Abstract
Background
Although it is clear that there are short-term effects of sodium intake on blood pressure, little is known about the most relevant timing of sodium exposure for the onset of hypertension. This question can only be addressed in cohorts with repeated measures of sodium intake.
Methods
Using up to 7 measures of dietary sodium intake and blood pressure between 1991 and 2009, we compared baseline, the mean of all measures, and the most recent sodium intake in association with incident hypertension, in 6578 adults enrolled in the China Health and Nutrition Survey aged 18 to 65 free of hypertension at baseline. We used survival methods that account for the interval-censored nature of this study, and inverse probability weights to generate adjusted survival curves and time-specific cumulative risk differences; hazard ratios were also estimated.
Results
For mean and most recent measures, the probability of hypertension-free survival was the lowest among those in the highest intake sodium group compared to all other intake groups across the entire follow-up. In addition, the most recent sodium intake measure had a positive dose-response association with incident hypertension [Risk Difference at 11 years of follow-up = 0.04 (95%CI −0.01, 0.09), 0.06 (0.00, 0.13), 0.18 (0.12, 0.24) and 0.20 (0.12, 0.27) for the second to fifth sodium intake groups compared to the lowest group respectively]. Baseline sodium intake was not associated with incident hypertension.
Conclusion
These results suggest caution when using baseline sodium intake measures with long-term follow up.
Keywords: China, sodium intake, incident hypertension, interval-censored, adjusted survival curves
Evidence supporting a positive association between sodium intake and blood pressure (BP) comes from a wide range of randomized trials, animal-based and observational studies.1 Findings suggest that BP responds relatively quickly to sodium intake; meta-analyses of randomized trials with sodium intake reduction have shown declines in BP as early as 4 days to 36 months.2,3 However, little is known about the most relevant associated time-frame for sodium exposure in relation to incident hypertension. He et al. found that the effects of an 18-month sodium reduction intervention trial had lasting effects on incident hypertension over 7 years post-intervention, even if the individuals did not maintain a low sodium intake after the intervention.4 Similarly, the effects on BP of a sodium reduction intervention during the first 6 months of life lasted over 15 years later.5 These results suggest that sodium might have a long-term influence on hypertension. However, to date, no study has compared the effects of sodium intake across different time-frames in the same subjects. This question can only be addressed in cohorts with repeated measures of sodium.
China provides an excellent setting for such an investigation as hypertension is increased tremendously over the past decades6,7 and population sodium intake is high and therefore of particular concern. In the INTERSALT Study among 45 samples worldwide, the Chinese had the highest sodium intake.8 In 1997, the mean 24-hour urine sodium excretion in the north of China was between 6.1 to 6.3 g.9 To date, sodium intake remains high in China, although it decreased from about 6.6 g/d in 1991 to 4.7g/d in 2009 (possibly due to increases in supermarkets, modern refrigeration, and modern transportation that helped decrease the use of salt as a food preservative) (S Du, unpublished data, 2012).
We used data from the China Health and Nutrition Survey (CHNS), a large longitudinal study that includes very detailed repeated measures of dietary sodium intake. To assess the relation between hypertension and sodium intake from different time frames, we included all subjects that had at least two measures of sodium intake. In the same subjects, we compared the cumulative risk and hazard ratios (HR) for hypertension-free survival across 3 different models: 1) sodium intake at baseline (most distal exposure), 2) the mean sodium intake across the entire follow-up, and 3) the last available measure of sodium of the follow-up (most recent exposure).
METHODS
Study population
The CHNS is an ongoing study with detailed income, employment, education, demographic, health, and nutritional information that started in 1989 with detailed follow-up across 20 years. A multistage, random cluster process was used to draw the sample in 9 provinces. This study was approved by the institutional review committees of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention. Participants provided their written, informed consent. Additional details regarding the CHNS data are provided elsewhere.10
We used 1991, 1993, 1997, 2000, 2004, 2006 and 2009 exams. Eligible subjects (N = 13,764) were those that entered the cohort before 2006 and that at baseline were both 18–65 years old and free of hypertension. We excluded those that have one or fewer measurement of BP (N=3,020), did not have at least two waves of sodium during their follow-up (before the event or last BP measure) (N=4,107) and had missing covariates at baseline (N=59). The final sample size was 6,578 (eFigure 1). Attrition is complex because participants missing in one wave may come back later and new participants are recruited as replenishment samples. Major reasons for loss to follow-up are migrant work, natural disasters, major redevelopment of housing and relocations and refusal to participate in clinical exams.10 In addition, for our analysis, even if subjects had complete data in many waves, some were excluded because they developed hypertension at the earlier visits and hence, did not have at least two waves of sodium measurement before the hypertension event. Those excluded were younger, a higher proportion resided in urban areas, had higher education and income, lower physical activity and entered the cohort later (eTable 1). Because the potential for selection bias was important, we conducted two sensitivity analyses: 1) we computed inverse probability (IP) weights using all eligible excluded subjects that had complete data for the covariates (N= 5581 out of a total of 7186 excluded); 2) we included all subjects with at least one measure of sodium available (N=10,017). This analysis were not performed for mean or recent sodium, because the majority of excluded had only baseline sodium. [eTable 3. (A, B)].
Measurement of variables
All rounds of the CHNS collected identical data from the community and household. The data was collected by trained and certified health workers.
Dietary intake
Nutrients, including sodium, were estimated based on a combination of three consecutive 24-hour recalls at the individual level and a food inventory at household level performed over the same 3 day period. For the food inventory, all available foods at the household (purchased, stored or home produced) were weighed on a daily basis, with changes in inventory and food wastage used to estimate the total household food consumption which was then allocated to each individual based on the three 24-hour recalls. Condiments like salt, monosodium glutamate, and soy sauce were part of the items weighed directly at the household level.
The sodium content was based on a Chinese food composition table in which all foods, including processed foods, were measured with the Perkin-Elmer Analyst 800.11 However, this food composition table, as in all other countries, does not include all processed foods available in China. This limitation likely did not have substantial influence on our sodium measure, because although the proportion of sodium intake coming from processed foods has increased over time in China (1.8% in 1991 to 6.8% in 2009) processed food intake is still relatively low (S Du, unpublished data, 2012).
A validation study was undertaken to evaluate the accuracy of estimated sodium and potassium intake in one of the survey provinces (but not with CHNS participants). The same methodology of dietary measurement was conducted by CHNS interviewers and 24-hour urine samples were collected during three days (para-aminobenzoic acid was used as a marker of completeness of 24-hour urine samples). The correlation coefficient between the estimated dietary sodium and potassium intakes and urine sodium and potassium excretion was 0.58 and 0.59 respectively (p = 0.005) (S Du, unpublished data, 2012). In addition, in a previous study total energy intake was validated using doubly labeled water, this study was conducted in Beijing (not CHNS participants) and the same methodology of dietary measurement was conducted by interviewers trained by CHNS staff. Correlation coefficient between the two methods was 0.56 for men and 0.60 for women,12 which is high in comparison to other studies.
To assess which dietary variables were important for our analysis, we looked at the correlation between sodium intake and other dietary variables that are emphasized for hypertensive patients13 (eTable 2). We did not find large correlations, perhaps because in China most sodium is added during cooking. Nonetheless, we include energy and potassium intake in models, because both are validated and have well documented association with hypertension.
Hypertension
BP was based on the mean of 3 measurements collected after a 10-min seated rest. Standard mercury sphygmomanometers (measuring range: 0–300 mm Hg; graduation: 2 mm Hg) with regular adult cuffs were used, the equipment was constant in all waves. Incident hypertension was defined at the first wave that the subject reported taking anti-hypertension medicines, and/or had high BP (≥90/140 mmHg).14
Covariates
For physical activity we used Metabolic Equivalents per week derived from detailed time spent and intensity levels in occupational and domestic physical activities.15 Other demographic and lifestyle covariates included gender, age, BMI, geographical region, urban/rural residence, education level, income, smoking status and alcohol intake.
Data analysis
Because not all individuals entered the study in 1991, the year zero of follow-up was defined at the entry point of each participant. In addition, the data is interval-censored because the outcome was ascertained only at specific waves and we therefore did not know the exact date of hypertension occurrence. We coded the time to event with two variables defining the interval in which the outcome was known to have occurred (between the wave at which the first hypertension status was found and the previous available BP measure). For example, if an individual entered in 1993, remained free of hypertension in both 1997 and 2000 but was classified as hypertensive in 2004, his interval was between 2000 and 2004 (or between 7 and 11 years of follow-up). If that same individual had BP measurement missing in 2000 then his interval was between 4 and 11 years of follow-up. We did not exclude subjects with missing BP at any waves, to avoid restricting more our analytic sample. However, because of this, many intervals for hypertension onset were considerably wide; therefore we conducted a sensitivity analysis excluding subjects with interim missing BP data points [eTable 3. (C)].
As our main effect measure, we present time-specific differences in the cumulative probability of hypertension-free survival. We used Turnbull’s approach16 to estimate the nonparametric maximum likelihood estimates of the cumulative probability via the SAS 9.2 procedure LIFEREG (SAS Institute Inc., Cary, NC). To account for confounding, we used IP weights. We fitted a multinomial logistic regressions to estimate the probability of exposure, Pr(X=x), as well as the probability of exposure conditional on the confounders, Pr(X=x | Z). We calculated stabilized IP weights as Pr(X=x)/Pr(X=x | Z)17 for each exposure (sodium at baseline, mean and recent), and incorporated these to estimate the adjusted survival curves (Figure 1) and adjusted cumulative risks (Table 2). We used a nonparametric bootstrap approach to estimate the standard error of the risk difference. Specifically, we drew 200 simple random samples, each of size 6578, with replacement; we then estimated the IP weights and calculated the risk difference in each of the 200 samples; finally we took the standard deviation of the 200 estimates as the estimate of the standard error for the risk difference. We note that, with interval censored data, Turnbull’s estimator is not regular asymptotically linear, so the standard theoretical justification for the bootstrap does not hold. However, the bootstrap performed well in both simulations and prior examples.18,19
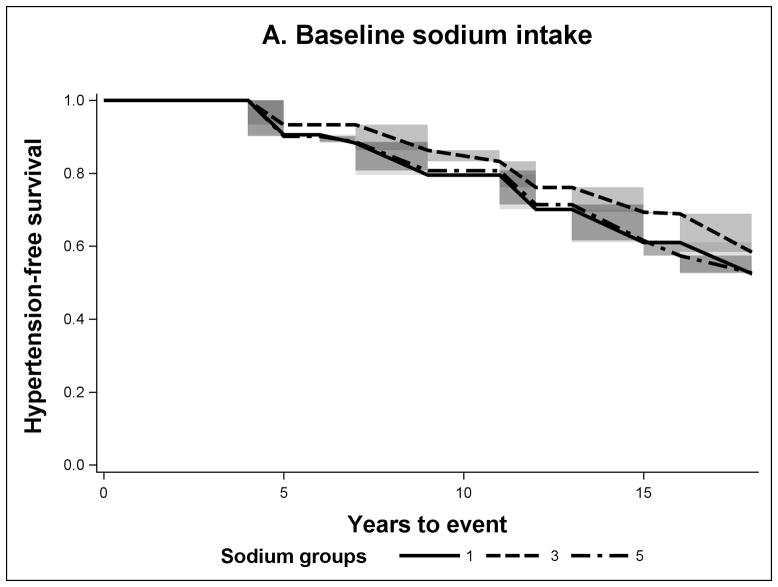
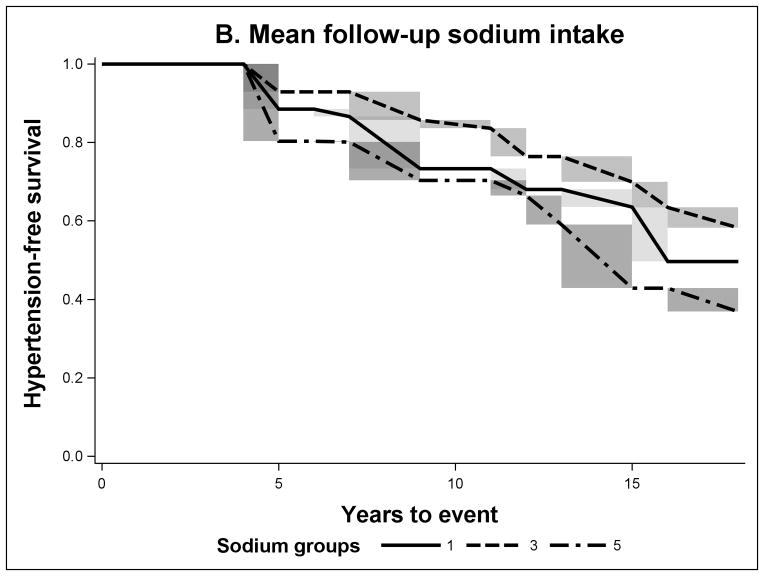
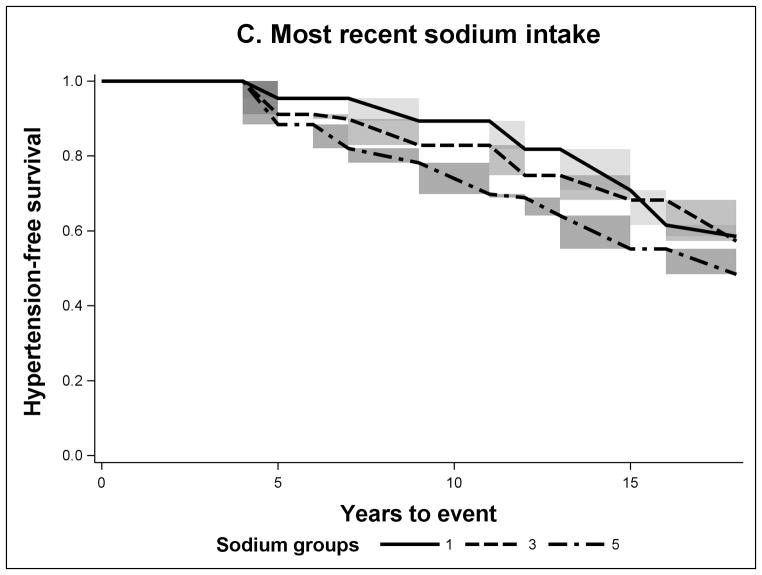
FIGURE 1.
Non-parametric Adjusted Survival Probability Curves for Incident Hypertension by Baseline Sodium Intake (A), Mean Sodium Intake (B) and Recent Sodium Intake (C) for sodium groups 1 (lowest intake), 3 and 5 (highest intake).
TABLE 2.
Cumulative Risk Difference at 11 years of follow-up for Incident Hypertension Comparing to the Lowest Intake Group.
| Baseline sodium intake
|
Mean sodium intake
|
Recent sodium intake
|
||||
|---|---|---|---|---|---|---|
| Cumulative Risk | Cumulative Risk Difference (95% CI) | Cumulative Risk | Cumulative Risk Difference (95% CI) | Cumulative Risk | Cumulative Risk Difference (95% CI) | |
| Crude estimate | ||||||
| G1 ( <3.7 g/d)a | 0.21 | 0 | 0.23 | 0 | 0.12 | 0 |
| G2 ( 3.7–5.2 g/d) | 0.19 | −0.02 (−0.07, 0.03) | 0.13 | −0.09 (−0.15, −0.03) | 0.15 | 0.03 (−0.01, 0.07) |
| G3 (5.2–6.6 g/d) | 0.16 | −0.05 (−0.10, 0.00) | 0.16 | −0.07 (−0.13, −0.01) | 0.16 | 0.05 (0.01, 0.09) |
| G4 (6.6–8.7 g/d) | 0.18 | −0.03 (−0.09, 0.02) | 0.18 | −0.04 (−0.11, 0.02) | 0.28 | 0.16 (0.11, 0.22) |
| G5 ( >8.7 g/d) | 0.18 | −0.03 (−0.07, 0.01) | 0.31 | 0.08 (−0.02, 0.18) | 0.32 | 0.20 (0.12, 0.28) |
| Adjustedb | ||||||
| G1 ( <3.7 g/d)a | 0.21 | 0 | 0.26 | 0 | 0.11 | 0 |
| G2 ( 3.7–5.2 g/d) | 0.20 | 0.00 (−0.05, 0.05) | 0.13 | −0.12 (−0.19, −0.06) | 0.15 | 0.04 (0.00, 0.08) |
| G3 (5.2–6.6 g/d) | 0.17 | −0.04 (−0.09, 0.01) | 0.17 | −0.09 (−0.15, −0.02) | 0.17 | 0.06 (0.02, 0.11) |
| G4 (6.6–8.7 g/d) | 0.18 | −0.02 (−0.08, 0.04) | 0.22 | −0.04 (−0.11, 0.03) | 0.29 | 0.18 (0.12, 0.24) |
| G5 ( >8.7 g/d) | 0.19 | −0.01 (−0.05, 0.03) | 0.30 | 0.05 (−0.06, 0.15) | 0.30 | 0.20 (0.12, 0.27) |
Reference category
Adjusted using Inverse Probability Weights for covariates at baseline: age, BMI, gender, region, urban/rural, education level, income, physical activity, smoking, alcohol intake and wave of entry; and for energy and potassium intake at baseline, mean or recent period accordingly.
CI, confidence interval; G, group.
As an alternative, we also present the more familiar regression-adjusted HRs, these were estimated using flexible parametric models for survival-time data [stpm in STATA 12.1 (StataCorp, College Station, TX)], because this procedure can handle interval-censored data. This method runs spline-smoothed versions of log-logistic or proportional hazards Weibull models. As previously suggested,20 we selected the log-logistic and 4 spline knots based on the lowest Akaike Information Criterion.
Exposure periods
The main exposure was sodium intake from three different exposure periods. 1) Baseline sodium was the first available measure of sodium; in 8% of the sample sodium at baseline (year 0 of follow-up) was missing, so we use the next available sodium intake measure. 2) Mean sodium was the arithmetic mean of all available sodium measurements during follow-up (i.e. mean of sodium available from year 0 to 6 of follow-up, if year 9 was the last BP measure). 3) Recent sodium was the last measure of sodium (i.e. sodium at year 6 of follow-up, if year 9 was the last BP measure). Because our aim was to compare different time frames of sodium intake in the same sample, we restricted our analysis to individuals with at least two measures of sodium before the event or censoring. For each exposure period we ran a separate model. Baseline sodium was categorized in quintiles, and mean and recent sodium were categorized in five groups using the cutoff values of the quintiles at baseline; this was done to maintain comparability across models. Therefore in this manuscript we refer to “quintiles” when is specific to baseline intake and to “groups” if otherwise. Lastly, we evaluated the effect of different combinations of baseline and recent sodium exposures. We ran a model including baseline and recent sodium simultaneously and computed the HR and 95% confidence intervals for several linear combinations of the coefficients.
Confounders
We selected potential confounders based on known factors associated with hypertension, and on the association of covariates with sodium intake and the outcome in our data. We controlled for covariates at baseline including age, BMI, gender, geographical region, urban/rural residence, education level, income, physical activity, smoking, alcohol intake and wave of entry. Energy and potassium intake were defined at baseline, mean of follow-up, or recent according to each type of sodium assessed.
RESULTS
The median follow-up was 11 years (range: 5–18), the average number of sodium measurements per individual was 3.3 (range: 2–6). The mean time between the recent sodium measurement and the event or censoring was 3.5 years (range: 2–16). This last wide range was due to the inclusion of subjects with missing BP at waves of data collection. In our third sensitivity analysis, in which we found similar results, this range was 2 to 4 years [eTable 3. (C)].
Sodium intake at baseline and the incidence of hypertension tended to be higher in males, central geographic region or rural residence, lower education level, higher energy intake, higher physical activity, smokers and in subjects entering the cohort in 1991. In addition, incidence of hypertension was higher in those with lower income, older age and higher BMI. Sodium was slightly higher in younger subjects and in individuals who had higher alcohol intake (Table 1).
TABLE 1.
Age-standardized Means or Percents for Baseline Characteristics of Participants by Quintiles of Sodium Intake at Baseline and Incident Hypertension (n=6,578), China Health and Nutrition Survey, 1991–2009.
| Variable | All
|
Baseline sodium quintiles
|
Incident hypertension
|
|||||
|---|---|---|---|---|---|---|---|---|
| (n=6578) | 1(lowest) <3.7 g/d |
2 3.7–5.2 g/d |
3 5.2–6.6 g/d |
4 6.6–8.7 g/d |
5(highest) >8.7 g/d |
Yes (n=1971) | No (n=4607) | |
| Baseline sodium intake (g/d) | 6.2 | 2.7 | 4.4 | 5.9 | 7.6 | 10.7 | 6.5 | 6.2 |
| Mean sodium intake (g/d) | 5.9 | 4.3 | 5.2 | 5.8 | 6.5 | 7.8 | 6.2 | 5.8 |
| Recent sodium intake (g/d) | 5.4 | 5.0 | 5.3 | 5.4 | 5.6 | 5.9 | 5.8 | 5.2 |
| Gender, % | ||||||||
| Female | 55 | 60 | 58 | 57 | 54 | 49 | 49 | 57 |
| Male | 45 | 40 | 42 | 43 | 46 | 51 | 51 | 43 |
| Age (years) | 36.8 | 37.5 | 37.0 | 36.5 | 36.8 | 36.4 | 41.5 | 34.9 |
| Body mass index (kg/m2) | 22.6 | 22.6 | 22.5 | 22.6 | 22.7 | 22.7 | 23.3 | 22.3 |
| Region, % | ||||||||
| North | 17 | 28 | 20 | 15 | 13 | 12 | 19 | 17 |
| Central | 33 | 25 | 26 | 34 | 35 | 43 | 37 | 31 |
| South | 50 | 47 | 54 | 51 | 52 | 45 | 44 | 52 |
| Residence, % | ||||||||
| Rural | 73 | 70 | 71 | 74 | 75 | 75 | 76 | 71 |
| Urban | 27 | 30 | 29 | 26 | 25 | 25 | 24 | 29 |
| Highest level of education attained, % | ||||||||
| None | 26 | 22 | 23 | 27 | 28 | 28 | 28 | 24 |
| Primary school | 23 | 24 | 23 | 22 | 23 | 23 | 25 | 22 |
| ≥Lower middle school | 51 | 54 | 54 | 51 | 49 | 49 | 47 | 54 |
| Income, % | ||||||||
| Low | 33 | 33 | 33 | 33 | 33 | 35 | 37 | 32 |
| Medium | 33 | 33 | 33 | 33 | 35 | 33 | 34 | 33 |
| High | 33 | 34 | 34 | 34 | 32 | 32 | 29 | 35 |
| Energy intake (kcal/d) | 2592 | 2321 | 2473 | 2606 | 2662 | 2899 | 2695 | 2561 |
| Potassium intake (g/d) | 1.6 | 1.5 | 1.6 | 1.7 | 1.7 | 1.7 | 1.6 | 1.6 |
| Physical activity, % | ||||||||
| Low | 33 | 38 | 36 | 33 | 30 | 31 | 29 | 36 |
| Medium | 33 | 32 | 33 | 33 | 34 | 35 | 35 | 33 |
| High | 33 | 31 | 31 | 33 | 36 | 35 | 36 | 32 |
| Current smoker, % | ||||||||
| No | 68 | 71 | 70 | 70 | 67 | 64 | 63 | 70 |
| Yes | 32 | 29 | 30 | 30 | 33 | 36 | 37 | 30 |
| Alcohol intake, % | ||||||||
| <3 times/week | 87 | 89 | 89 | 87 | 86 | 83 | 85 | 88 |
| ≥3 times/week | 13 | 11 | 11 | 13 | 14 | 17 | 15 | 12 |
| Wave of entry, % | ||||||||
| 1991 | 57 | 47 | 52 | 61 | 62 | 66 | 67 | 53 |
| 1993 | 9 | 8 | 10 | 8 | 8 | 9 | 9 | 9 |
| 1997 | 18 | 25 | 21 | 16 | 17 | 14 | 16 | 19 |
| 2000 | 10 | 12 | 10 | 10 | 9 | 8 | 6 | 12 |
| 2004 | 6 | 8 | 7 | 6 | 4 | 3 | 3 | 7 |
The sodium intake across the three different temporal exposure measures was higher in individuals with incident hypertension (Table 1). According to the adjusted hypertension-free survival probability curves for mean and recent sodium exposures, the 5th group (highest intake) had the lowest survival (Figures 1B and 1C). However for mean sodium intake the survival probability of the 3rd group was slightly higher than that of the 1st group (lowest sodium intake). In the case of baseline sodium (Figure 1A), all quintiles were closer to each other in survival. In Table 2, we present the cumulative risk and cumulative risk difference at the median time of follow-up (11 years). For the baseline sodium measure the cumulative risk was around 20% for all sodium intake groups. But for mean and recent sodium measures the highest sodium intake groups had a cumulative risk of hypertension of 30% and the third group had a cumulative risk of 17%, the key difference between the mean and the recent was that the lowest group had 26% and 11% respectively. The HRs presented in Table 3, in this study, have a similar interpretation. Adjusting for all measured covariates did not meaningfully affect the results.
Finally, we assessed the effect of change in sodium intake from baseline to recent period (Table 4). The predicted hazard was higher among individuals who remained in the 5th (high) intake group at baseline and at the most recent exam, compared with individuals who remained in the 1st (low) intake group at both points [HR=1.98 (95% CI= 1.47, 2.67)]. However, the estimated effect was slightly stronger when comparing subjects who increased from low to high sodium intake, with subjects that decreased from high to low [2.27 (1.64, 3.14)]. The estimate that compared high vs. low intake at the recent sodium controlling for baseline intake was 2.12 (1.69, 2.67), this estimate was similar to that shown in Table 3, where no adjustment by baseline sodium was done [2.10 (1.67, 2.63)].
TABLE 4.
Hazard Ratiosa for Incident Hypertension Comparing Different Combinationsb of High (>8.7 g/d) and Low (<3.7 g/d) Sodium Intake at Baseline and at the Most Recent Exam
| HR (95% CI) | |
|---|---|
| High-baseline, High-recent vs. Low-baseline, Low-recent | 1.99 (1.48, 2.68) |
| Low-baseline, High-recent vs. High-baseline, Low-recent | 2.42 (1.74, 3.35) |
| High-recent vs. Low-recent; baseline held constant | 2.19 (1.74, 2.76) |
| High-baseline vs. Low-baseline; recent held constant | 0.91 (0.73, 1.12) |
Estimates are linear combinations of coefficients from a model with baseline and recent sodium intake groups and covariates at baseline: age, BMI, gender region, urban/rural, education level, income, physical activity, smoking, alcohol intake, wave of entry; and energy and potassium intake at baseline and recent period. No interaction term was added (p-value=0.64 of Likelihood Ratio Test comparing model with and without baseline by recent interaction term).
Number of subjects in each combination: High-baseline, High-recent (N=215); Low-baseline, Low-recent (N=436); Low-baseline, High-recent (N=98); High-baseline, Low-recent (N=312); High-recent (N=765); Low-recent (N=1831); High-baseline (N=1316); Low-baseline (N=1315).
CI, confidence interval; HR, Hazard Ratio.
TABLE 3.
Hazard Ratios for Incident Hypertension Comparing to the Lowest Intake Group.
| Baseline sodium intake
|
Mean sodium intake
|
Recent sodium intake
|
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Crude estimate | |||
| G1 ( <3.7 g/d)a | 1 | 1 | 1 |
| G2 ( 3.7–5.2 g/d) | 0.83 (0.67, 1.01) | 0.75 (0.58, 0.95) | 1.21 (1.01, 1.44) |
| G3 (5.2–6.6 g/d) | 0.70 (0.57, 0.86) | 0.79 (0.62, 1.01) | 1.29 (1.06, 1.57) |
| G4 (6.6–8.7 g/d) | 0.77 (0.63, 0.94) | 0.99 (0.78, 1.27) | 1.89 (1.53, 2.34) |
| G5 ( >8.7 g/d) | 0.90 (0.74, 1.10) | 1.47 (1.10, 1.95) | 1.90 (1.53, 2.36) |
| Adjustedb | |||
| G1 ( <3.7 g/d)a | 1 | 1 | 1 |
| G2 ( 3.7–5.2 g/d) | 0.83 (0.67, 1.02) | 0.79 (0.61, 1.01) | 1.27 (1.05, 1.52) |
| G3 (5.2–6.6 g/d) | 0.72 (0.58, 0.89) | 0.90 (0.70, 1.16) | 1.47 (1.20, 1.80) |
| G4 (6.6–8.7 g/d) | 0.77 (0.62, 0.95) | 1.17 (0.90, 1.52) | 2.03 (1.63, 2.52) |
| G5 ( >8.7 g/d) | 0.99 (0.80, 1.22) | 1.68 (1.23, 2.29) | 2.16 (1.72, 2.71) |
Reference category
Adjusted by covariates at baseline: age, BMI, gender, region, urban/rural, education level, income, physical activity, smoking, alcohol intake and wave of entry. Energy and potassium intake were included in the model as baseline, mean or recent accordingly.
CI, confidence interval; G, group; HR, Hazard Ratio.
DISCUSSION
In this longitudinal study we found that the baseline measure of sodium intake had no association with increased risk of incident hypertension in a median follow-up of 11 years. These results are comparable to other studies that only studied sodium intake at baseline. In a Taiwanese study with a median follow-up period of 7.9 years, there was a weak J-shape relationship of sodium intake at baseline with incident hypertension.21 In a European study with a median follow-up of 6.5 years, the risk of incident hypertension did not increase across tertiles of urinary sodium measured at baseline.22
In models using only a baseline sodium exposure, the assumption is that either sodium intake remains constant over time and the whole follow-up period is the relevant time frame, or that the specific point in time (baseline measurement) and not the entire follow-up period is associated with disease incidence. Therefore, even if there were subsequent changes in diet, baseline exposure could be associated with the outcome. Some studies rely on this latter reasoning. For example, a low-sodium intervention during infancy (first 6 months of life) had effects on BP 15-years later even when the estimated sodium intake at follow-up was no longer different among intervention groups.6 Similarly, 7 years following the Trials of Hypertension Prevention, Phase 1, the sodium intake was no longer different between the groups, but those who were in the sodium reduction intervention group had a 35 percent reduced odds (OR= 0.65, 95%CI= 0.25, 1.69) of incident hypertension.4 These studies suggest that even when one measure of sodium does not represent long-term exposure, it still can have long-term effects on hypertension. In our study, this was not the case; a possible explanation is that the effects of an intervention, in which sodium intake is dramatically and intentionally reduced for a period of time, is different than the effect of a typical intake at a specific point long time ago.
If diet changes over time and the hypothesis is that relevant time-frame is the entire follow-up period, a better way to test this is to collect repeated measures of the exposure and examine their average over time. This measure however, could reduce measurement error due to intra-individual variation and therefore result in a stronger association.23 Surprisingly, in our analysis even if the mean measure had a lower degree of error, the mean sodium intake measure did not have a stronger association compared to recent intake.
In our final analysis, we compared different combinations of baseline and recent intake. The strongest estimate of those studied was found when comparing low-baseline, high-recent vs. high-baseline, low-recent; suggesting that there might be a deleterious effect related to increasing sodium intake (from baseline to recent), and a beneficial effect of decreasing it, however all these estimates were imprecise as demonstrated by wide 95% CIs.
It is important to acknowledge that we observed high variability in our estimate of sodium intake over time. As shown in Table 1, lower and higher sodium values at baseline were followed in the recent period by values closer to the mean; this regression to the mean is expected when measurements have random error.24 A key source of random error might be related to the day-to-day variability not captured by our dietary measure, which is based on 3-days of intake only. However, because sodium is not an episodically consumed nutrient, day-today variability might be less important compared to other micronutrients/foods. Indeed, most sodium literature is based on 24-hr urine which is a short-term measure that also misses day-today variability; still, consistent associations between sodium intake and blood pressure and cardiovascular diseases have been reported in the literature.2,25 Furthermore, most sodium in China is added during cooking (with high household-to-household variability), so the fact that we include household weighing in our methodology strengthens our measure, long-term instruments, such as food frequency questionnaires, that do not include weighing of foods and condiments will hardly capture sodium intake. Still, we cannot know how much of the variability over time in our measure, is due random error and how much is due to true changes in diet. Nevertheless, random error generally attenuates the strength of association,26 so even if our estimates are attenuated we were still able to address our research question regarding the comparison of associations between sodium intake and incident hypertension across varying time frames.
A strength of our analysis is the use of IP weights that allowed us to estimate cumulative risk difference as an effect measure and to present adjusted survival curves that retain a marginal interpretation.17 HRs can be misleading because of their non-collapsibility27 and because a single HR averaged over the duration of the follow-up might be inadequate if the HR changes over time.28 The adjusted survival curves, serve to overcome these problems and have the additional advantage of providing absolute risks, which can have a more direct interpretation.
Another strength was the use of models that account for interval-censored data.20,29 This type of data is quite usual in prospective studies in which participants are evaluated at certain times and not continuously. Assuming that the event occurred at the end or mid-point of each interval, and apply standard survival methods, has been shown to produce biased estimates.30 Discrete-time hazard models could have been an alternative if our interval lengths were equal or did not overlap, but that was not the case in our data set. However, an important limitation of models that account for interval-censored data is that they cannot incorporate time-varying variables. This is a limitation particularly for the mean and recent sodium measures; because although these exposures happened at different years of follow-up (i.e. for one person recent sodium happened in year 6, for another it happened in year 13), these variables were time-fixed and had the same value during the entire follow-up. This could have introduced bias in our results, because when the probability of event was estimated in the interval 7 to 9 years, for example, the sodium groups compared included information not only for sodium consumed until year 7, but they included information from sodium consumed after because it included the recent or mean intake of those that remained in the study free of hypertension longer. As a sensitivity analysis we used only the first two sodium measures, which everyone had, this way the mean and recent sodium was from around the same years of follow-up for everyone. As could be expected, the mean sodium (mean of the first two measures) and the recent sodium (second measure) had a weaker association to incident hypertension, but consistent with our main results, their association was stronger compared to the baseline sodium [eTable 3. (D)].
Limitations of this study also include that although our definition of hypertension is what is commonly used in population studies21,25,31 the ideal clinical definition of hypertension often is based on the average of 2 or more BP readings on each of 2 or more office visits.14 Here, we had 3 measures, but they were taken in the same day. Sample selectivity is another key issue, our sample dropped from 13,764 eligible subjects to 6,578 with sample exclusions. Selection bias arises when, by analyzing only those included in the sample, we condition on common effects of the exposure and the outcome32. In our data it seemed that sodium intake at baseline was not related to being selected for the analytic sample (Table 1. Electronic Appendix), therefore selection bias was less likely to be present. In addition, the results of two different sensitivity analyses also suggested that our main analysis had minimal bias of this kind. However, we do not have any information of the mean and recent sodium intake among those excluded from our analysis. Although we do not have a reason to believe these would be any different than baseline sodium, we cannot evaluate to which extent the sodium-hypertension relationship was different among those excluded, particularly for mean and recent sodium.
In conclusion, we found that in this population prospective repeated measures of the exposure were fundamental to adequately study the sodium-hypertension relation. The fact that we observed a null association between baseline sodium intake and hypertension, suggests that baseline measures from a long-term follow up may not be the salient time frame for hypertension exposure.
Supplementary Material
Acknowledgments
Sources of funding: NIH (R01-HD30880, DK056350, R24 HD050924and R01-HD38700, R21-DK089306 and R01-HL108427) and the Fogarty International Center, NIH for financial support for the CHNS data collection and analysis files from 1989 to 2011. Carolina Batis was supported by a scholarship from the Mexican council Consejo Nacional para la Ciencia y Tecnologia.
This research uses data from China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, Carolina Population Center, the University of North Carolina at Chapel Hill. We also wish to thank Dr. Chirayath M. Suchindran for advice with interval-censored survival models, Ms. Frances L. Dancy for administrative assistance, Mr. Tom Swasey for graphics support, and Mr Daniel Blanchette for exception programming assistance.
Footnotes
Conflicts of Interest: None of the authors has conflict of interests of any type with respect to this manuscript.
References
- 1.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6):363–84. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 2.He FJ, MacGregor GA. The Cochrane Collaboration. 2008. Effect of longer-term modest salt reduction on blood pressure (Review) [DOI] [PubMed] [Google Scholar]
- 3.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens. 2003;17(7):471–80. doi: 10.1038/sj.jhh.1001575. [DOI] [PubMed] [Google Scholar]
- 4.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35(2):544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 5.Geleijnse JM, Hofman A, Witteman J, Hazebroek AAJM, Valkenburg HA, Grobbee DE. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension. 1997;29(4):913–917. doi: 10.1161/01.hyp.29.4.913. [DOI] [PubMed] [Google Scholar]
- 6.Gu D, Reynolds K, Wu X, Chen J, Duan X, Muntner P, Huang G, Reynolds RF, Su S, Whelton PK, He J Inter ACGTICSoCDiA. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40(6):920–7. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 7.Yan S, Li J, Li S, Zhang B, Du S, Gordon-Larsen P, Adair L, Popkin B. The expanding burden of cardiometabolic risk in China: the China Health and Nutrition Survey. Obesity Reviews. 2012 doi: 10.1111/j.1467-789X.2012.01016.x. no–no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamler R, Liu LS, Nichols R, Huang DX, Long ZP, Xie JX, Elliott P. Blood pressure and life style in the People’s Republic of China: three samples in the INTERSALT Study. J Hum Hypertens. 1993;7(5):429–35. [PubMed] [Google Scholar]
- 9.Zhao L, Stamler J, Yan LL, Zhou B, Wu Y, Liu K, Daviglus ML, Dennis BH, Elliott P, Ueshima H, Yang J, Zhu L, Guo D, Group IR. Blood pressure differences between northern and southern Chinese: role of dietary factors: the International Study on Macronutrients and Blood Pressure. Hypertension. 2004;43(6):1332–7. doi: 10.1161/01.HYP.0000128243.06502.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. International journal of epidemiology. 2010;39(6):1435–40. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Wang G, Pan X. China food composition 2002. Beijing: Peking University Medical Press; 2002. [Google Scholar]
- 12.Yao M, McCrory MA, Ma G, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr. 2003;77(6):1409–16. doi: 10.1093/ajcn/77.6.1409. [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH Group DA-SCR. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med. 2009;68(7):1305–14. doi: 10.1016/j.socscimed.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. Journal of the Royal Statistical Society. Series B (Methodological) 1976:290–295. [Google Scholar]
- 17.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Sun J. Variance estimation of a survival function for interval-censored survival data. Statistics in Medicine. 2001;20(8):1249–1257. doi: 10.1002/sim.719. [DOI] [PubMed] [Google Scholar]
- 19.Choi BY, Fine JP, Alan Brookhart M. Statistics in Medicine. Practicable confidence intervals for current status data. [DOI] [PubMed] [Google Scholar]
- 20.Royston P. Flexible parametric alternatives to the Cox model, and more. Stata Journal. 2001;1(1):1–28. [Google Scholar]
- 21.Chien KL, Hsu HC, Chen PC, Su TC, Chang WT, Chen MF, Lee YT. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community-based cohort study in Taiwan. Journal of hypertension. 2008;26(9):1750. doi: 10.1097/HJH.0b013e328306a0a7. [DOI] [PubMed] [Google Scholar]
- 22.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA European Project on Genes in Hypertension I. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 305(17):1777–85. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 24.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34(1):215–20. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 25.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willet W. Nutritional Epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 27.Greenland S. Absence of confounding does not correspond to collapsibility of the rate ratio or rate difference. Epidemiology. 1996;7(5):498–501. [PubMed] [Google Scholar]
- 28.Hernan MA. The hazards of hazard ratios. Epidemiology. 21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez G, Calle ML, Oller R, Langohr K. Tutorial on methods for interval-censored data and their implementation in R. Statistical Modelling. 2009;9(4):259. [Google Scholar]
- 30.Lindsey JC, Ryan LM. Methods for interval-censored data. Statistics in Medicine. 1998;17(2):219–238. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Lakoski SG, Herrington DM, Siscovick DM, Hulley SB. C-reactive protein concentration and incident hypertension in young adults: the CARDIA study. Archives of internal medicine. 2006;166(3):345. doi: 10.1001/archinte.166.3.345. [DOI] [PubMed] [Google Scholar]
- 32.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.