Abstract
This study was designed to evaluate and compare the infection rate and cyst formation in male Balb/c mice following intraperitoneal injection of protoscoleces and activated oncospheres. Protoscoleces were collected aseptically from the liver of naturally infected sheep. The eggs were obtained from three experimentally infected dogs and activated oncospheres were prepared as described by Heath and Smyth (1970) and Kyngdon et al. (2006). A total of 20 Balb/c mice were divided into two groups of ten animals each. The mice of first and second groups were intraperitoneally infected with 1,500 protoescoleces and 1,500 activated oncosphere respectively. After 8 months of infection, all mice were euthanized, necropsied and the hydatid cysts were removed, counted, measured and weighed. The results showed that nine of ten mice of first group were infected. The maximum number of cysts was 551 in one mouse with mean size of 3.78 ± 2.21 mm and total weight of 25.48 g. The minimum number of cysts was 13 in one mouse with mean size of 3.93 ± 2.18 mm and total weight of 0.564 g. In the second group, only one mouse was infected with two cyst with mean size of 11.15 ± 1.94 mm and total weight of 1.193 g. This study showed that the male Balb/c inbred mouse is more permissive to infection with intraperitoneal injection of protoscoleces than activated oncospheres.
Keywords: Hydatid cyst formation, Intraperitoneal, Protoscoleces, Oncospheres, Balb/c mice
Introduction
Hydatid disease is one of the most important zoonotic helminthic diseases worldwide (Moazeni and Alipour-Chaharmahali 2011). The causative agent, Echinococcus granulosus, exists as a series of genetic variants which differ in a wide variety of criteria that impact on life cycle pattern, host specificity, development rate, pathogenicity, antigenicity, sensitivity to chemotherapeutic agents, population biology, transmission dynamics, and control of the disease (McManus and Smyth 1986; Thompson and Lymbery 1995; Sharifiyazdi et al. 2011). E. granulosus is a cestode whose life cycle involves dogs and other canids as definitive hosts in which adult tapeworms attach to the intestinal epithelium and undergo sexual reproduction, leading to the development of eggs that are shed into the environment with the faeces. Intermediate hosts include humans as well as cattle, sheep, camels, horses and others that acquire the infection by oral uptake of cestode eggs. After ingestion by suitable intermediate host and subsequent passage through stomach and intestine, the oncosphere larva become activated, penetrate the mucosa, enter to the blood stream and lymphatic vessels and are disseminated in the body. After an undefined incubation period, E. granulosus metacestodes are formed (Moro and Schantz 2009; Walker et al. 2004). For developmental biology, drug efficacy, immunological and other analytical and descriptive studies on metacestode of E. granulosus, two alternative tools have been developed. One is in vitro culture and the other is laboratory animal models. (Nakaya et al. 2006; Zak and Sande 1999). Various species of animals (sheep, baboons, monkey, rabbits, mice, gerbils) and various ways of infection (intragasteric administration of eggs, intraperitoneal, intravenous or subcutaneous injection of activated oncospheres and intraperitoneal inoculation of protoscoleces or cysts) have been described (Zak and Sande 1999). The factors involved in innate susceptibility/resistance (s/r) to Echinococcus infections are largely unknown. Different strains of mice which were infected with eggs, hatched eggs, or activated oncospheres of E. granulosus showed differences in s/r (Zhang et al. 2008). Since Balb/c mice is a suitable experimental model for cystic echinococcosis (Rahimi et al. 2011), the aim of this study was to evaluate and compare the infection rate of cyst formation in male Balb/c mice following the intraperitoneal injection of live protoscoleces and activated oncospheres.
Materials and methods
Chemicals
Pepsin and pancreatin for artificial gastric and intestinal fluid preparation, RPMI-1640 medium, Benzyl penicillin and Streptomycin sulfate were purchased from Sigma-Aldrich Co (St. Louis, MO, USA).
Collection of protoscoleces
Protoscoleces of E. granulosus were collected aseptically from the liver hydatid cysts of natural infected sheep, slaughtered in two abattoirs located in Shiraz and Marvdasht, Fars Province, southern Iran. The protoscoleces were then washed several times with sterile 0.85 % NaCl and stored in RPMI 1640 medium overnight at 37 °C. Viability of protoscoleces was determined by 0.1 % eosin staining.
Eggs collection
Three dogs were infected orally with viable protoscoleces of E. granulosus, after 11 weeks post infection, the dogs were euthanized and parasitized intestines were opened, cut into pieces and soaked in normal saline 37 °C until all worms had been released. The experiment was approved by the local ethics committee of School of Veterinary Medicine, Shiraz University, in accordance with the ethics standards of “Principles of Laboratory Animal Care”. The worms were collected and washed ten times by warm saline, followed by two washes in warm 2 % (w/v) sodium bicarbonate to dissolve residual mucus, then with two final washes in PBS. The worms were homogenized by an electric blender to release eggs and then the suspension was sieved and washed. The eggs were stored in PBS containing 1,000 IU/ml benzyl penicillin and 1,000 μg/ml streptomycin sulfate at 4 °C (Zhang et al. 2001).
Activation of oncospheres
Approximately 500,000 eggs were placed in each tube. Eggs were centrifuged at 1,000×g for 2 min, the supernatant withdrawn, and replaced by 10 ml of sterile artificial gastric fluid (AIF), at 37 °C. Tubes were mixed on a rotator at 37 °C for 1 h. Eggs were centrifuged at 1,000×g for 10 min, the supernatant was discarded and 10 ml of sterile artificial intestinal fluid (AIF) at 37 °C was added. Eggs were mixed on a rotator at 37 °C for 30 min, followed by centrifugation at 1,000×g for 10 min. The supernatant was discarded, and the pellet containing oncospheres was washed twice with 10 ml of sterile PBS, centrifuging for 2 min at 1,000×g each time. The pellet was finally suspended in an appropriate volume of sterile PBS (Heath and Smyth 1970, Kyngdon et al. 2006). The number of activated and unactivated oncospheres was then estimated by placing entire volume of 2 × 10 microlitre of suspension onto both sides of a Neubauer slide. The ×200 magnification was used to distinguish and count unactivated oncospheres from those free of their oncospheral membranes (activated oncospheres) (Heath and Lawrence 1996).
Infection of mice
Male Balb/c mice of 6 weeks age were used in this experiment. The mice were inbred and supplied by Laboratory Animal Research Center, Shiraz University of Medical Sciences, and were housed in a controlled temperature (20–24 °C), light-cycle 12 h light and 12 h dark and humidity 45–55 % with food and water ad libitum. A total of 20 Balb/c mice divided into two groups of ten mice each. In first group each mouse was experimentally infected by intraperitoneal injection of 1,500 protoscoleces dissolved in 0.2 ml of medium RPMI 1640 and in second group each mouse was experimentally infected by intraperitoneal injection of 1,500 activated oncosphere dissolved in 0.2 ml of PBS. The study was approved by the local ethics committee of School of Veterinary Medicine, Shiraz University, in accordance with the ethics standards of “Principles of Laboratory Animal Care”. After 8 months of infection the mice were euthanized and necropsy was carried out immediately thereafter. At necropsy, the peritoneal cavity was opened and different visceral organs were inspected and sliced carefully, and the hydatid cysts were removed and weighed. The cysts were carefully separated from each other and were photographed by a Sony digital still camera (Model NO: DSC-HX9V, Serial NO 2291889, Japan). Sizes of the cysts were determined based on the scaled ruler, using adobe Photoshop CS3. At first a pixel distance for each millimeter of scaled ruler was determined in the image and the diameter of each cyst was then determined in pixel. The cyst’s size was calculated in millimeter by dividing the diameter in pixel by each millimeter in pixel.
Results
In the first group which were infected with protoscoleces, nine of ten mice were infected, most of the cysts formed in peritoneal cavity, other site of cyst formation was lung (only in one mice) and subcutaneous, site of injection, in two mice. Weight and mean size of the cysts in every mouse are shown in Table 1. In second group which were infected with oncospheres, only two cysts were seen in peritoneal cavity of one mouse. There was no cyst in other organs. In the first group (protoscoleces), the maximum number of cysts was 551 in one mouse. Total weight of all 551 cysts was 25.48 g. The mean size of these cysts was 3.78 ± 2.21 mm. The minimum number of cysts was 13 in one mouse. Total weight of all 13 cysts was 0.564 g. The mean size of these cysts was 3.93 ± 2.18 mm (Fig. 1a). In the second group (oncospheres), total weight of two cysts was 1.193 g. The mean size of these cysts was 11.15 ± 1.94 mm (Fig. 1b).
Table 1.
Hydatid cyst formation in mice infected intraperitoneally with 1,500 protoscoleces (group 1) and 1,500 activated oncospheres (group 2)
| Mouse no. | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| Cyst number | Mean size (mm) | Weight (g) | Cyst number | Mean size (mm) | Weight (g) | |
| 1 | 551 | 3.78 ± 2.21 | 25.48 | 2 | 11.15 ± 1.94 | 1.193 |
| 2 | 30 | 3.97 ± 2.11 | 1.6215 | – | – | – |
| 3 | 27 | 4.67 ± 3.26 | 2.83 | – | – | – |
| 4 | 280 | 3.94 ± 2.18 | 14.16 | – | – | – |
| 5 | 43 | 3.87 ± 1.34 | 2.548 | – | – | – |
| 6 | 56 | 4.09 ± 1.96 | 2.52 | – | – | – |
| 7 | 13 | 3.93 ± 2.18 | 0.564 | – | – | – |
| 8 | 247 | 2.52 ± 1.37 | 4.24 | – | – | – |
| 9 | 52 | 2.07 ± 0.76 | 0.376 | – | – | – |
| 10 | – | – | – | – | – | – |
Fig. 1.
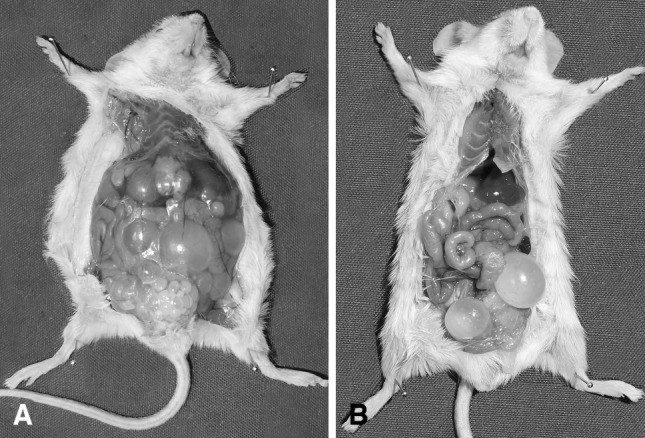
Hydatid cysts developed in peritoneal cavity of the mouse which received 1,500 protoscoleces (a) and 1,500 activated oncospheres (b)
Discussion
Cystic echinococcosis can develop in two types of infection. Primary infection occurs by ingestion of oncospheres, while secondary infection is caused by dissemination of protoscoleces after accidental rupture of fertile cysts (Mourglia-Ettlin et al. 2011). Breijo et al. (2008) showed that establishment and survival of hydatid cyst is associated with the control of the early inflammatory response which depends on down regulation of complement activation. In different host species the local reaction to the cyst is diverse, ranging from a severe granulomatous type lesion to a non-infiltrated collagenous capsule, which the first one is associated with cyst degeneration and eventual death, while the second one correlates with a stable host-parasite relationship that allows the development of fertile cysts (Breijo et al. 2008). Echinococcus metacestodes in suitable intermediate host are characterized by rapid growth and profuse production of protoscoleces, whereas in less suitable species growth is delayed and protoscoleces formation is impaired or absent (Zak and Sande 1999). Even different strains of mice infected with eggs, hatched eggs, or activated oncospheres of E. granulosus showed differences in susceptibility/resistance (Zhang et al. 2008). In an experimental infection, CF1 white mice were infected orally with E. granulosus eggs, 66 % of mice became infected. Although the lungs were frequently parasitized, the liver was the favored site of election. Cyst growth was greater for abdominal cysts than for thoracic. Protoscoleces and brood capsules were first seen at 195 days post infection (Colli and Schantz 1974). In another study, after oral infection of Kunming strain of mouse with E. granulosus eggs, resulting cysts were distributed in liver (72 %) and lung or thoracic cavity (28 %), cyst resulting from i.v. injection of activated oncospheres were restricted to lung and thoracic cavity but the majority of cysts were found in the peritoneal cavity following i.p. inoculation of activated oncospheres (Zhang et al. 2001). Dempster et al. infected DBA/2J, CBA/J, Balb/cJ, C57/B16J and CF-1 mice orally and parenterally with eggs, hatched eggs or activated oncospheres. Less than 1 % of the oral dose resulted in cyst establishment and approximately 80 % of eggs were shed unhatched, this may be due to inability of mouse bile to stimulate oncosphere activation. Intraperitoneal injection of 600 activated oncospheres into Balb/cJ mice resulted in considerable cyst formation (15–51 cysts per mouse). When activated oncospheres were injected intraperitoneally into Balb/cJ, DBA/2J and CF-1 mice, cysts were restricted to the peritoneal cavity while in intravenous injection of activated oncospheres, the cysts lodged almost exclusively in the lung and thoracic cavity, except in DBA/2J mice where 55 % of cysts were lodged in the liver (Dempster et al. 1991). In this study infection of male Balb/c inbred mice with intraperitoneal injection of protoscoleces resulted in a higher infection rate compared to activated oncospheres. In E. granulosus initial reorganization of the oncosphere and formation of the germinal and laminated layers occur rapidly (14 days) but because of host-parasite interplay dynamic, the outcome of infection depends on the balance achieved by the combination of the different variables involved with the host immunity and the parasite avoidance strategies include virulence of parasite species or strains, susceptibility/resistance of the host, parasite burden, infection frequency, etc. (Conchedda et al. 2004). Cystic echinococcosis (CE) enhances both humoral and cellular (Th1 and Th2) responses in infected intermediate host (Rahimi et al. 2011). The results of our study showed higher infection rate after intraperitoneal injection of protoscoleces, in comparison with intraperitoneal injection of activated oncospheres and the most common site of the cyst formation was peritoneal cavity. Our results are in accordance with Dempster et al. report (1991). In conclusion our study showed that the male Balb/c inbred mouse is more permissive to infection with intraperitoneal injection of protoscoleces than activated oncospheres.
References
- Breijo M, Anesetti G, Martinez L, Sim RB, Ferreira AM. Echinococcus granulosus: the establishment of the metacestode is associated with control of complement-mediated early inflammation. Exp Parasitol. 2008;118:188–196. doi: 10.1016/j.exppara.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Colli CW, Schantz PM. Growth and development of Echinococcus granulosus from embryophores in an abnormal host (Mus musculus) J Parasitol. 1974;60:53–58. doi: 10.2307/3278678. [DOI] [PubMed] [Google Scholar]
- Conchedda M, Gabriele F, Bortoletti G. Immunobiology of cystic echinococcosis. Parassitologia. 2004;46:375–380. [PubMed] [Google Scholar]
- Dempster RP, Berridge MV, Harrison GBL, Heath DD. Echinococcus granulosus: development of an intermediate host mouse model for use in vaccination studies. Int J Parasitol. 1991;21:549–554. doi: 10.1016/0020-7519(91)90059-G. [DOI] [PubMed] [Google Scholar]
- Heath DD, Lawrence SB. Antigenic polypeptides of Echinococcus granulosus oncospheres and definition of protective molecules. Parasite Immunol. 1996;18:347–357. doi: 10.1046/j.1365-3024.1996.d01-114.x. [DOI] [PubMed] [Google Scholar]
- Heath DD, Smyth JD. In vitro cultivation of Echinococcus granulosus, Taenia hydatigena, T. ovis, T.pisiformis and T. serialis from oncosphere to cystic larva. Parasitology. 1970;61:329–343. doi: 10.1017/S0031182000041184. [DOI] [PubMed] [Google Scholar]
- Kyngdon CT, Gauci CG, Rolfe RA, Velásquez Guzmán JC, Farfán Salazar MJ, Verástegui Pimentel MR, Gonzalez AE, Garcia HH, Gilman RH, Strugnell RA, Lightowlers MW. In vitro oncosphere-killing assays to determine immunity to the larvae of Taenia pisiformis, Taenia ovis, Taenia saginata and Taenia solium. J Parasitol. 2006;92:273–281. doi: 10.1645/GE-619R.1. [DOI] [PubMed] [Google Scholar]
- McManus DP, Smyth JD. Hydatid disease (hydatidosis): changing concepts in epidemiology and speciation. Parasitol Today. 1986;2:163–168. doi: 10.1016/0169-4758(86)90147-X. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Alipour-Chaharmahali MR. Echinococcus granulosus: in vitro effectiveness of warm water on protoscolices. Exp Parasitol. 2011;127:14–17. doi: 10.1016/j.exppara.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Mourglia-Ettlin G, Marque JM, Chabalgoity JA, Dematteis S. Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl Trop Dis. 2011;5:1–11. doi: 10.1371/journal.pntd.0001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya K, Mamuti W, Xiao N, Sato MO, Wandra T, Nakao M, Sako Y, Yamasaki H, Ishikawa Y, Craig PS, Schantz PM, Ito A. Usefulness of severe combined immunodeficiency (scid) and inbred mice for studies of cysticercosis and echinococcosis. Parasitol Int. 2006;55:91–97. doi: 10.1016/j.parint.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Rahimi HR, Sarkari B, Mohammadzadeh T, Sadjjadi SM. Immune responses to antigens of in vitro reared Echinococcus granulosus adult worms in Balb/c mice. Iran J Immunol. 2011;8:236–243. [PubMed] [Google Scholar]
- Sharifiyazdi H, Oryan A, Ahmadnia S, Valinezhad A. Genotypic characterization of Iranian camel (Camelus deromdarius) isolates of Echinoccocus granulosus. J Parasitol. 2011;97:251–255. doi: 10.1645/GE-2642.1. [DOI] [PubMed] [Google Scholar]
- Thompson RCA, Lymbery AJ. Echinococcus and hydatid disease. In: Thompson RCA, editor. Biology and systematics of echinococcus. 1. Wallingford: CAB International; 1995. pp. 1–50. [Google Scholar]
- Walker M, Rossignol JF, Torgerson P, Hemphill A. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J Antimicrob Chemother. 2004;54:609–616. doi: 10.1093/jac/dkh386. [DOI] [PubMed] [Google Scholar]
- Zak O, Sande MA. Hand book of animal models of infection. In: Romig T, Bilger B, editors. Animal models for echinococcosis. 1. San Diego, London, Boston: Academic press; 1999. pp. 877–884. [Google Scholar]
- Zhang W, You H, Zhang Z, Turson G, Hasyet A, McManus DP. Further studies on an intermediate host murine model showing that primary Echinococcus granulosus infection is protective against subsequent oncospheral challenge. Parasitol Int. 2001;50:279–283. doi: 10.1016/S1383-5769(01)00086-1. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ross AG, McManus DP. Mechanisms of immunity in hydatid disease: implications for vaccine. J Immunol. 2008;181:6679–6685. doi: 10.4049/jimmunol.181.10.6679. [DOI] [PubMed] [Google Scholar]


