Abstract
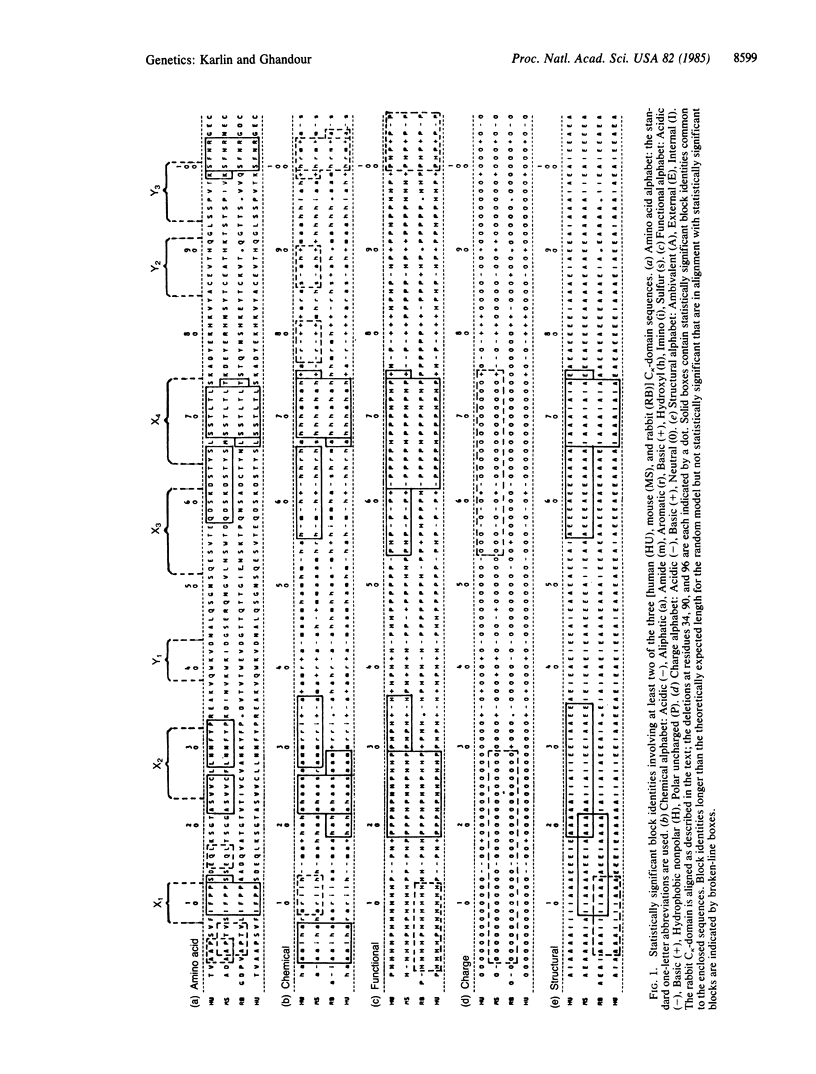
We compare the amino acid sequences of the constant domains of the immunoglobulin kappa chain of human, mouse, and rabbit by using four classification schemes ("alphabets") of the 20 amino acids based on their chemical, functional, charge, and structural properties. The comparison reveals three regions of pronounced similarity across the three species, independent of allotype. Two of these regions (residues 65-73 and 99-103) entail a high degree of identity at the DNA level and are distinguished from the rest of the constant domain in codon usage and in the dinucleotide sequence at abutting sites of adjacent codons. Residues 22-29 are highly conserved among the three species in the chemical and functional alphabets but do not show any three-sequence significant amino acid block identities. These results are discussed in terms of transcript processing, effector functions, and structural interactions within the constant domain and with the heavy chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emorine L., Dreher K., Kindt T. J., Max E. E. Rabbit immunoglobulin kappa genes: structure of a germline b4 allotype J-C locus and evidence for several b4-related sequences in the rabbit genome. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5709–5713. doi: 10.1073/pnas.80.18.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L., Sogn J. A., Trinh D., Kindt T. J., Max E. E. A genomic gene encoding the b5 rabbit immunoglobulin kappa constant region: implications for latent allotype phenomenon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1789–1793. doi: 10.1073/pnas.81.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M. An improved method of testing for evolutionary homology. J Mol Biol. 1966 Mar;16(1):9–16. doi: 10.1016/s0022-2836(66)80258-9. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W. Use of Chou-Fasman amino acid conformational parameters to analyze the organization of the genetic code and to construct protein genealogies. J Mol Evol. 1977 Sep 20;10(1):7–47. doi: 10.1007/BF01796133. [DOI] [PubMed] [Google Scholar]
- Grantham R. Amino acid difference formula to help explain protein evolution. Science. 1974 Sep 6;185(4154):862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Koshland D. E., Jr An evaluation of the relatedness of proteins based on comparison of amino acid sequences. J Mol Biol. 1970 Jun 28;50(3):617–639. doi: 10.1016/0022-2836(70)90089-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Ozeki H. Codon usage and transfer RNA contents: organism-specific codon-choice patterns in reference to the isoacceptor contents. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1087–1097. doi: 10.1101/sqb.1983.047.01.123. [DOI] [PubMed] [Google Scholar]
- Karlin S., Ghandour G. Alignment maps and homology analysis of the J-C intron in human, mouse, and rabbit immunoglobulin kappa gene. Mol Biol Evol. 1985 Jan;2(1):53–65. doi: 10.1093/oxfordjournals.molbev.a040337. [DOI] [PubMed] [Google Scholar]
- Karlin S., Ghandour G. Comparative statistics for DNA and protein sequences: multiple sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6186–6190. doi: 10.1073/pnas.82.18.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Ghandour G., Foulser D. E. DNA sequence comparisons of the human, mouse, and rabbit immunoglobulin kappa gene. Mol Biol Evol. 1985 Jan;2(1):35–52. doi: 10.1093/oxfordjournals.molbev.a040336. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Skurla R. M., Jr, Mage R. G., Bernstein K. E. Kappa-chain allotypes and isotypes in the rabbit: cDNA sequences of clones encoding b9 suggest an evolutionary pathway and possible role of the interdomain disulfide bond in quantitative allotype expression. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1794–1798. doi: 10.1073/pnas.81.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Repeating sequences and gene duplication in proteins. J Mol Biol. 1972 Mar 14;64(2):417–437. doi: 10.1016/0022-2836(72)90508-6. [DOI] [PubMed] [Google Scholar]


