Abstract
A survey of parasites of freshwater fishes in Harike, Kanjali and Ropar wetland of Punjab (India) revealed the presence of two new and two already known myxosporean species belonging to the genus Thelohanellus Kudo, 1933 parasitizing fins and gills respectively. Spores of the first species, T. kalavatae n. sp. measure 11.5 × 4.9 μm, elongately oval in valvular view having rounded blunt anterior end and rounded posterior end with lateral walls almost parallel to each other. Polar capsule is globular in shape and measure 5.2 × 3.3 μm in size. Anterior end of the polar capsule terminate into a small distinct neck. Spores of the second species, T. kalbensi n. sp. measure 9.5 × 4.9 μm, egg shaped to ovoid in valvular view having narrower anterior end with a prominent pore and broad rounded posterior end. Polar capsule is globular in shape with a short distinct tubular neck, measure 4.8 × 3.16 μm and occupies almost two-third of the spore body cavity. Spores of third species, T. avijiti Basu and Haldar, 2003 measure 10.1 × 6.6 μm, egg shaped in valvular having tapering, bluntly pointed anterior end and broad rounded posterior end. Polar capsule is rounded to sub-spherical in shape, measure 3.3 × 3.0 μm and is situated anteriorly. Spores of fourth species, T. gangeticus Tripathi, 1952 measure 13.3 × 4.8 μm, elongately pyriform in valvular view having tapering anterior end and rounded posterior end. Polar capsule is also elongately pyriform in shape measure 6.6 × 3.1 μm with thin neck and occupy half of the spore body cavity.
Keywords: Gills, Harike wetland, Plasmodia, Punjab
Introduction
Wetlands are the ecotones or transitional zones between permanently aquatic and dry terrestrial ecosystems. Ramsar convention has defined wetlands as “areas of marsh, peat land or water, whether natural or artificial, temporary or permanent, with water that is static or flowing, fresh, brackish or salty including area of marine water, the depth of which at low tide does not exceed six meter”. In Punjab, there are 12 natural, 10 man-made wetlands covering 15,500 Ha area and only 3 main wetlands are included in Ramsar list of International importance i.e., Harike, Kanjali and Ropar wetlands. State has 2 other wetlands of national importance and 5 of state importance (ENVIS Centre, PSCST, Punjab; The Tribune, Feb 04, 2008). These wetlands have extremely rich biodiversity as they support a variety of plant and animal life. Flora is comprised of variety of trees, bushes, shrubs, herbs, grasses and fauna consists of different types of fishes, amphibians, reptiles and birds. Harike wetland is the largest freshwater wetlands in northern India occupying 4,100 Ha of area harbour 16 species of freshwater fishes (Punjab State Council for Science and Technology Chandigarh, 2002). Kanjali wetland with an area of 185 Ha support diversity of resident and migratory birds, nurture large number of fish fauna with as many as 17 species of fishes. Ropar wetland is an important habitat of many species and has tremendous ecological value, spread over 1,365 Ha, this wetland support as many as 35 species of fishes. These fishes are vulnerable to various parasitic infections, out of which Myxozoa is emerging as the major group. They cause production loss and deaths and some fish have to be discarded because they are unsightly and not considered to be fit for human consumption.
Up till now, Phylum Myxozoa include 4 malacosporean and 2,180 myxosporean species to a total of 62 genera (Lom and Dykova 2006). However, three more genera (Soricimyxum, Gadimyxa, Thelohanelloid) with type species S. fegati Prunescu et al. 2007 from liver of Sorex araneus; G. atlantica Koie et al. 2007 from urinary system of Gadus morhua and T. bengalensis Sarkar 2009 from gall bladder of Arius sagor have been described subsequently. Phylum Myxozoa has been studied by only limited number of workers in Indian subcontinent. Most of the work on this phylum has been done on fresh water as well as marine fishes of mainly two states, West Bengal and Andhra Pradesh.
In north India, Gupta and Khera (1987, 1988a, b, c, d, 1989a, b, 1990, 1991) recorded 25 species belonging to genera Myxobolus, Henneguya, Myxidium, Thelohanellus and Unicauda infecting freshwater fishes.
There is an addition in the existing number of myxosporean species particularly those belonging to Myxobolus since 2007 in India alone. Recently, Kaur and Singh (2008, 2009, 2010a, b, 2010/2011, 2011a, b, c, d, e, f, 2012a) have contributed 17 new species to the genus Myxobolus and 1 new species of the genus Triangular from freshwater fishes in wetlands of Punjab. Kaur and Singh (2012b) also compiled and published a synopsis of 131 nominal species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) reported from India and a revised dichotomous key of 59 genera of the Phylum Myxozoa (class Myxosporea).
There are very few numbers of species of the genus Thelohanellus reported all over the world. In a monograph, Lom and Dykova (1992) enlisted 39 species of this genus. Basu and Haldar (1999) described a new species of Thelohanellus from gills of hybrid carps and gave a checklist of its different species described from Indian fishes. Basu et al. (2006) provided a synopsis of 32 indian species belonging to the genus Thelohanellus including one new species- T. disporomorphus infecting Indian major carp, Cirrhina mrigala. Kalavati and Nandi (2007) gave a compilation of 27 species of genus Thelohanellus infecting Indian fishes.
During the present study on the fishes of Harike, Kanjali and Ropar wetlands of Punjab (India), two new species, T. kalavati sp. nov., T. kalbensi sp. nov. and two already known species T. avijiti Basu and Haldar 2003 and T. gangeticus Tripathi 1952 collected from fins and gills of C. reba, L. bata and L. calbasu respectively. The description has been prepared in accordance with the guidelines of Lom and Arthur (1989).
Materials and methods
Fishes collected from Harike, Kanjali and Ropar wetlands were brought to the laboratory and examined for myxozoan infections. Plasmodia when found were removed and teased on slide and covered with cover slip and examined under the oil immersion for the presence of myxospores. Fresh spores were treated with 8 % KOH solution for the extrusion of polar filaments. For permanent preparation, air-dried smears were stained with Ziehl-Neelsen and Iron-haematoxylin. Drawings were made from stained material with the aid of camera lucida. Measurements of spores were done with the aid of a calibrated ocular micrometer. All measurements are presented in μm as range values followed by mean ± SD in parentheses. The abbreviations used in the paper are as follows: LS, Length of spore; WS, Width of spore; LPC, Length of polar capsule; WPC, Width of polar capsule; NC, Number of coils of polar filaments; SD, Standard deviation.
Results and discussion
T. kalavatae n. sp. (Figs. 1a, b, 2a, b, 3a, b)
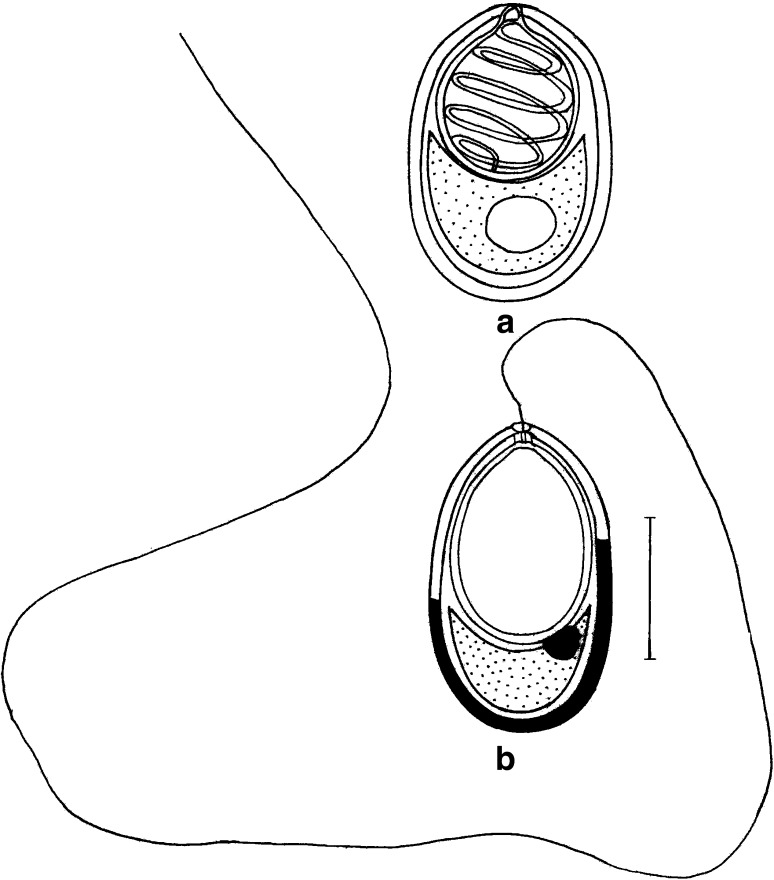
Fig. 1.
T. kalavatae sp. nov. a spore stained in Ziehl-Neelsen (valvular view), b spore stained in Iron-haematoxylin (extruded polar filament) Scale bar = 0.005 mm
Fig. 2.
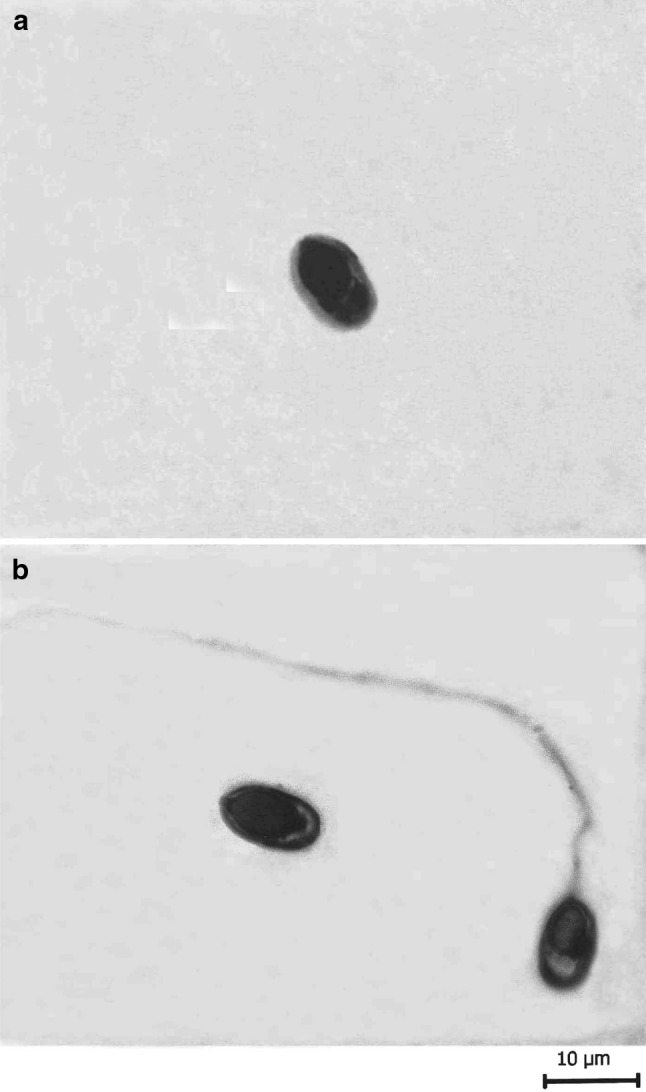
T. kalavatae sp. nov. a spore stained in Ziehl-Neelsen, b spores stained in Iron-haematoxylin (extruded polar filament)
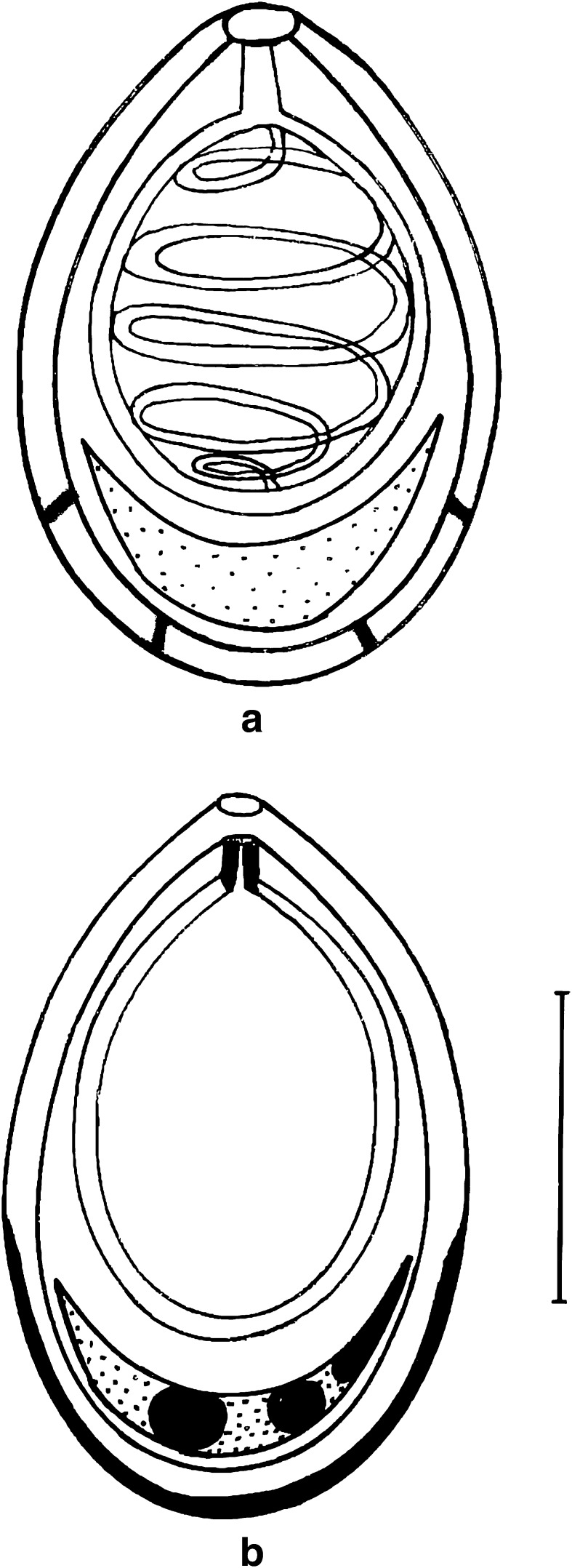
Fig. 3.
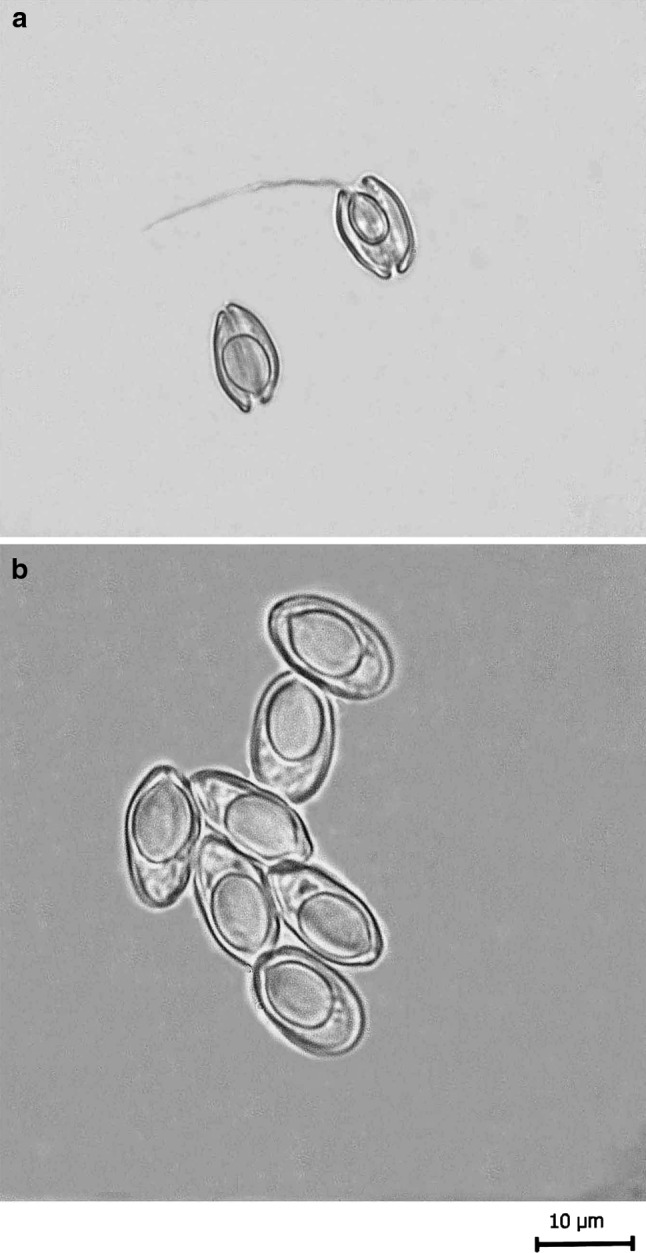
a, b Fresh spores of T. kalavatae sp. nov. a spores in side view (polar filament everted), b spores in valvular view
Plasmodia
White, spherical to rounded, present on the caudal fin, 3–4 in number and measure 0.6–0.8 mm in diameter. 7–8 spores are present per plasmodium.
Spore (Table 1) measurements based on 7–8 spores in frontal view
Table 1.
Measurements (μm) and ratio of T. kalavatae n. sp.
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 11.0–12.0 | 11.5 | 0.70 |
| WS | 4.3–5.5 | 4.9 | 0.84 |
| LPC | 4.7–5.7 | 5.2 | 0.70 |
| WPC | 2.9–3.7 | 3.3 | 0.56 |
| Ratio: LS/WS | 2.3 | ||
| NC | 5–6 | ||
| Parietal folds | Absent |
The spores are histozoic, measure 11.5 × 4.9 μm, elongately oval in valvular view having rounded blunt anterior end and rounded posterior end with lateral walls almost parallel to each other. Shell valves are thick, smooth, symmetrical and measure 0.65 μm in thickness. Shell valves at the posterior end of the spore appear thicker (which stain dark blue with Heidenhain’s Iron-haematoxylin) than the rest on the spore body. Parietal folds are absent. Polar capsule is globular in shape and measure 5.2 × 3.3 μm in size. Anterior end of the polar capsule terminate into a small distinct neck. Polar filament form 5–6 coils arranged slightly oblique to the polar capsule axis and measure 53–54 μm in length after eversion. One capsulogenic nucleus is present beneath the polar capsule measuring 0.33 μm in diameter. Sporoplasm is agranular, homogenous, half moon shaped and occupy whole of the extracapsular space behind the polar capsule. An iodinophilous vacuole is present and measure 3.1 μm in diameter.
Taxonomic characters
- Type host:
Cirrhina reba (Ham.) vern. chunni, mori, kursa
- Type locality:
Harike wetland, Punjab, India
- Type specimen:
Paratypes are spores stained in Ziehl-Neelsen and Iron-haematoxylin, deposited in the museum of department of Zoology, Punjabi University, Patiala, India. Slide no. TH/ZN/12.04.1010 and TH/IH 12.04/1010
- Site of infection:
Caudal fin
- Prevalence of infection:
25 % (3/12)
- Etymology:
The specific epithet kalavatae has been given after the name of the Dr. C. Kalavati, an eminent worker of the department of Zoology, Andhra University, Waltair, India
Discussion
The present species of Thelohanellus was compared with T. niloticus Gurley 1893 from skin of head of Labeo niloticus; T. seni (Southwell and Prashad 1918) Chakravarty and Basu 1948 from branchiae of Catla catla; T. mrigalae Tripathi 1952 from skin on the head of C. mrigala; T. nikolski Akhmerov 1955 from fin of Cyprinus carpio haematopterus; T. potaili Lalitha Kumari 1969 from fin of L. potail; T. sanjibi Sarkar and Ghosh 1990 from kidney of Mystus gulio; T. sudevi Sarkar and Ghosh 1990 from kidney of Amblypharyngodon mola; T. caudatus Pagarkar and Das 1993 from between rays of caudal fin and anal fin of L. rohita; T. orissae Haldar et al. 1997 from gills of C. mrigala and T. avijiti Basu and Haldar 2003 from dorsal fin of L. rohita; T. habibpuri Acharya and Dutta 2007 from pectoral fin of L. rohita and T. imphlaensis Hemananda et al. 2010/2011 from gills of L. rohita but differ from all of the above species in morphological and morphometric characteristics (Table 2).
Table 2.
Comparative description of T. kalavatae n. sp. with morphologically similar species (measurements are in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| T. kalavatae n. sp. (present study) | Cirrhina reba | Caudal fin | Harike wetland, Punjab (India) | 11.5 × 4.9 | 5.2 × 3.3 |
| T. niloticus Gurley 1893 | Labeo niloticus | Skin of head | Nile (Egypt) | 5.0 × 3.5 | – |
| T. seni (Southwell and Prashad 1918) Chakravarty and Basu 1948 | Catla catla | Branchiae | West Bengal (India) | 12.48–14.94 | 8.56 |
| T. mrigalae Tripathi 1952 | C. mrigala | Skin on the head | West Bengal (India) | 10.8–12.0 × 6.3–7.2 | 5.4–7.2 × 3.6–5.0 |
| T. nikolski Akhmerov 1955 | Cyprinus carpio haematopterus | Fin | Amur basin (Russia) | 19.0–20.0 × 12.0 | 7.0 × 5.0–6.0 |
| T. potaili Lalitha Kumari 1969 | L. potail | Fin | Andhra Pradesh (India) | 13.0 × 8.2 | 5.9 × 4.3 |
| T. sanjibi Sarkar and Ghosh 1990 | Mystus gulio | Kidney | West Bengal (India) | 12.52 × 8.27 | 4.52 × 4.0 |
| T. sudevi Sarkar and Ghosh 1990 | Amblypharyngodon mola | Kidney | West Bengal (India) | 14.05 × 5.87 | 5.17 × 2.65 |
| T. caudatus Pagarkar and Das 1993 | L. rohita | Between rays of caudal fin and anal fin | West Bengal (India) | 13.8 × 9.0 | 7.02 × 5.07 |
| T. orissae Haldar et al. 1997 | C. mrigala | Gills | Orissa (India) | 7.29 × 3.11 | 3.72 × 2.32 |
| T. avijiti Basu and Haldar 2003 | L. rohita | Dorsal fin | West Bengal (India) | 14.0 × 9.7 | 6.0 × 4.0 |
| T. habibpuri Acharya and Dutta 2007 | L. rohita | Pectoral fin | West Bengal (India) | 13.0–14.3 (13.9) × 8.0–9.0 (8.5) | 6.0–6.5 (6.0) × 4.1–5.0 (4.9) |
| T. imphlaensis Hemananda et al. 2010/2011 | L. rohita | Gills | Imphal, Manipur (India) | 20.4–22.1 (21.33) × 8.5–10.2 (9.43) | 10.2–11.05 (10.79) × 3.40–4.25 (3.78) |
The present species have spores elongately oval in shape with rounded, blunt anterior end and rounded posterior end with lateral walls almost parallel to each other. Polar capsule is globular in shape. The anterior end of the polar capsule terminate into a distinct neck and the posterior end is rounded in outline. Morphologically, the present species is comparable with the spores of T. sanjibi, T. sudevi and T. nikolski. However, spores in T. sanjibi are egg to ovoidal in shape and more pointed anteriorly; elongately ellipsoidal spores with slightly acuminated anterior end in T. sudevi and egg to ovoidal shaped spore with variable shape and size in T. nikolski differentiate all of them from the present species.
Furthermore, the shell valves at the posterior end of the spore appear thicker (stain dark blue with Heidenhain’s Iron-haematoxylin) than the rest on the spore body in the species under study.
In view of the above differences, the present species under study is proposed as new to the science and named as T. kalavatae n. sp. through this communication.
T. kalbensi n. sp. (Figs. 4a, b, 5a, b, 6)
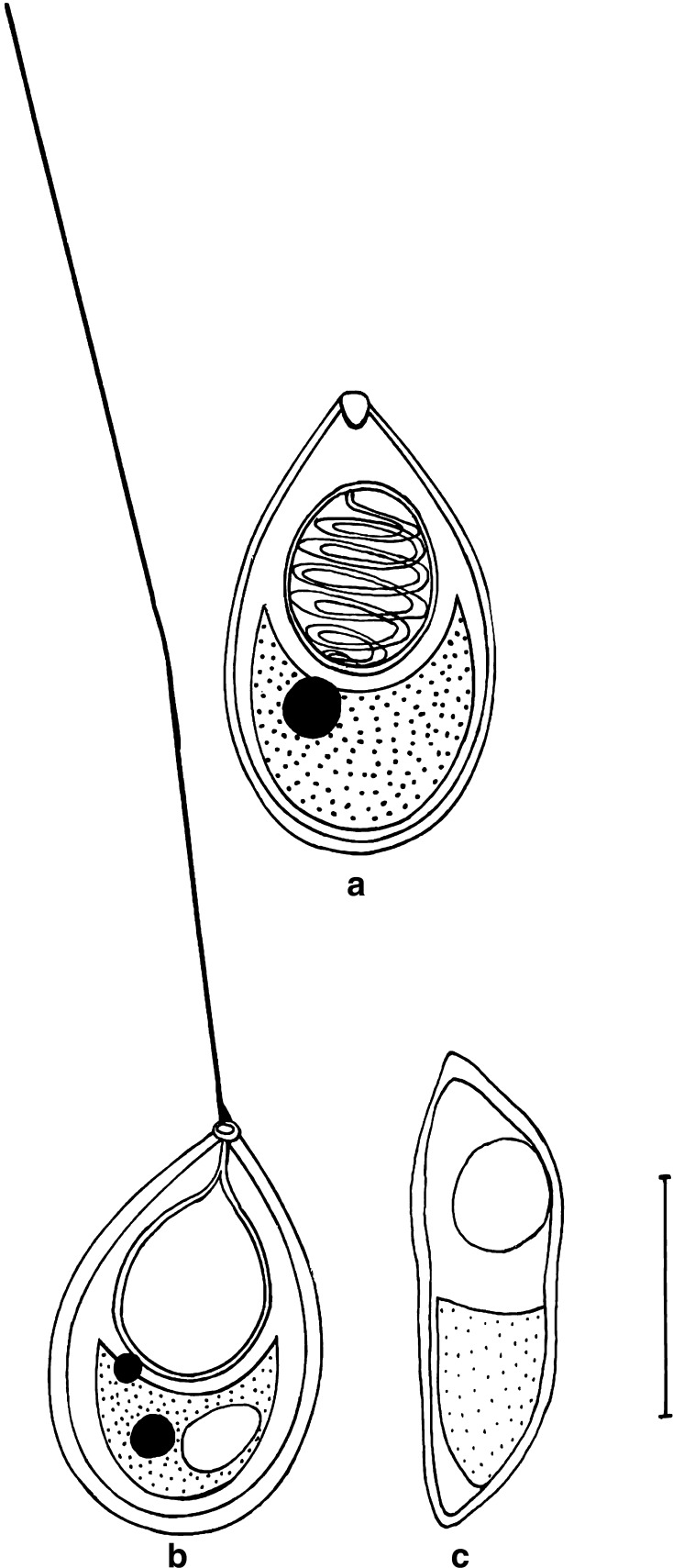
Fig. 4.
T. kalbensi sp. nov. a spore stained in Ziehl-Neelsen (valvular view), b spore stained in Iron-haematoxylin Scale bar = 0.005 mm
Fig. 5.
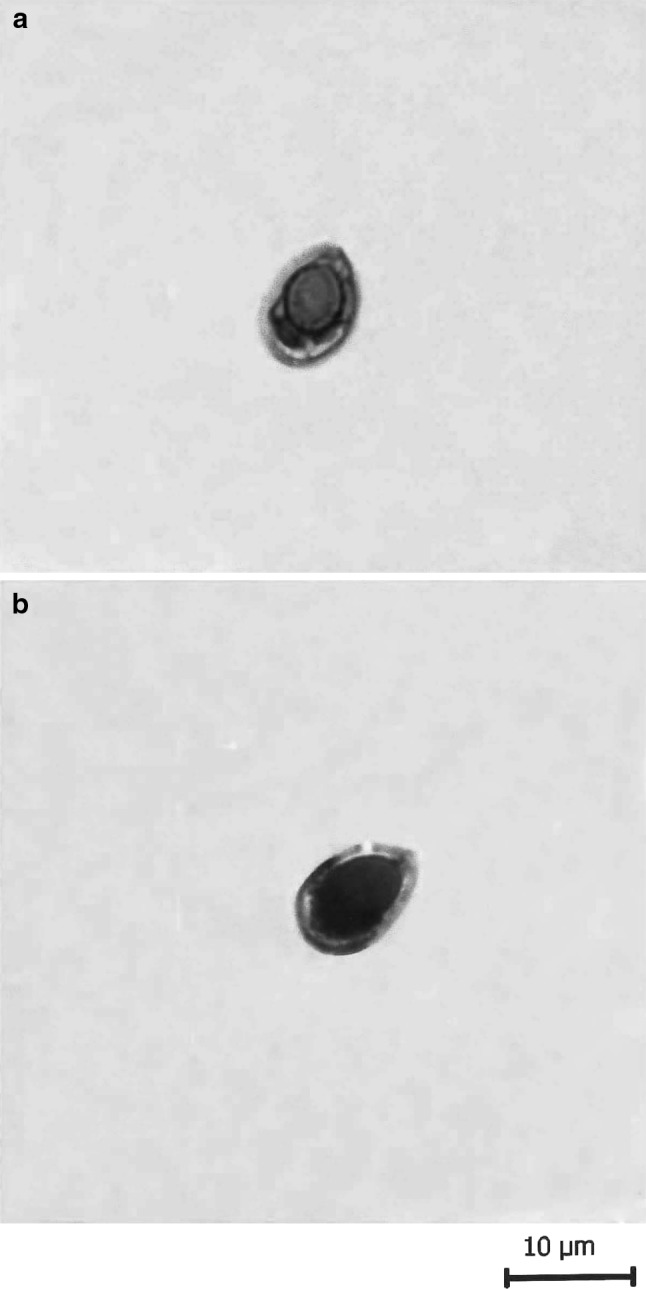
T. kalbensi sp. nov. a spore stained in Ziehl-Neelsen, b spore stained in Iron-haematoxylin
Fig. 6.
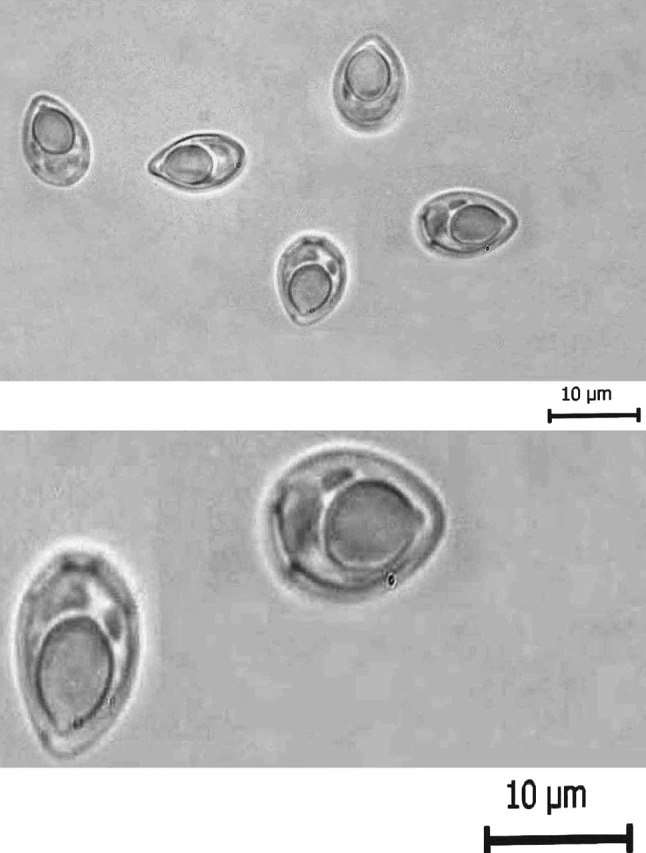
Fresh spores of T. kalbensi sp. nov. a Normal view, b enlarged view showing parietal folds
Plasmodia
Minute, present in the gills, 6–8 spores are present per plasmodium.
Spore (Table 3) measurements based on 6–8 spores in frontal view
Table 3.
Measurements (μm) and ratio of T. kalbensi n. sp.
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 9.0–10.0 | 9.5 | 0.70 |
| WS | 4.4–5.4 | 4.9 | 0.70 |
| LPC | 4.4–5.2 | 4.8 | 0.56 |
| WPC | 2.8–3.4 | 3.16 | 0.42 |
| Ratio: LS/WS | 1.9 | ||
| NC | 5–6 | ||
| Parietal folds | Absent |
The spores are histozoic, measure 9.5 × 4.9 μm, egg shaped to ovoid in valvular view having narrower anterior end with a prominent pore and broad rounded posterior end. Shell valves are thick, smooth, symmetrical and measure 0.5 μm in thickness. Parietal folds are absent. Shell valves at the posterior end of the spore appear thicker (which stains dark blue with Heidenhains, Iron-haematoxylin) than the rest on the spore body. Polar capsule is globular in shape with a short distinct tubular neck, measure 4.8 × 3.16 μm and occupies almost two-third of the spore body cavity. Polar filament form 5–6 coils arranged perpendicular to the polar capsule axis. Sporoplasm is agranular, homogenous, half moon shaped and occupies whole of the extracapsular space behind the polar capsule. Sporoplasm contain three nuclei, larger one measuring 1.0 μm and other two 0.8–0.9 μm in diameter. An iodinophilous vacuole is absent.
Taxonomic characters
- Type host:
Labeo calbasu (Ham.) vern. kalbans
- Type locality:
Kanjali wetland, Punjab, India
- Type specimen:
Paratypes are spores stained in Ziehl-Neelsen and Iron-haematoxylin, deposited in the museum of department of Zoology, Punjabi University, Patiala, India. Slide no. LC/I/ZN 01.02.2010 and LC/I/IH 01.02.2010
- Site of infection:
Gills
- Prevalence of infection:
20 % (2/10)
- Etymology:
The specific epithet kalbensi has been given after the vernacular name of the fish
Discussion
The present species under study was compared with T. niloticus Gurley 1893 from skin of head of Labeo niloticus; T. rohitae Southwell and Prashad 1918 from gills of L. rohita; T. seni Southwell and Prashad 1918 from gills, fin of Catla catla; T. calbasui Tripathi 1952 from scales of L. calbasu; T. mrigalae Tripathi 1952 from skin on the head of C. mrigala; T. nikolski Akhmerov 1955 from fin of Cyprinus carpio haematopterus; T. caudatus Pagarkar and Das 1993 from the rays, caudal and anal fin of Labeo rohita; T. andhrae Qadri, 1962 from gills of L. fimbriatus; T. boggoti Qadri 1962 from gills of L. boggot; T. shortii Qadri 1967 from fin of L. fimbriatus; T. batae Lalitha Kumari 1969 from gill filaments of L. bata; T. potaili Lalitha Kumari 1969 from fin of L. potail; T. qadrii Lalitha Kumari, 1969 from gill filaments of L. potail;T. bengalensis Sarkar and Raychaudhuri 1986 from gall bladder of Catla catla; T. chilkensis Kalavati and Vaidehi 1991 from gall bladder of L. rohita; T. assambai Fomena et al. 1994 of Labeo sp.; T. costeae Sakiti 1997 from gills of L. senegalensis; T. ndjamenaensis Kostoingue et al. 1999 from gills of L. parvus; T. bicornei Kabre et al. 2002 from intestine of L. coubie; T. avijiti Basu and Haldar 2003 from dorsal fin of L. rohita; T. chandannagarensis Basu and Haldar 2003 from gill filament of Catla catla; T. endodermitus Mukhopadhyay and Haldar 2004 from under scales of L. rohita and T. habibpuri Acharya and Dutta 2007 from pectoral fin of L. rohita; T. zahrahae Szekely et al. 2009 from gills of Barbonymus gonionotus and T. imphlaensis Hemananda et al. 2010/2011 from gills of L. rohita but differ from all of the above species in morphological and morphometric characteristics (Table 4).
Table 4.
Comparative description of T. kalbensi n. sp. with morphologically similar species (measurements are in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| T. kalbensi n. sp. (present study) | Labeo calbasu | Gills | Harike wetland, Punjab (India) | 9.5 × 4.9 | 4.8 × 3.16 |
| T. niloticus Gurley 1893 | Labeo niloticus | Skin of head | Nile (Egypt) | 5.0 × 3.5 | – |
| T. rohitae Southwell and Prashad 1918 | L. rohita | Gills | West Bengal (India) | 30.0–33.0 × 10.0–13.0 | 16.0–20.0 × 7.8–24.0 |
| T. seni Southwell and Prashad 1918 | Catla catla | Gills, fin | West Bengal (India) | 12.48–14.94 × 8.56 | 6.42 × 4.52 |
| T. calbasui Tripathi 1952 | L. calbasu | Scales | West Bengal (India) | 9.0–10.8 × 7.2 | 5.4 × 34 |
| T. mrigalae Tripathi 1952 | Cirrhina mrigala | Skin on the head | West Bengal (India) | 10.8–12.0 (11.4) | 5.4–7.2 × 3.6–5.0 |
| T. nikolski Akhmerov 1955 | Cyprinus carpio haematopterus | Fin | Amur basin (Russia) | 19.0–20.0 × 12.0 | 7.0 × 5.0–6.0 |
| T. caudatus Pagarkar and Das 1993 | L. rohita | Rays, caudal, anal fin | West Bengal (India) | 13.8 × 9.0 | 7.02 × 5.07 |
| T. andhrae Qadri, 1962 | L. fimbriatus | Gills | Andhra Pradesh (India) | 11.2–14.5 × 4.5–5.5 | 6.0–8.0 × 2.0–2.5 |
| T. boggoti Qadri 1962a | L. boggot | Gills | Andhra Pradesh (India) | 11.5 × 6.75 | 6.2 × 3.8 |
| T. andhrae Qadri 1962b | L. fimbriatus | Gills | Andhra Pradesh; Kerala (India) | 11.2–14.5 × 4.5–5.5 | 6.0–8.0 × 2.0–2.5 |
| T. shortii Qadri 1967 | L. fimbriatus | Fin | Andhra Pradesh (India) | 12.53 × 6.91 | 7.07 × 4.2 |
| T. batae Lalitha Kumari 1969 | L. bata | Gill filaments | Andhra Pradesh (India) | 12.3 × 6.2 | 7.7 × 3.0 |
| T. potaili Lalitha Kumari 1969 | L. potail | Fin | Andhra Pradesh (India) | 13.0 × 8.2 | 5.9 × 4.3 |
| T. qadrii Lalitha Kumari 1969 | L. potail | Gill filaments | Andhra Pradesh (India) | 14.7 × 5.4 | 8.2 × 3.8 |
| T. bengalensis Sarkar and Raychaudhuri 1986 | Catla catla | Gall bladder | West Bengal (India) | 10.95 × 6.59 | 5.42 × 3.47 |
| T. chilkensis Kalavati and Vaidehi 1991 | L. rohita | Gall bladder | Orissa (India) | 26.7 × 8.7 | 17.54 × 7.01 |
| T. assambai Fomena et al. 1994 | Labeo sp. | – | Africa | 10.5 × 6.0 | 7.5 × 2.7 |
| T. costeae Sakiti 1997 | L. senegalensis | Gill | Benin (Africa) | 8.5–10.5 (9.4) × 5.0–6.5 (5.6) | 4.0–5.5 (4.8) × 2.0–3.0 (2.6) |
| T. ndjamenaensis Kostoingue et al. 1999 | L. parvus | Gill | Chad (Central Africa) | 10.0–11.0 (10.0) × 7.0–8.0 (7.3) | 4.0–5.0 (4.2) × 3.0–5.0 (3.2) |
| T. bicornei Kabre et al. 2002 | L. coubie | Intestine | Burkina Faso (Africa) | 13.0–14.0 (13.5) × 8.0–9.0 (8.4) | 6.5–8.0 (7.2) × 3.5–4.0 (3.7) |
| T. avijiti Basu and Haldar 2003 | L. rohita | Dorsal fin | West Bengal (India) | 14.0 × 9.7 | 6.0 × 4.0 |
| T. chandannagarensis Basu and Haldar 2003 | Catla catla | Gill filaments | West Bengal (India) | 12.5 × 6.7 | 5.1 × 3.1 |
| T. endodermitus Mukhopadhyay and Haldar, 2004 | L. rohita | Under scales | West Bengal (India) | 13.66 × 5.35 | 7.14 × 3.0 |
| T. habibpuri Acharya and Dutta 2007 | L. rohita | Pectoral fin | West Bengal (India) | 13.9 × 8.5 | 6.0 × 4.9 |
| T. zahrahae Szekely et al. 2009 | Barbonymus gonionotus | Gills | Malaysia | 23.8 × 9.0 | 9.9 × 6.3 |
| T. imphlaensis Hemananda et al. 2010/2011 | L. rohita | Gills | Imphal (India) | 20.4–22.1 (21.33) × 8.5–10.2 (9.43) | 10.2–11.05 (10.79) × 3.40–4.25 (3.78) |
The present species (LS/WS: 1.9) under study have spores egg shaped to ovoid in outline. Anterior end of the spore is narrower ending into a wide, prominent pore and, posterior end is broad and rounded. In this respect, it is comparable with spores of T. avijiti, T. boggoti, T. habibpuri and T. caudatus. But the anterior end is slightly acuminated and the polar capsule occupy almost half of the spore body cavity in T. avijiti (LS/WS:1.4); polar capsule is flask-shaped in T. boggoti (LS/WS:1.70); larger spores in T. habibpuri (LS/WS: 1.6) containing polar capsule occupying less than half of the spore body cavity and anteriorly pointed spores containing a pyriform polar capsule in T. caudatus (LS/WS: 1.5) differentiates all of them from the present species.
Furthermore, the polar capsule is globular in shape with a distinct tubular neck occupying almost two-third of the spore body cavity in the species under consideration. In addition, the presence of 4–5 parietal folds in present species separates it from all of the above species (see fresh spores). In this respect, the present species can be compared with T. andhrae Qadri, 1962 in which the spore as well the polar capsule are elongated pyriform with tapering anterior end in contrast to egg shaped to ovoid spores having globular polar capsule with a short distinct tubular neck. Shell valves at posterior end of the spore appear thicker (which stains dark blue with Heidenhains Iron-haematoxylin) than the rest on the spore body.
In view of the above differences, the present species is proposed as new to the science and named as T. kalbensi n. sp. through this communication.
T. avijiti Basu and Haldar 2003 (Figs. 7a–c, 8a, b)
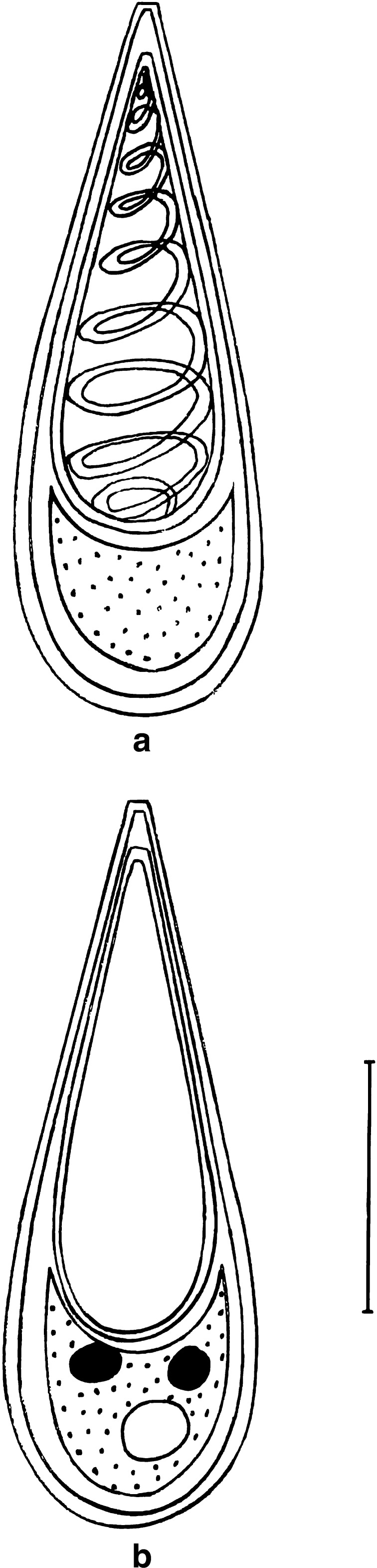
Fig. 7.
T. avijiti Basu and Haldar 1981a. a spore stained in Ziehl-Neelsen (valvular view), b spore stained in Iron-haematoxylin (extruded polar filament), c spore in side view, Scale bar = 0.005 mm
Fig. 8.
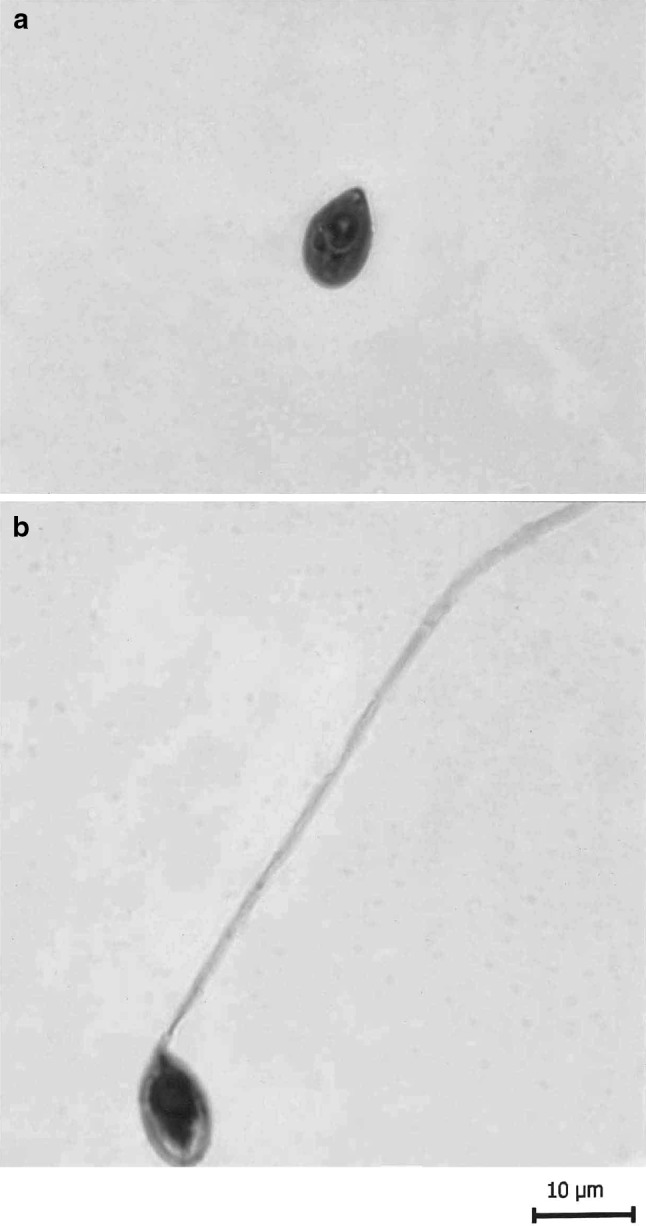
T. avijiti Basu and Haldar 1981a. a spore stained in Ziehl-Neelsen, b spore stained in Iron-haematoxylin (extruded polar filament)
Plasmodia
Small, white, rounded to spherical, present on the pelvic fin, 2–3 in numbers and measure 0.6–0.8 mm in diameter. 8–10 spores are present per plasmodium.
Spore (Table 5) measurements based on 8–9 spores in frontal view
Table 5.
Measurements (μm) and ratio of T. avijiti Basu and Haldar 2003
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 9.9–10.3 | 10.1 | 0.28 |
| WS | 6.0–7.2 | 6.6 | 0.84 |
| LPC | 2.9–3.7 | 3.3 | 0.56 |
| WPC | 2.9–3.1 | 3.0 | 0.14 |
| Ratio: LS/WS | 1.5 | ||
| NC | 6–7 | ||
| Parietal folds | Absent |
The spores are histozoic, measure 10.1 × 6.6 μm, egg shaped in valvular having tapering, bluntly pointed anterior end and broad rounded posterior end. Shell valves are thin, smooth, symmetrical and measuring 0.16 μm in thickness. Parietal folds are absent. Polar capsule is rounded to sub-spherical in shape, measure 3.3 × 3.0 μm and is situated anteriorly. It occupies almost half of the spore body cavity and contain 6–7 coils of polar filament arranged obliquely to the polar capsule axis. Polar filament is thick and thread-like measuring 70 μm in length, extrude through a prominent pore anteriorly. One capsulogenic nucleus is present beneath the polar capsule and measure 0.65 μm in diameter. Sporoplasm occupies whole of the extracapsular space behind the polar capsule and contain a nucleus measuring 0.95 μm. An iodinophilous vacuole measuring 2.8 μm in diameter is present.
Taxonomic characters
- Host:
Labeo bata (Ham.) vern. bata
- Locality:
Ropar wetland, Punjab, India
- Site of infection:
Pelvic fin
- Prevalence of infection:
20 % (3/15)
Remarks
The present observations (LS/WS: 1.5) on T. avijiti Basu and Haldar 2003 are in conformity with original description (LS/WS: 1.4), however, spore and polar capsule are smaller in size in the present species. In addition, a prominent pore is present at the anterior end of the spore of the present species. Earlier, this parasite was recorded from dorsal fin of Labeo rohita. A new location- pelvic fin and a new locality- Ropar wetland are recorded for this parasite (Table 6).
Table 6.
Comparative description of T. avijiti Basu and Haldar 2003 with original species (measurements are in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| T. avijiti (present study) | Labeo bata | Pelvic fin | Ropar wetland, Punjab (India) | 10.1 × 6.6 | 3.3 × 3.0 |
| T. avijiti Basu and Haldar 2003 | L. rohita | Dorsal fin | West Bengal (India) | 14.0 × 9.7 | 6.0 × 4.0 |
T. gangeticus Tripathi 1952 (Figs. 9a, b, 10a, b)
Fig. 9.
T. gangeticus Tripathi 1952. a spore stained in Ziehl-Neelsen (valvular view), b spore stained in Iron-haematoxylin, Scale bar = 0.005 mm
Fig. 10.
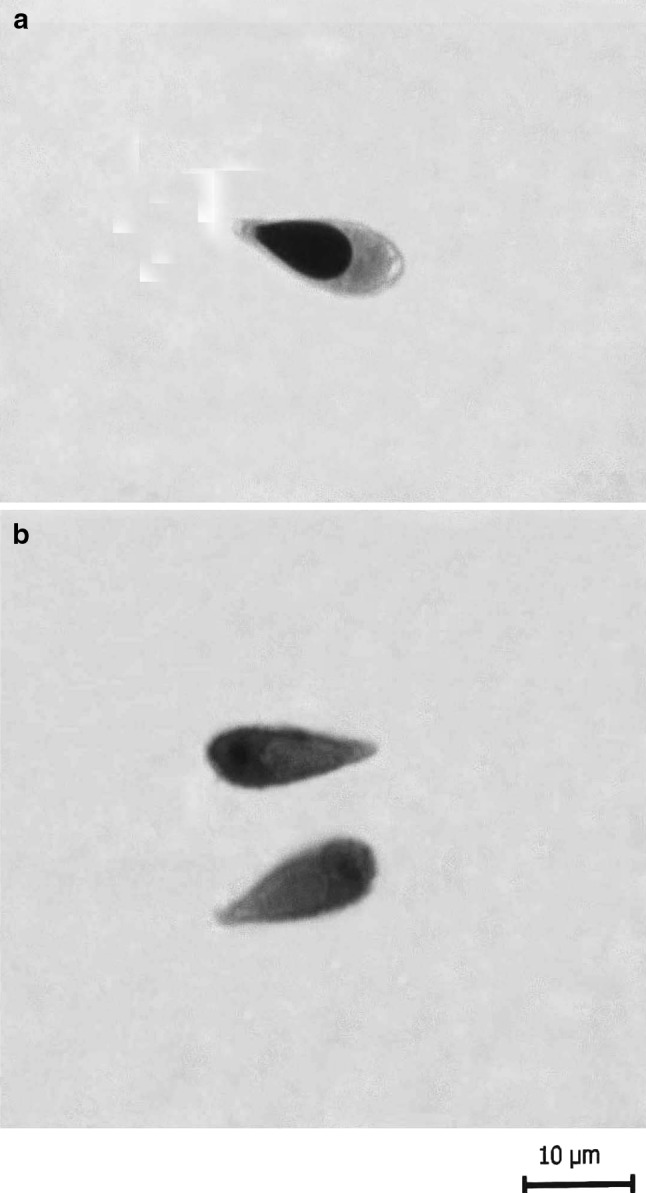
T. gangeticus Tripathi 1952. a spore stained in Ziehl-Neelsen, b spore stained in Iron-haematoxylin
Minute, present in the gills, 4–5 spores are present per plasmodium.
Spore (Table 7) measurements based on 8–10 spores in frontal view
Table 7.
Measurements (μm) and ratio of T. gangeticus Tripathi, 1952
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 13.0–13.6 | 13.3 | 0.42 |
| WS | 4.6–5.0 | 4.8 | 0.28 |
| LPC | 6.2–7.0 | 6.6 | 0.56 |
| WPC | 2.9–3.3 | 3.1 | 0.28 |
| Ratio: LS/WS | 2.7 | ||
| NC | 9–10 | ||
| Parietal folds | Absent |
The spores are histozoic, measure 13.3 × 4.8 μm, elongately pyriform in valvular view having tapering anterior end and rounded posterior end. Shell valves are thin, smooth, symmetrical and measure 0.41 μm in thickness. Parietal folds are absent. Polar capsule is also elongately pyriform in shape measure 6.6 × 3.1 μm with thin neck and occupy half of the spore body cavity. Polar filament form 9–10 coils arranged obliquely to the polar capsule axis. Sporoplasm occupies whole of the extracapsular space behind the polar capsule and contain two sporoplasmic nuclei measuring 1.2–1.3 μm in diameter. An iodinophilous vacuole is present measuring 1.6 μm in diameter.
Taxonomic characters
- Host:
Labeo calbasu (Ham.) vern. kalbans
- Locality:
Kanjali wetland, Punjab, India
- Site of infection:
Gills
- Prevalence of infection:
20 % (2/10)
- Plasmodia
Remarks
The present observations (LS/WS: 2.7) on T. gangeticus Tripathi, 1952 are in conformity with the original description (LS/WS: 3.1) except some variations in the size of spore and polar capsule. Earlier, this parasite was recorded from muscles of Chela bacaila. A new host- Labeo calbasu, a new location- gills and a new locality- Kanjali wetland are recorded for this parasite (Table 8).
Table 8.
Comparative description of T. gangeticus Tripathi 1952 with original species (measurements are in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| T. gangeticus (present study) | Labeo calbasu | Gills | Kanjali wetland, Punjab (India) | 13.3 × 4.8 | 6.6 × 3.1 |
| T. gangeticus Tripathi 1952 | Chela bacaila | Muscles | West Bengal (India) | 16.2–17.5 × 5.4 | 7.2 × 2.5 |
Acknowledgments
The authors express thanks to University Grants Commission (UGC) for the financial support.
Contributor Information
Ranjeet Singh, Email: ranjitsrana@gmail.com.
Harpreet Kaur, Email: harpreet_bimbra@yahoo.com.
References
- Acharya S, Dutta T. Thelohanellus habibpuri sp. n. (Myxozoa: Bivalvulida) from the tropical freshwater fish rohu, Labeo rohita (Hamilton-Buchann, 1882) in West Bengal, India: Light and electron microscope observations. Anim Biol. 2007;57(3):293–300. doi: 10.1163/157075607781753119. [DOI] [Google Scholar]
- Akhmerov AK. Ways of the origin of myxosporidian species of the genus Thelohanellus Kudo from Amur wild carp. Doklad kad Nauk SSSR. 1955;105:1129–1132. [Google Scholar]
- Basu S, Haldar DP. Thelohanellus bifurcata n. sp. a new species of the genus Thelohanellus from hybrid carps and checklist of the species of the genus described from Indian fishes. Proc Zool Soc Calcutta. 1999;52(1):115–124. [Google Scholar]
- Basu S, Haldar DP. Observations on two new thelohanelloid species (Myxozoa: Bivalvulida) from Indian major carps of West Bengal. India J Parasitol Appl Anim Biol. 2003;12(1&2):15–24. [Google Scholar]
- Basu S, Modak BK, Haldar DP. Synopsis of the Indian species of the genus Thelohanellus Kudo, 1933 along with description of Thelohanellus disporomorphus sp. n. J Parasitol Appl Anim Biol. 2006;15(1–2):81–94. [Google Scholar]
- Chakravarty M, Basu MS. Observations on some myxosporidians parasitic in fishes, with an account of nuclear cycles in one of them. Proc Zool Soc Bengal. 1948;1:23–33. [Google Scholar]
- Fomena A, Marques A, Bouix G, Njine T (1994) Myxobolus bilongi n. sp., Thelohanellus assambai n. sp., T. sanagaensis n. sp., Myxosporidies parasites de Labeo sp. (Teleosteen, Cyprinidae) du Bassin de la Sanaga au Cameroun (Afrique Centrale). Annales de la Faculte des Sciences de Yaounde 131–142
- Gupta S, Khera S. On the genera Henneguya Thelohan, 1892 and Unicauda Davis, 1944. Res Bull (Sci) Panj Univ. 1987;38:153–163. [Google Scholar]
- Gupta S, Khera S. Review of the genus Myxobolus Bütschli, 1882. Res Bull (Sci) Panj Univ. 1988;39(I–II):45–48. [Google Scholar]
- Gupta S, Khera S. On a new myxozoan parasite (Myxozoa) Lomosporus indicus gen. sp. n. from fresh water fishes, Labeo calbasu (Ham.) Acta Protozool. 1988;27:171–175. [Google Scholar]
- Gupta S, Khera S. On a new species, Myxidium labeonis from freshwater fishes of Punjab, India. Arch Protistenkd. 1988;136:393–396. doi: 10.1016/S0003-9365(88)80020-4. [DOI] [Google Scholar]
- Gupta S, Khera S. On one new and one already known species of the genus Myxobolus from freshwater fishes of India. Res Bull (Sci) Panj Univ. 1988;39(III-IV):173–179. [Google Scholar]
- Gupta S, Khera S. Observations on Myxobolus haldari sp. nov. (Myxozoa: Myxosporea) from freshwater fishes of North India. Res Bull (Sci) Panj Univ. 1989;40:281–291. [Google Scholar]
- Gupta S, Khera S. Observations on Myxobolus punjabensis sp. nov. (Myxozoa: Myxobolidae), parasitic on gills and fins of Labeo dyocheilus. Riv Parasitol. 1989;50(1–2):131–138. [Google Scholar]
- Gupta S, Khera S. On three species of the genus Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea) from freshwater fishes of Northern India. Indian J Parasitol. 1990;14:1–8. [Google Scholar]
- Gupta S, Khera S. On some species of the genus Myxobolus (Myxozoa: Myxosporea) from freshwater fishes of India. Indian J Parasitol. 1991;15(1):35–47. [Google Scholar]
- Gurley RS. On the classification of the Myxospora group of protozoan parasites infesting fishes. Bull US Fish Comm. 1893;11:407–431. [Google Scholar]
- Haldar DP, Das MK, Sharma BK. Studies on Protozoan Parasites from Fishes. Four New Species of the Genera Henneguya Thelohan, 1892. Thelohanellus Kudo, 1933 and Myxobolus Butschli, 1892. Arch Protistenkd. 1983;127:283–296. doi: 10.1016/S0003-9365(83)80023-2. [DOI] [Google Scholar]
- Haldar DP, Samal KK, Mukhopadhyay D. Studies in the protozoan parasites of fishes in Orissa: five new species of the genera Henneguya,Thelohanellus and Unicauda (Myxozoa: Bivalvulida) J Bengal Nat Hist Soc. 1997;16(2):50–63. [Google Scholar]
- Hemananda T, Mohilal N, Bandyopadhyay PK, Mitra AK (2010/2011) Thelohanellus imphalensis sp. nov. (Myxozoa) infecting gills of a major carp Labeo rohita Hamilton 1822 from Thoubal, Manipur, India. Protistology 6 (4):280–283
- Kabre GB, Sakiti NG, Marques A, Sawadogo I. Thelohanellus bicornei sp. n., (Myxosporea, Bivalvulida) a gill parasite of Labeo coubie Ruppel, 1832 (Osteichthyes, Cyprinidae) from Burkina Faso, West Africa. Parasite. 2002;9:219–223. doi: 10.1051/parasite/2002093219. [DOI] [PubMed] [Google Scholar]
- Kalavati C, Nandi NC. Handbook of Myxosporidean parasites of Indian fishes. Kolkata: ZSI; 2007. p. 293. [Google Scholar]
- Kalavati C, Vaidehi J. A new myxosporidian, Thelohanellus chilkensis n. sp. from the gills of the common carp, Labeo rohita in Chilka lake, Orissa, India. Uttar Pradesh J Zool. 1991;11(1):73–78. [Google Scholar]
- Kaur H, Singh R (2008) Observations on one new species, of Genus Myxobolus- M. naini and rediscription of M. magauddi recorded from freshwater fishes of Kanjali Wetland of Punjab, India. In: Proceedings 20th National Congress Parasitol, NEHU, Shillong, pp 75–79
- Kaur H, Singh R. A new myxosporean species, Myxobolus eirasi sp nov, a known species M. venkateshi Seenappa, Manohar (1981) from the Indian major carp fish Cirrhina mrigala (Ham) Protistology. 2009;6(2):126–130. [Google Scholar]
- Kaur H, Singh R (2010/2011) Two new species of Myxobolus (Myxosporea, Bivalvulida) from the Indian major carp Labeo rohita Hamilton, 1822. Protistology 6(4):264–270
- Kaur H, Singh R. One new myxosporidian species, Myxobolus slendrii sp. nov., one known species, M. punjabensis Gupta, Khera (1989) infecting freshwater fishes in wetlands of Punjab, India. India Parasitol Res. 2010;106(5):1043–1047. doi: 10.1007/s00436-010-1746-9. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R. Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) from freshwater fishes of Punjab Wetlands (India) J Parasit Dis. 2011;35(1):33–41. doi: 10.1007/s12639-011-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Singh R. Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting an Indian major carp in Ropar and Kanjali wetlands (Punjab) J Parasit Dis. 2011;35(1):23–32. doi: 10.1007/s12639-011-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Singh R. Myxobolus harikensis sp. nov. (Myxozoa: Myxobolidae) infecting fins of Cirrhina mrigala (Ham.)—an Indian major carp in Harike Wetland, Punjab (India) Parasitol Res. 2011;109(6):1699–1705. doi: 10.1007/s00436-011-2445-x. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R (2011d) Two new and one already known species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting gill lamellae of Indian major carp fishes in Ropar and Harike wetlands (Punjab). In: Proceeding 22nd National Congress of parasitology, University of West Bengal, Kalyani, pp 81–90
- Kaur H, Singh R. Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting Indian freshwater fishes in Punjab Wetlands (India) Parasitol Res. 2011;108(5):1075–1082. doi: 10.1007/s00436-011-2307-6. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R. Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting an Indian major carp and a cat fish in wetlands of Punjab, India. J Parasit Dis. 2011;35(2):169–176. doi: 10.1007/s12639-011-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Singh R. One new myxosporean species, Triangula cirrhini sp. nov., and one known species, T. ludhianae (Syn. M. ludhianae Gupta and Khera, 1991) comb. n. (Myxozoa: Myxosporea), infecting Indian major carp in Harike wetland of Punjab. Anim Biol. 2012;62:129–139. [Google Scholar]
- Kaur H, Singh R. A synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Bivalvulida) parasitizing Indian fishes and a revised dichotomous key to myxosporean genera. Syst Parasitol. 2012;81:17–37. doi: 10.1007/s11230-011-9321-z. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R, et al. A new myxosporean species Myxobolus sclerii sp. nov. and one known species M. stomum Ali et al. (2003) from two Indian major carp fishes. J Parasit Dis. 2010;34(1):33–39. doi: 10.1007/s12639-010-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koie M, Karlsbakk E, Nylund A. A new genus Gadimyxa with three new species (Myxozoa, Parvicapsulidae) parasitic in marine fish (Gadidae) and the two-host life cycle of Gadimyxa atlantica n. sp. J Parasitol. 2007;93(6):1459–1467. doi: 10.1645/GE-1256.1. [DOI] [PubMed] [Google Scholar]
- Kostoingue B, Fall M, Faye N, Toguebaye BS. Three new myxosporidian (Myxozoa: Myxosporea) parasites of freshwater fishes from Chad (Central Africa) Acta Protozool. 1999;38:323–326. [Google Scholar]
- Kudo R. A taxonomic consideration of Myxosporidia. Trans Am Microsc Soc. 1933;52:195–216. doi: 10.2307/3222254. [DOI] [Google Scholar]
- Lalitha Kumari PS. Studies on parasitic protozoa (Myxosporidia) of fresh water fishes of Andhra Pradesh, India. Riv Parasitol. 1969;30:153–226. [PubMed] [Google Scholar]
- Lom J, Arthur JR. A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis. 1989;12:151–156. doi: 10.1111/j.1365-2761.1989.tb00287.x. [DOI] [Google Scholar]
- Lom J, Dykova I. Myxosporidia (Phylum Myxozoa) In: Lom J, Dykova I, editors. Protozoan parasites of fishes. Developments in aquaculture and fisheries. Amsterdam: Elsevier; 1992. pp. 159–235. [Google Scholar]
- Lom J, Dykova I. Myxozoan genera: Definition and notes on taxonomy, life- cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1–36. doi: 10.14411/fp.2006.001. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Haldar DP. Thelohanellus endodermitus sp. n. A new Myxozoan (Myxozoa: Bivalvulida) from the major carp, Labeo rohita (Hamilton-Buchanon) in a sewage farm in West Bengal, India. Environ Ecol. 2004;22(1):139–142. [Google Scholar]
- Pagarkar AU, Das M. Two new species of myxozoa, Thelohanellus caudatus n. sp. and Myxobolus serrata n. sp. from cultural carps. J Inland Fish Soc India. 1993;25(1):30–35. [Google Scholar]
- Prunescu CC, Prunescu P, Pucek Z, Lom J. The first finding of myxosporean development from plasmodia to spores in terrestrial mammals: Soricimyxum fegati gen. et sp. n. (Myxozoa) from Sorex araneus (Soricomorpha) Folia Parasitol. 2007;54:159–164. doi: 10.14411/fp.2007.022. [DOI] [PubMed] [Google Scholar]
- Qadri SS. A new Myxosporidian Thelohanellus boggoti n. sp. from an Indian fresh water fish Labeo boggot. Arch Protistenkd. 1962;106:218–222. [Google Scholar]
- Qadri SS. New Myxosporidia frm freshwater fish, Labeo fimbriatus II Thelohanellus andhrae sp. n. Arch Protistenkd. 1962;21:517–520. doi: 10.1007/BF00260256. [DOI] [PubMed] [Google Scholar]
- Qadri SS. On a new Myxosporidian, Thelohanellus shortti n. sp. from a fresh water fish, Labeo fimbricatus of Andhra Pradesh, India. Protozoology. 1967;2:207–218. [Google Scholar]
- Sakiti GN (1997) Myxosporidies et Myxosporidies des poisons du Benin: faunistique, ultrastructure, biologie. These de doctorat d’E tat, Universite National du Benin, pp 296
- Sarkar NK. Thelohanelloid bengalensis gen. and sp. nov. (Myxosporea: Thelohanellidae) from the gall bladder of marine catfish of the Bay of Bengal, India. Uttar Pradesh J Zool. 2009;29(2):251–254. [Google Scholar]
- Sarkar NK, Ghosh S. Two new myxozoan parasite of the genus Thelohanellus Kudo, 1933 (Myxosporea: Myxobolidae) from freshwater fishes of West Bengal, India. New Agriculturist. 1990;1(1):35–38. [Google Scholar]
- Sarkar NK, Raychaudhuri S. Thelohanellus bengalensis sp. n. and Myxidium mystuium sp. n. (Myxozoa): Two new Myxosporidia from Indian freshwater teleost. Acta Protozool. 1986;25(3):359–362. [Google Scholar]
- Southwell T, Prashad B. On some Mxosporidia. Parasites of Indian fishes with a note on the carcinoma in the climbing perch. II. Rec Indian Mus. 1918;15:341–355. [Google Scholar]
- Szekely C, Shaharom-Harrison F, Cech G, Mohamed K, Molnar K. Myxozoan pathogens of Malaysian fishes cultured in ponds and net-cages. Dis Aquat Organ. 2009;83:49–57. doi: 10.3354/dao01990. [DOI] [PubMed] [Google Scholar]
- Tripathi YR. Studies on the parasites of Indian fishes. I. Protozoa. Myxosporidia together with a Checklist of parasitic protozoa described from Indian fishes. Rec Indian Mus. 1952;50:63–88. [Google Scholar]