et al. 2006) and India (Kochar et al. 2007a, b). In our study generalized tonic–clonic convulsions were observed in two patients infected with P. falciparum. Other reported neurologic manifestations include acute inflammatory demyelinating polyneuropathy (Chakravarty et al. 2004) and post-malaria neurologic syndrome causing bilateral facial paralysis (Kochar et al. 2007a, b), have not been reported in the present study.
Pulmonary syndromes associated with P. vivax malaria include acute non-cardiogenic pulmonary edema, ARDS, acute pulmonary injury, and interstitial pneumonia (Parren et al. 1998; Tanios et al. 2001; Habib and Singh 2004; Kochar et al. 2005; Taylor et al. 2006). These syndromes are also associated with high mortality rates for P. falciparum malaria and were the most commonly observed P. vivax-associated complications in the study in Papua New Guinea (Genton et al. 2008). Anemia was the most commonly observed complication in a recent study from Indonesia (Tjitra et al. 2008). Recent observations have shown evidence of sequestration of parasites in lung vasculature during evaluation of lung injury in P. vivax malaria (Nicholas et al. 2007). Cerebral dysfunction in P. vivax malaria may occur through generation of nitric oxide (Beg et al. 2002).Cytokines and leukotrienes may be responsible for severe anaemia and hemostatic complications (Clark and Cowden 1999). A report of P. vivax infection in pregnancy that resulted in low birth weight babies suggested sequestration of P. vivax in the placenta (Nosten et al. 1999). A recent study that analyzed malaria severity in P. vivax infection clearly demonstrated enhanced aggregation, erythrocyte clumping, and reduced deformability affecting microcirculation (Jayavanth and Park 2007). Shock has been reported as a complication of P.vivax malaria Kumar et al. (2007). In the present study 6 patients infected with P.vivax presented in shock.
Severe manifestations in P. falciparum malaria can develop within 12–24 h and may lead to death, if it is not treated promptly and adequately. Severe malaria is characterized by one or more of the following features: impaired consciousness/coma, repeated generalized convulsions, renal failure (serum creatinine >3 mg/dl), jaundice (serum bilirubin >3 mg/dl), severe anaemia (hemoglobin <5 g/dl), pulmonary oedema/acute respiratory distress syndrome, hypoglycaemia (plasma glucose <40 mg/dl), metabolic acidosis, circulatory collapse/shock (systolic BP <80, <50 mmHg in children), abnormal bleeding and dic, haemoglobinuria, hyperpyrexia (temperature >106°F or >42 °C), hyperparasitaemia (>5 % parasitized RBCs).The recent years have witnessed an increasing trend of severe malaria caused by P. vivax. In the present study, the manifestations of severity were strongly associated with P. vivax which included malaria 68.75 % of the total number of cases in the study group.
Conclusions
The present study reflects the association of severe malaria with P. vivax. It clearly shows that patients infected with P. vivax also present with manifestations of severe malaria like serum bilirubin >3 mg/dl, serum creatinine >3 mg/dl, severe anaemia (hemoglobin <5 mg/dl), acute kidney injury, metabolic injury, shock, generalized tonic–clonic convulsions and hypoglycemia. The severity of these symptoms shown by P. vivax malaria make it quite evident that it must be considered as a cause of severe malaria, which until recently was assumed to be a manifestation of P. falciparum malaria.
Acknowledgments
We the authors thank the management of Chattrapati Shivaji Subharti Hospital for allowing us to carry out our research work. We declare that we have not received funding from any source/body for carrying out our research work.
References
- Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–232. doi: 10.4269/ajtmh.2002.67.230. [DOI] [PubMed] [Google Scholar]
- Chakravarty A, Ghosh B, Bhattacharyya R, Sengupta S, Mukherjee S. Acute inflammatory demyelinating polyneuropathy following Plasmodium vivax malaria. Neurol India. 2004;52:130–131. [PubMed] [Google Scholar]
- Clark IA, Cowden WB. Why is the pathology of falciparum worse than that of vivax malaria. Parasitol Today. 1999;15:458–461. doi: 10.1016/S0169-4758(99)01535-5. [DOI] [PubMed] [Google Scholar]
- Dhingra N, Jha P, Sharma VP, et al., for the Million Death Study Collaborators (2010) Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet 376:1768–1774 [DOI] [PMC free article] [PubMed]
- Diamond-Smith N, Singh N, Gupta RK, et al. Estimating the burden of malaria in pregnancy: a case study from rural Madhya Pradesh, India. Malar J. 2009;8:24. doi: 10.1186/1475-2875-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton B, D’Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:1–9. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AG, Singh KS. Respiratory distress in non-immune adults with imported malaria. Infection. 2004;32:356–359. doi: 10.1007/s15010-004-4060-6. [DOI] [PubMed] [Google Scholar]
- Jayavanth S, Park BC. Microrheologic dysfunctions in blood during malaria. Indian J Exp Biol. 2007;45:111–120. [PubMed] [Google Scholar]
- Kochar DK, Agarwal RP, Kochar SK, Jain R, Rawat N, Pokharna RK, Kachhawaha S, Srivastava T. Hepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malaria. QJM. 2003;96:505–512. doi: 10.1093/qjmed/hcg091. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Singh P, Agarwal P, Kochar SK, Pokharna R, Sareen PK. Malarial hepatitis. J Assoc Physicians India. 2003;51:1069–1072. [PubMed] [Google Scholar]
- Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar DK, Kaswana K, Kochar SK, Sirohi P, Pal M, Kochar A, Agarwal RP, Das A. Comparative study of regression of jaundice in patients of malaria and acute viral hepatitis. J Vector Borne Dis. 2006;43:123–129. [PubMed] [Google Scholar]
- Kochar DK, Pakalapati D, Kochar SK, Sirohi P, Khatri MP, Kochar A, Das A. An unexpected cause of fever and seizures. Lancet. 2007;370:908. doi: 10.1016/S0140-6736(07)61417-2. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Sirohi P, Kochar SK, Bindal D, Kochar A, Jhajharia A, Goswami J. Post-malaria neurological syndrome—a case of bilateral facial palsy after Plasmodium vivax malaria. J Vector Borne Dis. 2007;44:227–229. [PubMed] [Google Scholar]
- Kumar S, Melzer M, Dodds P, Watson J, Ord R. P. vivax malaria complicated by shock and ARDS. Scand J Infect Dis. 2007;39:255–256. doi: 10.1080/00365540600904787. [DOI] [PubMed] [Google Scholar]
- Mehta KS, Halankar AR, Makwana PD, Torane PP, Shah VB. Severe acute renal failure in malaria. J Postgrad Med. 2001;47:24–26. [PubMed] [Google Scholar]
- Mohapatra MK, Padhiary KN, Mishra DP, Sethy G. Atypical manifestations of Plasmodium vivax malaria. Indian J Malariol. 2002;39:18–25. [PubMed] [Google Scholar]
- Murray Christopher L, Rosenfield Lisa C, Stephen LS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- National Malaria Research Institute, New Delhi (2012) Guidelines for the diagnosis and treatment of malaria in India
- Nautiyal A, Singh S, Parmeshwaran G, Disalle M. Hepatic dysfunction in a patient with Plasmodium vivax infection. Med Gen Med. 2005;7(1):8. [PMC free article] [PubMed] [Google Scholar]
- Nicholas MA, Handojo T, Michael CF, Tjitra E, Price RN, Graeme PM. Lung injury in vivax malaria: pathological evidence for pulmonary vascular sequestration and post treatment alveolar inflammation. JID. 2007;195:589–596. doi: 10.1086/510756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/S0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- Ozen M, Gungor S, Atambay M, Dalal N. Cerebral malaria owing to Plasmodium vivax : case report. Ann Trop Paediatr. 2006;26:141–144. doi: 10.1179/146532806X107494. [DOI] [PubMed] [Google Scholar]
- Parren A, Beretta F, Schubarth P. ARDS in Plasmodium vivax malaria. Schweiz Med Wochenschr. 1998;128:1020–1023. [PubMed] [Google Scholar]
- Tanios MA, Kogelman L, McGovern B, Hassoun PM. Acute respiratory distress syndrome complicating Plasmodium vivax malaria. Crit Care Med. 2001;29:665–667. doi: 10.1097/00003246-200103000-00037. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Canon V, White NJ. Pulmonary manifestations of malaria: recognition and management. Treat Respir Med. 2006;5:419–428. doi: 10.2165/00151829-200605060-00007. [DOI] [PubMed] [Google Scholar]
- Tjitra E, Nicholas MA, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. Multi drug resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:1–10. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]