Abstract
Personalized medicine applies knowledge about the patient’s individual characteristics in relation to health and intervention outcomes, including treatment response and adverse side-effects, to develop a tailored treatment plan. For women with breast cancer, personalized medicine has substantially improved the rate of survival, however, a high proportion of these women report multiple, co-occurring psychoneurological symptoms over the treatment trajectory that adversely affect their quality of life. In a subset of these women, co-occurring symptoms referred to as symptoms clusters, can persist long after treatment has ended. Over the past decade, research from the field of nursing and other health sciences has specifically examined the potential underlying mechanisms of the psychoneurological symptom cluster in women with breast cancer. Recent findings suggest that epigenetic and genomic factors contribute to inter-individual variability in the experience of psychoneurological symptoms during and after breast cancer treatment. While nursing research has been underrepresented in the field of personalized medicine, these studies represent a shared goal; that is, to improve patient outcomes by considering the individual’s risk of short- and long-term adverse symptoms. The aim of this paper is to introduce a conceptual model of the individual variations that influence psychoneurological symptoms in women with breast cancer, including perceived stress, hypothalamic-pituitary adrenocortical axis dysfunction, inflammation, as well as epigenetic and genomic factors. The proposed concepts will help bring nursing research and personalized medicine together, in hopes that this hitherto neglected and understudied area of biomedical research convergence may ultimately lead to the development of more targeted clinical nursing strategies in breast cancer patients with psychoneurological symptoms.
Keywords: Breast cancer, epigenetics, genetics, hypothalamic-pituitary-adrenal axis, personalized medicine, psychoneuroimmunology, psychoneurological symptoms, symptom clusters
1. Introduction
Although advances in treatment have substantially increased rates of survival for women with breast cancer (BCA), the women often report a number of distressing symptoms that worsen over the course of treatment, and may persist in survivorship [1, 2]. These distressing symptoms can prohibit recovery of pre-diagnosis levels of function and life satisfaction. This is a significant and global health care concern because a majority of BCA survivors are young (< 64 years of age) and these women comprise the largest group of cancer survivors in the world [3]. The most common, severe, and distressing symptoms that co-occur throughout the treatment trajectory and after treatment include depressive symptoms, anxiety, cognitive dysfunction, fatigue, sleep disturbance, and pain. This set of symptoms, which has been termed the psychoneurological (PN) symptom cluster [4], has been reliably associated with reduced quality of life [5–7]. Research to identify the physiological mechanisms underlying these co-occurring symptoms has been ongoing in the hope that it may lead to the development of more targeted symptom management strategies.
Several studies have found an association between symptoms and dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis [8–10]. In addition, studies using a cytokine-induced “sickness behavior” framework have provided some evidence for the association of inflammation with physical and emotional symptoms commonly experienced by women with BCA [11–13]. More recently, research focused on variability in symptom severity and persistence led to investigations focused on the contributions of genetic factors and epigenetic modifications that occur due to psychosocial stressors, the disease process and treatment [14, 15].
Personalized medicine applies knowledge about the patient’s individual characteristics that influence patient outcomes, including treatment response and adverse side-effects, to develop a tailored treatment approach. While nursing research has been underrepresented in the field of personalized medicine, these studies represent a shared goal that crosses disciplines; that is, to improve patient outcomes by considering the individual’s risk of short- and long-term adverse symptoms.
The aim of this paper is to introduce a conceptual model of the individual characteristics that influence psychoneurological symptoms in women with breast cancer, including perceived stress, hypothalamic-pituitary adrenocortical axis dysfunction, inflammation, as well as epigenetic and genomic factors. Genetic variants and epigenetic modifications will be described for each of the candidate mechanisms proposed to influence variability in the symptom experience.
2. Individual Characteristics that Influence Psychoneurological Symptoms
Over the past decade, research from the field of nursing and other health sciences have examined potential underlying mechanisms of the psychoneurological (PN) symptom cluster in women with breast cancer. Collectively, these studies suggest that both psychosocial and biological characteristics of the individual are important in understanding the risk of experiencing PN symptoms during treatment and in survivorship. The individual characteristics of women with BCA and related mechanisms that contribute to PN symptoms are discussed.
2.1 Perceived Stress
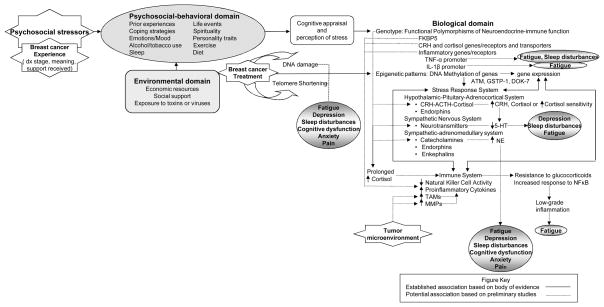
Seminal research on symptom clusters in women with BCA derived from the perspective of psychoneuroimmunology (PNI) to describe the relationships among multiple psychological stressors encountered during treatment and their reported symptoms [8–13, 16]. An important goal of PNI is to explain the effect of perceived stress on psychosocial-behavioral, neuro-immune and environmental interactions and the underlying mechanisms that influence health outcomes. We have expanded upon the tenets of PNI to incorporate genetic and epigenetic candidate mechanisms of symptoms in the conceptual model (Figure 1).
Figure 1. Psychoneuroimmunology Model Integrating Epigenetic and Genomic Factors with a view to personalized medicine in nursing for psychoneurological symptom clusters in women with breast cancer.
Genetic polymorphisms that have been associated with specific symptom phenotypes are depicted in the model as well as potential pathways initiated by prolonged stress, BCA treatment and the tumor microenvironment that may lead to symptom clusters. CRH = corticotropin releasing hormone; TNF-α = tumor necrosis factor – alpha; IL-1β = interleukin 1 – beta; ACTH = adrenocorticotropic hormone; 5-HT = 5-hydroxytryptamine (serotonin); NE = norepinephrine; TAMs = tumor-associated macrophages; MMPs = matrix metalloproteinases.
The individual’s genome provides a blueprint for the coordination of neuroendocrine-immune interactions [17]. Variability in the stress response system can occur due to genetic polymorphisms that alter neuroendocrine-immune signaling pathways, thereby leading to disruption in homeostatic regulation and increased vulnerability to persistent symptoms [18–20]. The epigenome contributes another layer of variability by regulating the genes that are “turned on” or “turned off” [21]. Although epigenetic patterns are inherited, modifications can occur in response to extreme or prolonged psychological stress [22, 23] and in association with cancer development and progression [24].
Recent studies on gene-environment interactions demonstrate how genetic variants that regulate the stress response system can influence persistent symptoms. The FK506 binding protein 5 (FKBP5) gene codes for the protein that binds to glucocorticoid receptors and modulates glucocorticoid sensitivity [25]. In addition to regulating the nuclear factor kappa-beta (NFkB) pathway, FKBP5 is involved in the processes of tumorigenesis and chemoresistance. Polymorphisms of the FKBP5 gene were recently shown to predict anxiety and depression in newly diagnosed patients with cancer who reported high levels of perceived stress [26]. In adults with a history of childhood trauma, DNA methylation of one of the FKBP5 polymorphisms was found to increase the risk of developing psychiatric disorders, including symptoms of post-traumatic stress disorder [27].
Chronic stress is associated with low-grade inflammation fostered by immune cells that have acquired resistance to cortisol [28]. NFkB plays a significant role in stress-induced cortisol resistance in addition to regulating the expression of cytokines, inducible nitric oxide synthase, cyclooxygenase 2, growth factors and inhibitors of apoptosis [29]. Activation of the NFkB pathway contributes to tumorigenesis and protects tumor cells from apoptosis by oxidative stress thereby providing a mechanism of cellular immortality [30]. Chronically high levels of perceived stress are associated with increased expression of nuclear factor kappa-B (NFkB) in monocytes, decreased expression of glucocorticoid receptors and fatigue in BCA survivors and caregivers [31, 32].
2.2 Hypothalamic-pituitary-adrenocortical axis dysfunction
Stressful events in childhood and genetic factors have been associated with increased HPA axis responses due to hyperactivity of the corticotropin releasing hormone (CRH) system [33]. A history of childhood trauma was associated with increased CRH concentrations in cerebrospinal fluid along with increased mRNA expression of CRH and CRH receptor type 1 [34, 35]. In addition, CRH polymorphisms and elevated CRH levels are associated with enhanced stress responses to psychosocial stressors [36] and depressive symptoms [37]. An enhanced stress response could lead to cortisol resistance and inflammation, thereby promoting an environment conducive to tumorigenesis [29, 30] Current studies are evaluating whether enhanced stress responses increase the risk of BCA in women with and without a positive family history.
Cortisol receptors provide substantial variability in the HPA response and are likely the most studied component of the HPA axis. Genetic variants of the two central corticosteroid receptors, high-affinity mineralocorticoid receptor (MR) and the lower-affinity glucocorticoid receptor (GR), modify HPA axis responses at several levels [38]. Both receptors regulate corticosteroid-mediated feedback on the HPA axis and deficits in the activity of either one may alter the response to cortisol, resulting in an increased or decreased stress response. Specifically, the GR polymorphism TthIIII (rs10052957) is associated with higher basal cortisol levels, while N363S (rs6195) and BclI site (rs41423247) polymorphisms result in increased cortisol sensitivity [39]. Conversely, polymorphisms in NR3C1-1 (rs10482605), ER22/23EK (rs6189/6190), and A3669G (rs6198), result in decreased cortisol sensitivity [40]. The MR polymorphisms -2G/C (rs2070951) and I180V (rs5522) also result in decreased cortisol sensitivity [38].
To our knowledge, there have not been any studies evaluating the influence of CRH or cortisol receptor polymorphisms on BCA symptoms. However, some studies support the notion that alterations in cortisol secretion and glucocorticoid receptor sensitivity may increase the risk of experiencing persistent symptoms. Lower levels of morning cortisol and flattened diurnal variation of cortisol have been reported in BCA survivors with persistent fatigue [41]. In addition, cortisol dysregulation and reduced levels of serotonin were described in women with BCA who reported symptoms of fatigue, depression, and sleep disturbances [9, 42].
2.3 Pro- and anti-inflammatory cytokines
The observation of adaptive behaviors to infection in animals was initially described by Hart, who coined the term “sickness behaviors” [43]. This set of behaviors, which includes fever, fatigue, loss of appetite, hypersomnia, and depression, has been compared to symptoms observed in patients with cancer [44]. Similar symptom clusters are reported in human studies with experimental stimulation of pro-inflammatory cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α) [45, 46] and with treatment involving interleukins [47, 48].
Elevated levels of pro-inflammatory cytokines have been reported in patients with cancer who report distressing symptoms prior to treatment [12, 49, 50] suggesting that the tumor microenvironment may influence symptoms via stimulation of cytokines. Tumor cells release norepinephrine which induces increased production of proinflammatory cytokines by tumor-associated macrophages (TAMs) [14]. In addition, breast cancer treatment with surgery, chemotherapy, radiation and hormone therapy is associated with elevated levels of proinflammatory cytokines [51]. IFN-γ, administered for the treatment of a variety of cancers, is associated with fatigue, depression and cognitive dysfunction mediated by activation of indolamine (IDO) which generates cytotoxic kynurenine metabolites [13]. Sustained elevation of IDO during BCA treatment was recently reported among women with early-stage BCA [27].
A subgroup of women with BCA report severe persistent symptoms over the treatment trajectory and in survivorship. Several studies have been conducted to explore the influence of cytokine polymorphisms on symptom patterns in patients with cancer. Both an IL-6 polymorphism and TNF-α promoter polymorphism were associated with sleep disturbance and fatigue in 288 persons with cancer and their care providers [52, 53]. In addition, Collado-Hidalgo et al. [54] found a significant relationship between promoter polymorphisms in IL-1beta and persistent fatigue in BCA survivors. Another recent study of fatigue in BCA survivors reported up-regulation of NF-κB genes associated with pro-inflammatory transcription factors [55]. Several genes encoding pro-inflammatory cytokines (IL-1A, IL-1B, IL-6, Oncostatin M) and chemokine signaling pathways (CXCL2, CXCR5, CCL20) were also found to be upregulated.
Polymorphisms of genes that regulate inflammation have also been linked with variability in pain perception. The influence of 59 SNPs in 37 inflammation genes on pain severity was recently investigated in 667 newly diagnosed persons with lung cancer [56]. Three SNPs with functional effects on nociceptive transmission were found to be significantly associated with pain severity (PTGS2 [rs5275], TNFA [rs1800629], and NFKBIA [rs8904]). In the general population and in specific pain syndromes functional polymorphisms of sensory nerve calcium channels are associated with increased pain perception [57].
Although less studied, anti-inflammatory genetic polymorphisms and their interactions with other polymorphisms may play a role in vulnerability to symptom development and persistence. Gene variants of IL1-receptor 2, IL-10 and TNF-α were associated with depressive symptom trajectories in 167 oncology patients [58]. Along with age and performance status, the polymorphisms predicted membership in a resilient versus subsyndromal classification.
2.4 Oxidative Stress and Telomere Shortening
Breast cancer and treatment with chemotherapy may also contribute to symptom development and persistence through their influence on oxidative stress and telomere shortening. Telomeres are non-coding segments of DNA at the end of chromosomes that prevent the loss of genetic data and protect them from fusing together during cell division [59]. During each cell division a cell loses 30 to 200 base pairs resulting in a gradual decline in telomere length over time, however, factors such as oxidative stress and chemotherapy can accelerate telomere shortening [60]. When the telomere gets shortened to a certain length, this signals senescence and the cell undergoes apoptosis, better known as programmed cell death.
Telomere damage, including shortening and mutations can result from endogenous and exogenous toxic exposures and lead to p53 activation, a major component of DNA repair. However, p53 activation can also impair mitochondrial function [61]. Mitochondrial dysfunction results in the production of reactive oxygen species (ROS) leading to oxidant stress, which further damages pre- and post-telomeric DNA. This cyclical process of DNA damage and mitochondrial dysfunction ultimately results in cellular and metabolic senescence, apoptosis and metabolic changes that are associated with aging and disease, including cancer. In addition, genetic mutations of the telomerase system, which is classically known to maintain and even lengthen telomeres, have also been shown to play a role in stem-cell homeostasis. Without a healthy stem cell reservoir to replenish somatic cells, functional decline of tissues and organs occurs, thus leading to aging [60].
Telomere shortening may be linked with the symptom experience. Associations among mood disorders, including major depression, oxidative stress and accelerated telomere shortening have been reported [62, 63]. However, when telomeres reach a critical stage of shortening and induce cellular senescence, an altered pattern of gene expression can cause the cell to function aberrantly [59]. Although not directly tested, the accumulation of dysfunctional senescent cells in BCA survivors may play a role in symptom persistence. A recent study testing the effect of a psychosocial counseling intervention in cervical cancer survivors found that a longitudinal decrease in perceived stress was not only associated with increased telomere length in peripheral blood mononuclear cells (PBMCs) but also an increase in the naïve T cell population [64]. The results suggest a potential role for such interventions in cancer prevention and in patients receiving chemotherapy to reduce the rate of telomere attrition and damage.
2.5 DNA Damage
In addition to telomere shortening, oxidative stress is a common cause of DNA damage resulting in single- or double-strand breaks that can increase the risk of cancer [59]. Evidence of oxidative DNA damage has been reported in PBMCs of women with BCA following chemotherapy. In fact, many chemotherapeutic agents achieve their effect by causing DNA damage to block replication of cancer cells and induce apoptosis. Although the goal is to eradicate tumor cells, the DNA in normal cells can also be affected resulting in many of the side-effects observed. As a plausible mechanism of persistent symptoms in cancer survivors, clinical research studies are underway to examine the relationship between DNA damage and the symptom trajectory [49].
Deficiencies in DNA repair mechanisms that increase BCA risk may also contribute to the symptom trajectory through an accumulation of DNA damaged cells. Ataxia Telangiectasia Mutated (ATM) kinase is a protein that acts a master controller of cell cycle checkpoint signaling pathways that are required for cellular response to DNA damage and genome stability. Methylation of the ATM gene is associated with increased risk of BCA [65]. It was recently reported that miR-18a, a microRNA than functions in transcriptional and post-transcriptional regulation of gene expression, impairs the DNA damage response by downregulating ATM kinase [66]. Promoter CpG methylation of glutathione S-transferase 1 (GSTP1) which results in accumulation of oxygen radicals and DNA damage has also been identified as a biomarker of BCA risk [67]. The DOK-7 protein participates in activating the protein MuSK that maintains acetylcholine receptor (AChR) in the neuromuscular junction. Methylation of DOK-7 has been associated with an increased risk of BCA [68] and may be a plausible mechanism of fatigue, the most frequently reported symptom over the disease trajectory and in survivorship.
3. Outlook
The body of research on symptom clusters in women with BCA suggests that psychological and biological factors are relevant to understanding the symptom experience and trajectory. The proposed conceptual model provides a framework to guide the generation of hypothesis on the mechanisms of symptom cluster phenotypes in women with BCA. It emphasizes the unique stressors confronted by women with BCA including psychosocial stressors arising from the BCA experience, biological stressors from the tumor microenvironment and environmental stressors related to treatment with surgery, chemotherapy, radiation and hormone therapy. Based on a growing body of evidence, the model incorporates potential contributions of genetic and epigenetic factors on stress responses and vulnerability to symptom phenotypes.
Further research to test the relationships posited by the model and examine other gene-gene and gene-environment interactions is needed to advance predictive accuracy and begin the development of interventions to target the pathways contributing to symptom phenotypes. A deeper understanding of the gene-gene interactions and epigenetic modifications that influence symptoms may provide new methods to predict the most effective treatment with the least risk of symptoms. Eventually this information could be used to provide women with BCA more personalized information on their symptom risk profile, which may assist in decision-making regarding treatment options.
Conclusions
Personalized medicine and nursing research share a common goal of improving patient outcomes in women with breast cancer. Recent studies in nursing and other areas of health science suggest there are individual characteristics that influence the patient’s symptom experience during and after breast cancer treatment. Although nursing research has been underrepresented in the field of personalized medicine, this knowledge is prerequisite for providing a personalized approach to symptom management. The proposed concepts help, in part, to bring nursing research and personalized medicine together in hopes that this area of research may ultimately lead to the development of more personalized and targeted symptom management strategies.
Table 1.
Genetic polymorphisms associated with psychoneurological (PN) symptom cluster.
| Candidate gene; SNP (rs), tSNP numbers, and/or non-SNP polymorphism | PN-based Symptoms | Function | References |
|---|---|---|---|
| HPA Axis Dysfunction: Corticotropin Releasing Hormone and Cortisol genes/receptors; Glucocorticoid genes/receptors; Mineralocorticoid genes/receptors; Monoamine genes/receptors and transporters | |||
| NR3C1; rs10052957, rs6195, rs41423247 rs10482605, rs6189, rs6190, rs6198 |
Depression Anxiety |
Glucocorticoid receptors Increased basal cortisol levels Increased cortisol sensitivity Decreased cortisol sensitivity |
[17, 36] |
| MR; rs2070951, rs5522 | Depression | Mineralocorticoid receptor Decreased cortisol sensitivity |
[37] |
| MAOA; functional variable number tandem repeat (VNTR) | Depression | Metabolizes 5-HT and norepinephrine | [18, 19, 59, 60] |
| SERT (5-HTTLPR); rs25531, rs3794808, functional insertion/deletion | Depression Fatigue Cognitive dysfunction |
Serotonin transporter | [18, 37] |
| Immune Reactivity: Inflammatory genes/receptors | |||
| NFKB; functional insertion/deletion rs8904 |
Depression, Fatigue Pain |
Inflammatory transcription/signaling | [55*, 56] |
| IL1B -511CT; rs16944 | Fatigue | Inflammatory signaling | [54*] |
| IL-6 -174GC; rs1800795 IL-6 -6101AT;rs4719714 |
Fatigue | Inflammatory signaling | [52*, 54*] |
| TNFA -308GA; rs1800629 | Fatigue Sleep disturbances Pain |
Inflammatory signaling | [53*, 56*] |
research studies that included women with breast cancer
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R01 NR012667, Lyon/Jackson-Cook (MPI), 10/01/10–8/30/15, “Epigenetics and Psychoneurologic Symptoms in Women with Breast Cancer.” P30 NR011403, Grap (PI), 8/10/2009–05/1/2014) Biobehavioral Science Core, CBCR Center of Excellence.
List of Abbreviations
- AChR
acetylcholine receptor
- ATM
Ataxia Telangiectasia Mutated
- BCA
breast cancer
- CRH
corticotropin releasing hormone
- DNA
deoxyribonucleic acid
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenocortical
- IDO
indolamine
- IL
interleukin
- MR
mineralocorticoid receptor
- mRNA
messenger ribonucleic acid
- NFkB
nuclear factor kappa-beta
- PBMCs
peripheral blood mononuclear cells
- PN
psychoneurological
- PNI
psychoneuroimmunology
- SNP
single-nucleotide polymorphism
- TAM
tumor-associated macrophages
- TNF-α
tumor necrosis factor-alpha
Footnotes
Conflict of Interest
Non declared/applicable.
Contributor Information
Angela R. Starkweather, Virginia Commonwealth University School of Nursing.
Debra E. Lyon, Virginia Commonwealth University School of Nursing.
R. K. Elswick, Jr., Virginia Commonwealth University School of Nursing
Alison J. Montpetit, Virginia Commonwealth University School of Nursing.
Yvette Conley, University of Pittsburgh School of Nursing
Nancy L. McCain, Virginia Commonwealth University School of Nursing.
References
- 1.Kim HJ, Barsevick AM, Tulman L. Predictors of the intensity of symptoms in a cluster in patients with breast cancer. J Nurs Schol. 2009;41(2):158–165. doi: 10.1111/j.1547-5069.2009.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18(2):187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietman J, Dijkstra P, Debreczeni R, et al. Impairments, disabilities and health related quality of life after treatment of breast cancer: A follow-up study 2. 7 years after surgery. Disabil Rehabil. 2004;26(2):78–84. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 4.Kim E, Jahan T, Aouizerat BE, et al. Differences in symptom clusters identified using occurrence rates versus symptom severity rating in patients at the end of radiation therapy. Cancer Nurs. 2009;32(6):429–436. doi: 10.1097/NCC.0b013e3181b046ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nurs. 2010;14(1):101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montazeri A, Vahdaninia M, Harirchi I, et al. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow-up study. BMC Cancer. 2008;8(11):330–336. doi: 10.1186/1471-2407-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manag. 2008;35(2):162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LE, Campbell TS, Garland SN, Grossman P. Associations among salivary cortisol, melatonin, catecholamines, sleep quality and stress in women with breast cancer and health controls. J Behavior Med. 2007;30(1):45–58. doi: 10.1007/s10865-006-9082-3. [DOI] [PubMed] [Google Scholar]
- 9.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35(4):635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- 10.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: Covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29(3):333–337. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosomatic Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lyon DE, McCain NL, Walter J, Schubert C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs Research. 2008;57(1):51–58. doi: 10.1097/01.NNR.0000280655.58266.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 14.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosomatic Med. 2011;73:724–730. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veeck J, Esteller M. Breast cancer epigenetics: from DNA methylation to microRNAs. J Mammary Gland Biol Neoplas. 2008;15(1):5–17. doi: 10.1007/s10911-010-9165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCain NL, Gray DP, Walter JM, Robins J. Implementing a comprehensive approach to the study of health dynamics using the psychoneuroimmunology paradigm. Adv Nurs Sci. 2005;28(4):320–332. doi: 10.1097/00012272-200510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wüst S, Federenko IS, Van Rossum EFC, et al. A psychobiological perspective on genetic determinants of hypothalamus-pituitary-adrenal axis activity. Ann NY Acad Sci. 2004;1032(1):52–62. doi: 10.1196/annals.1314.005. [DOI] [PubMed] [Google Scholar]
- 18.Jabbi M, Korf J, Kema IP, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT, and MAOA. Mol Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–271. [Google Scholar]
- 21.Mathews HL, Witek Janusek L. Epigenetics and psychoneuroimmunology: mechanisms and models. Brain Behav Immun. 2011;25(1):25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Morita K, Masuda K, et al. Gene expression signature in peripheral blood cells from medical students exposed to chronic psychological stress. Biol Psychol. 2007;76(3):147–155. doi: 10.1016/j.biopsycho.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Pyter LM, Pineros V, Galang JA, et al. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Nat Acad Sci, USA. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turan N, Katari S, Coutifaris C, Sapienza C. Explaining inter-individual variability in phenotype: is epigenetics up to the challenge? Epigenetics. 2010;5(1):16–19. doi: 10.4161/epi.5.1.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. British J Cancer. 2011;104:19–23. doi: 10.1038/sj.bjc.6606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JI, Chung HC, Jeung H, et al. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: A controlled and prospective study. Psychoneuroendocrin. 2012;37(9):1569–1576. doi: 10.1016/j.psyneuen.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neurosci. 2012;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutgendorf SK, Costanzo ES. Psychoneuroimmunology and health psychology: an integrative model. Brain Behav Immun. 2003;17:225–232. doi: 10.1016/s0889-1591(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 29.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappa B signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sfikas A, Batsi C, Tselikou E, et al. The canonical NFkB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage. Cell Signalling. 2012;24:2007–2023. doi: 10.1016/j.cellsig.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt MV, Sterlemann V, Müller MB. Chronic stress and individual vulnerability. Ann NY Acad Sci. 2008;1148(1):174–183. doi: 10.1196/annals.1410.017. [DOI] [PubMed] [Google Scholar]
- 33.Hillhouse EW, Grammatopoulous DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocrinol Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 34.Tyrka AR, Price LH, Gelernter J, et al. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63(4):398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.DeRijk RH. Single nucleotide polymorphisms related to HPA axis reactivity. Neuroimmunomod. 2009;16:340–352. [Google Scholar]
- 37.Ressler KJ, Bradley B, Mercer KB, et al. Polymorphisms in CRHR1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms. Amer J Med Genet B Neuropsych Genet. 2010;153B(3):812–824. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeRijk RH, Wust S, Meijer OC, et al. A common polymorphism in the mineralcorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006;91:5083–5089. doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- 39.Zobel A, Jessen F, von Widdern O, et al. Unipolar depression and hippocampal volume: impact of DNA sequence variants of the glucocorticoid receptor gene. Amer J Med Genet B Neuropsych Genet. 2008;147B:836–843. doi: 10.1002/ajmg.b.30709. [DOI] [PubMed] [Google Scholar]
- 40.Mormede P, Foury A, Barat P, et al. Molecular genetics of hypothalamic-pituitary-adrenal axis activity and function. Ann NY Acad Sci. 2011;1220:127–136. doi: 10.1111/j.1749-6632.2010.05902.x. [DOI] [PubMed] [Google Scholar]
- 41.Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychological stress in breast cancer survivors with persistent fatigue. Psychosomatic Med. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 42.Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33(4):775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- 43.Hart BL. Biological basis of the behavior of sick animals. Neurosci and Biobehav Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 44.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 45.Dantzer R, Capuron L, Irwin MR, et al. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrin. 2008;33(1):18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosomatic Res. 2007;62(2):207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Wichers MC, Maes M. Mechanisms of interferon-alpha-induced depressive symptoms. Acta Neuropsychiatrica. 2002;14(1):103–105. doi: 10.1034/j.1601-5215.2002.140301.x. [DOI] [PubMed] [Google Scholar]
- 49.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patient prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wefel JS, Lenzi R, Theriault R, et al. Chemobrain in breast carcinoma? A prologue Cancer. 2004;101:46–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 51.Lyon DE, Walter J, Starkweather AR, Schubert C, McCain NL. Tryptophan degradation in women with breast cancer: A pilot study. BMC Research Notes. 2011;4:156–164. doi: 10.1186/1756-0500-4-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miaskowski C, Dodd M, Lee K, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manag. 2010;40(4):531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11(1):27–44. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 54.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav Immun. 2008;22(8):1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reyes-Gibby CC, Spitz MR, Yennurajalingam S, et al. Role of inflammation gene polymorphisms on pain severity in lung cancer patients. Cancer Epid Biomarkers Prev. 2009;18(10):2636–2642. doi: 10.1158/1055-9965.EPI-09-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reimann F, Cox JJ, Belfer I, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Nat Acad Sci USA. 2010;107(11):5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn LB, Aouizerat BE, Langford DJ, et al. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. Eur J Oncol Nurs. 2012 doi: 10.1016/j.ejon.2012.10.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackburn EH. Walking the walk from genes through telomere maintenance to cancer risk. Cancer Prevent Res. 2011;4(4):473–475. doi: 10.1158/1940-6207.CAPR-11-0066. [DOI] [PubMed] [Google Scholar]
- 60.Calado R, Young N. Telomeres in disease. F1000 Med Rep. 2012;4:8. doi: 10.3410/M4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shammas MA. Telomeres, lifestyle, cancer and aging. Curr Opin Clin Nutr Metab Care. 2010;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress – Preliminary findings. PLoS ONE. 2011;6(3):e1737. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biegler KA, Anderson AKL, Wenzel LB, et al. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: Implications for cancer prevention. Cancer Prev Res. 2012;5(10):1173–1182. doi: 10.1158/1940-6207.CAPR-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan K, Garcia-Closas M, Orr N, et al. Intragenic ATM methylation in peripheral blood DNA as a biomarker of breast cancer risk. Cancer Res. 2012;72(9):2304–2313. doi: 10.1158/0008-5472.CAN-11-3157. [DOI] [PubMed] [Google Scholar]
- 66.Song L, Lin C, Wu Z, et al. miR-18a impairs DNA damage response through downregulatin of ataxia telangiectasia mutated (ATM) kinase. PLOS One. 2011;6(9):e25454. doi: 10.1371/journal.pone.0025454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van de Voorde L, Speeckaert R, van Gestel D, et al. DNA methylation-based biomarkers in serum of patients with breast cancer. Mutation Res. 2012;751:304–325. doi: 10.1016/j.mrrev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Heyn H, Carmona FJ, Gomez A, et al. DNA methylation profiling in breast cancer discordant identical twins identified DOK7 as novel epigenetic biomarker. Carcinogenesis. 2012;34(1):102–108. doi: 10.1093/carcin/bgs321. [DOI] [PMC free article] [PubMed] [Google Scholar]