Abstract
Drosophila eye development has been extensively studied, due to the ease of genetic screens for mutations disrupting this process. The eye imaginal disc is specified during embryonic and larval development by the Pax6 homolog Eyeless and a network of downstream transcription factors. Expression of these factors is regulated by signaling molecules and also indirectly by growth of the eye disc. Differentiation of photoreceptor clusters initiates in the third larval instar at the posterior of the eye disc and progresses anteriorly, driven by the secreted protein Hedgehog. Within each cluster, the combined activities of Hedgehog signaling and Notch-mediated lateral inhibition induce and refine the expression of the transcription factor Atonal, which specifies the founding R8 photoreceptor of each ommatidium. Seven additional photoreceptors, followed by cone and pigment cells, are successively recruited by the signaling molecules Spitz, Delta, and Bride of sevenless. Combinations of these signals and of intrinsic transcription factors give each ommatidial cell its specific identity. During the pupal stages, Rhodopsins are expressed, and the photoreceptors and accessory cells take on their final positions and morphologies to form the adult retina. Over the past few decades, the genetic analysis of this small number of cell types arranged in a repetitive structure has allowed a remarkably detailed understanding of the basic mechanisms controlling cell differentiation and morphological rearrangement.
Introduction
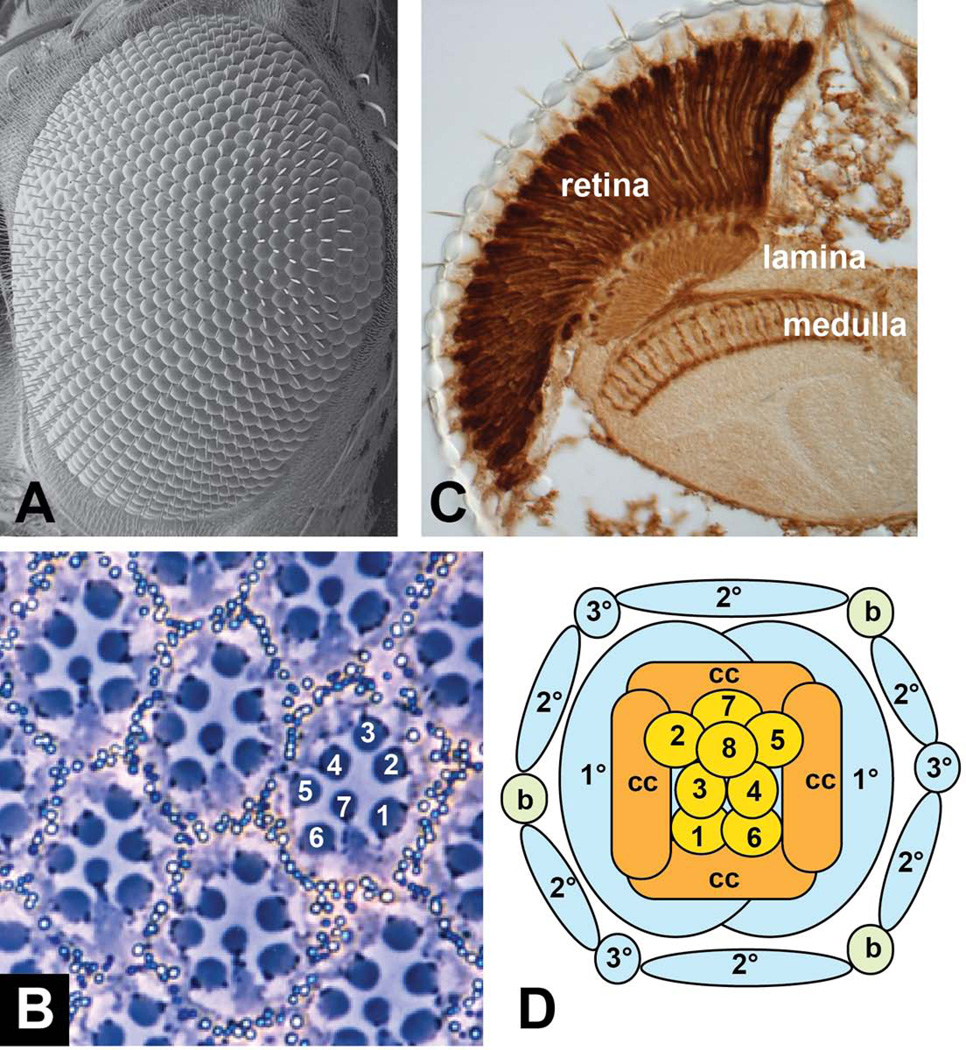
The adult Drosophila eye is a highly organized structure composed of approximately 800 ommatidial units arranged in a hexagonal lattice (Fig. 1A). Each ommatidium contains 8 photoreceptor cells, which extend their light-collecting rhabdomeres into the center of the ommatidium in a trapezoidal pattern (Fig. 1B). The outer photoreceptors R1-R6 have large rhabdomeres, express the rhodopsin Rh1, and project axons into the lamina, a region of the brain specialized for motion detection. The inner photoreceptors R7 and R8 have centrally located small rhabdomeres, with the R8 rhabdomere directly below R7, each express one of four rhodopsins (Rh3-Rh6), and project their axons into the medulla, the brain region responsible for color vision (Fig. 1C) 1. The photoreceptors are surrounded by four cone cells, which secrete the lens, and by two primary pigment cells, which contribute to isolating each ommatidial light-sensing unit. These ommatidial clusters are separated from each other by a lattice of secondary and tertiary pigment cells and mechanosensory bristles (Fig. 1D) 2. Because the eye has a highly repetitive structure and is not essential for survival of flies raised in the laboratory, it is well suited for genetic screens. The isolation of numerous mutations that affect the formation of the adult eye has led to a detailed mechanistic understanding of its development.
Figure 1. Structure of the adult Drosophila eye.
(A) shows a scanning electron micrograph of the surface of the eye, demonstrating the hexagonal packing of the ommatidia. (B) shows a tangential section through the eye, illustrating the characteristic trapezoidal arrangement of the rhabdomeres of photoreceptors R1-R7. The rhabdomere of R8 lies below that of R7. (C) shows a coronal section through the adult head of a fly expressing lacZ in all photoreceptors, stained with anti-β-galactosidase. This section shows the elongated shape of the photoreceptor cells in the retina and their axons extending to the lamina and medulla. (D) is a diagram of the arrangement of cell types found in each ommatidium. 1–8, photoreceptors R1-R8; cc, cone cells; 1°, 2°, 3°, pigment cells; b, mechanosensory bristle.
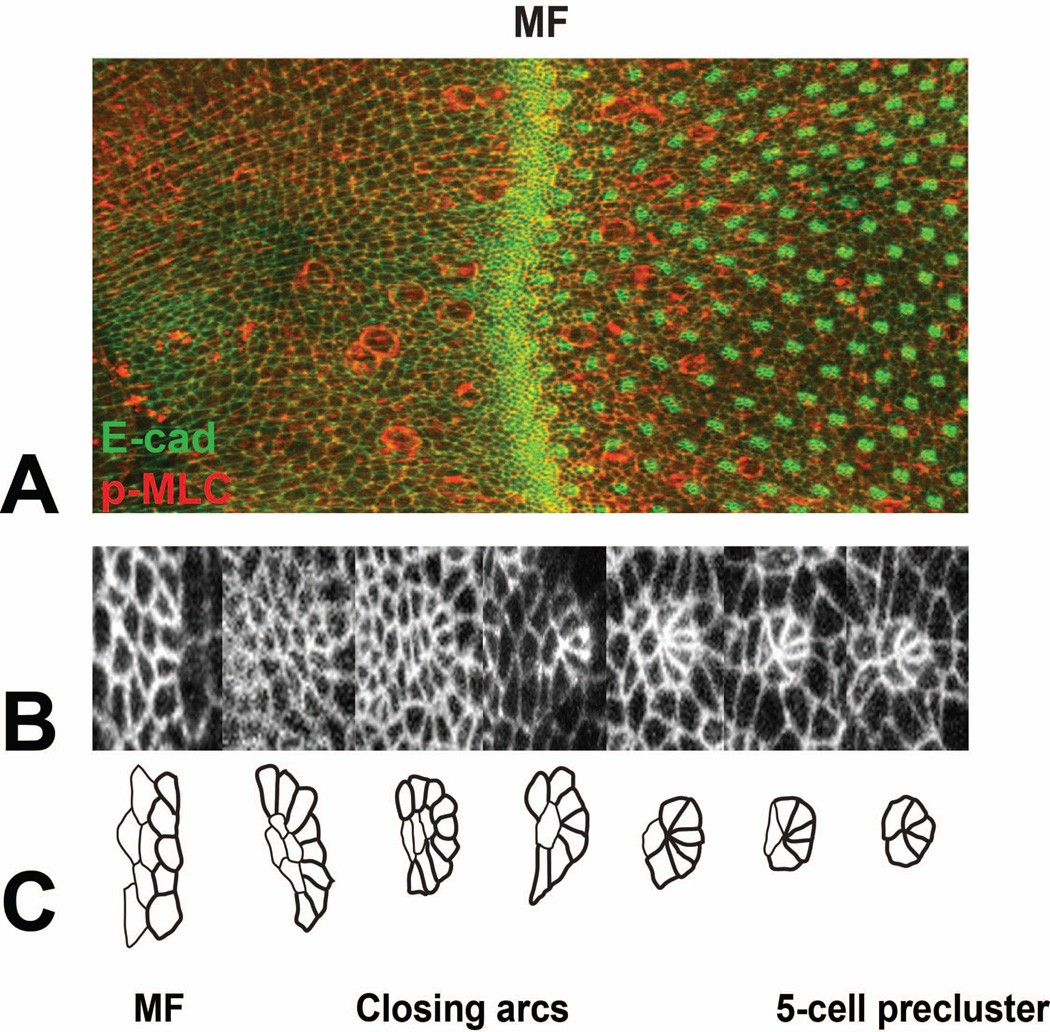
The eye develops from the eye imaginal disc, a bilayered epithelial tissue that invaginates from the embryonic epidermis, grows and differentiates inside the larva, and everts during metamorphosis. Retinal differentiation initiates at the posterior margin of the eye disc in the third larval instar and gradually progresses towards the anterior margin, reaching it after the first day of pupal development. The first overt sign of differentiation is a transient invagination of the disc surface known as the morphogenetic furrow 3 (Fig. 2). Anterior to this moving furrow, cells divide in an unpatterned manner. On the posterior side of the furrow, their apical profiles become organized into evenly spaced arcs. These arcs close up and finally transform into 5-cell preclusters (Fig. 2B, C), within which the photoreceptors R8, R2 and R5, and R3 and R4 differentiate in sequence, as revealed by their expression of neuronal markers 4–6. The cells that remain undifferentiated at this stage undergo a final round of division, the second mitotic wave, before differentiating as R1 and R6, R7, cone cells, and primary pigment cells 4, 5. During pupal development, some of the remaining cells surrounding the ommatidial clusters die, and the rest reorganize to form a hexagonal lattice, in which the sides are formed by secondary pigment cells and the vertices by tertiary pigment cells alternating with bristles 7. This review will describe the genetic and molecular mechanisms that underlie the process of retinal differentiation. Further information about all the genes discussed is available in Flybase (http://flybase.org/).
Figure 2. Pattern formation in the developing eye disc.
(A) shows part of an eye disc labeled with E-cadherin-GFP (E-cad-GFP, green) and anti-phosphorylated myosin light chain (p-MLC, red). Anterior is to the left. The morphogenetic furrow (MF) is indicated. (B) shows a series of E-cad-GFP-labeled cell clusters increasing in age from left to right, and (C) shows tracings of the same clusters. Unpatterned cells in the morphogenetic furrow transform into arcs, which close by removal of the central cells and ultimately become 5-cell preclusters. Figure kindly provided by Franck Pichaud.
The eye field is specified by a network of transcription factors
The compound eye-antennal imaginal disc gives rise not only to the eye, but also to much of the head cuticle 8. A cascade of transcription factors specifies first the entire disc and subsequently the eye field within it. During embryonic stages, the whole eye-antennal disc expresses Eyeless (Ey) and Twin of eyeless (Toy), two homologues of the Paired domain/homeodomain transcription factor Pax6 (Fig. 3A). Toy is also expressed earlier in a broad region of the embryonic head 9, 10. Although ey and toy are required for head development, some mutant alleles of these genes result in a specific loss of eye tissue 11, 12. Strikingly, misexpression of these genes in other imaginal discs can drive the development of ectopic eyes 9, 13, although this occurs only at positions where other factors necessary for eye development are present 14–18. Interestingly, Pax6 appears to be at the top of a hierarchy of factors that regulate eye development not only in Drosophila, but in almost every species that has been examined, suggesting that cells important for vision came under the control of this transcription factor at a very primitive stage of their evolution 19.
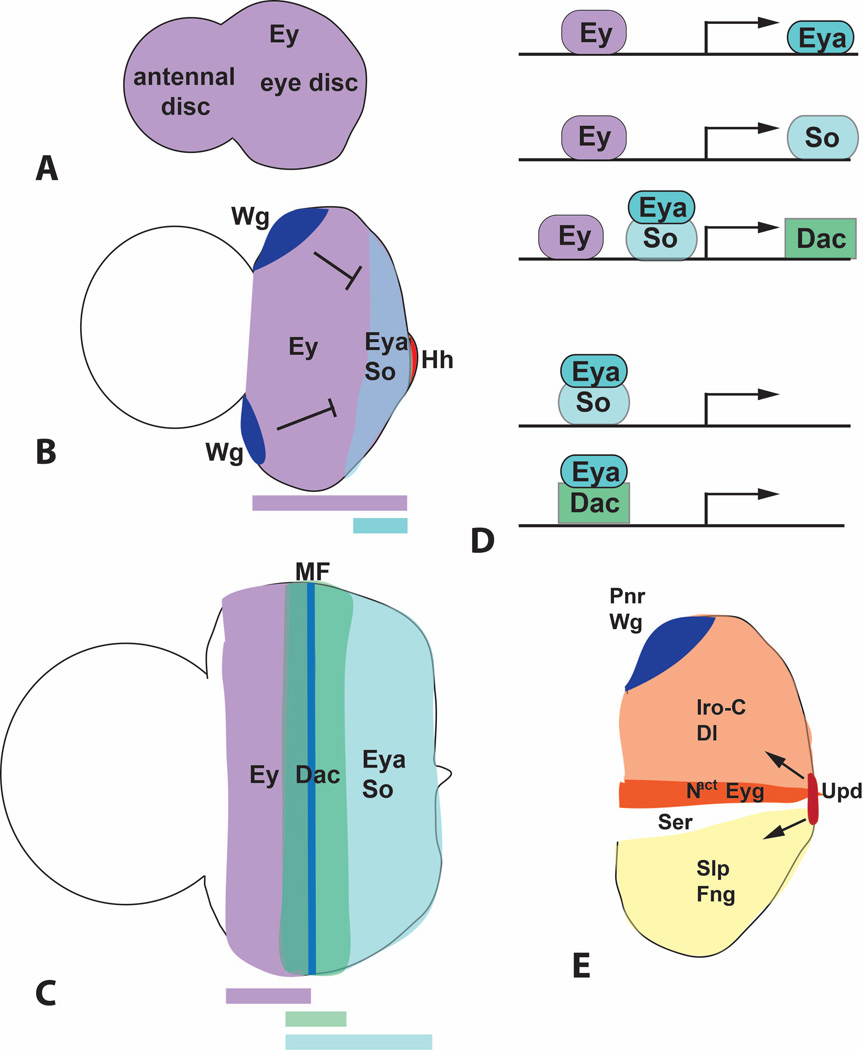
Figure 3. Retinal determination genes.
(A–C) are diagrams showing the expression pattern of Ey, Eya, So and Dac in first instar (A), second instar (B) and third instar (C) eye-antennal discs. Colored bars below the diagrams indicate the regions in which these expression domains overlap. Repression of eya by anterior Wg and its activation by posterior Hh is indicated in (B). MF, morphogenetic furrow. (D) represents the functional relationships between these transcription factors. Ey directly activates eya and so transcription, and Ey, Eya and So all contribute to dac activation. Eya can interact with the DNA-binding protein So to form a compound transcription factor that regulates downstream genes, and may also regulate gene expression in a complex with Dac. (E) shows the expression domains of some of the factors that drive dorsal-ventral compartmentalization and growth of the early eye disc. Dorsally expressed Pnr activates wg expression, and Wg then establishes the expression domains of the Iro-C and Slp transcription factors. These control the compartmentalized distribution of Notch ligands and modifying enzymes that lead to Notch activation at the dorsoventral midline. Downstream targets of Notch that regulate growth include the transcription factor Eyg and the long-range signaling molecule Upd.
During the second larval instar, ey expression is lost from the antennal disc and comes to define the eye field, while the transcription factor Cut specifies and maintains the antennal state 10, 20, 21. Ey is coexpressed with two other transcription factors, Homothorax (Hth) and Teashirt (Tsh), and acts in combination with them to promote the growth of the early eye disc 17, 22. Ey also induces the expression of eyes absent (eya) and sine oculis (so) at the posterior of the eye disc, acting directly through binding sites in the regulatory regions of these genes (Fig. 3B, D) 10, 23, 24. Eya and So can form a compound transcription factor that is targeted to specific sequences by the DNA-binding domain of So 25, 26. Eya and So are in turn required for the expression of Dachshund (Dac) 27, another protein that can both interact with Eya and bind to specific DNA sequences (Fig. 3D) 28, 29. Eya, So and Dac are all essential for eye differentiation 25, 30–33. When ectopically expressed, these downstream factors have a more limited ability to induce ectopic eye development, and this is accompanied by induction of ey expression, indicating the existence of feedback loops within the retinal determination network 25, 29, 34, 35. In addition to its role as a transcription factor, Eya has a second function in the cytoplasm as a tyrosine phosphatase enzyme. Although its phosphatase activity seems to contribute to eye specification, the mechanism of this effect is not fully understood 36–38.
eya expression is confined to the posterior part of the eye disc due to its regulation by localized signaling molecules. Posteriorly expressed Hedgehog (Hh) and Decapentaplegic (Dpp), a Bone Morphogenetic Protein (BMP) homologue, act as positive regulators of eya, and anteriorly expressed Wingless (Wg) as a negative regulator 18, 20, 39, 40. The lateral regions that express Wg do not form part of the eye field, but will instead give rise to head cuticle 41. Wg is both necessary and sufficient to promote the head cuticle fate; loss of Wg activity expands the eye field into the dorsal head, while ectopic activation of the Wg pathway within the eye field produces cuticular outgrowths 42–45. Because Wg acts at a long range, the disc must reach a certain size before Wg levels are low enough in its posterior region for eya expression to be initiated 20. Growth of the disc thus controls the timing of eya expression, and mutations that disrupt growth will secondarily block differentiation.
Growth of the eye disc depends on the subdivision of the disc into dorsal and ventral compartments, which allows activation of Notch signaling specifically at the dorsoventral boundary. This is achieved by partitioning the glycosyltransferase enzyme Fringe (Fng) specifically into the ventral compartment. Modification of Notch by Fng renders it insensitive to the ventrally expressed ligand Serrate (Ser), but sensitive to the dorsally expressed ligand Delta (Dl), ensuring that it is active only where the two domains meet 46–48 (Fig. 3E). The upstream regulators of Dl, Ser and fng expression are three homeodomain transcription factors known as the Iroquois complex (Iro-C) in the dorsal compartment, and two Sloppy-paired transcription factors, members of the Forkhead family, in the ventral compartment; mutual repression maintains this complementary arrangement 49, 50. Expression of the Iro-C genes is initiated by Wg, which is predominantly dorsal in the early eye disc, due to its activation by Pannier (Pnr), a transcription factor expressed at the dorsal margin of the eye disc, and ventral repression by the JAK/STAT pathway ligand Unpaired (Upd) 51–54. Interestingly, Upd expression is also induced by Notch activation at the midline of the posterior margin, a point from which it acts at a long range to stimulate growth throughout the eye disc 55–58. In addition, Notch signaling promotes growth autonomously through the downstream effectors Eyegone (Eyg) and Four-jointed 53, 55, 58.
Progressive photoreceptor differentiation is driven by autoregulatory loops
In the middle of the third larval instar, photoreceptor clusters begin to form at the posterior of the eye disc. As the morphogenetic furrow progresses anteriorly, successive rows of ommatidia differentiate approximately every two hours until the eye field is complete 24 hours after pupariation 3. The driving force behind this differentiation wave is the signaling molecule Hh, which is essential for both the initiation and progression of photoreceptor differentiation 59–61. Hh expression at the posterior margin of the early eye disc is established by three zinc finger transcription factors in the Odd-skipped family 62. Subsequently, Hh itself induces additional Hh expression in the photoreceptors as they differentiate, through an indirect autoregulatory loop. This gradual spread of Hh expression drives the progression of differentiation from posterior to anterior across the disc 59, 60. In the eye disc, Hh acts primarily to inactivate the repressor form of the downstream transcription factor Cubitus interruptus (Ci), allowing the expression of dpp 18, 63. dpp is expressed in a stripe in the morphogenetic furrow, immediately anterior to the zone of hh expression (Fig. 4A, B) 60. Posterior to the morphogenetic furrow, cells can no longer respond to Hh because Ci is degraded by a ubiquitin ligase complex containing Cullin3 and the adaptor protein Roadkill 64, 65. The morphogen Dpp acts at a long range to restrict the expression of the transcription factor Hth to the anterior of the eye disc, where it represses eya in combination with Ey and Tsh 17. Dpp signaling thus creates a preproneural zone in which cells express both ey and eya and are primed to respond to the shorter-range Hh signal (Fig. 4A, B). Hh and Dpp signaling, in combination with additional inputs, lead to the down-regulation of ey and tsh in differentiating cells and the up-regulation of so and dac as well as eya in the preproneural zone and more posteriorly 40.
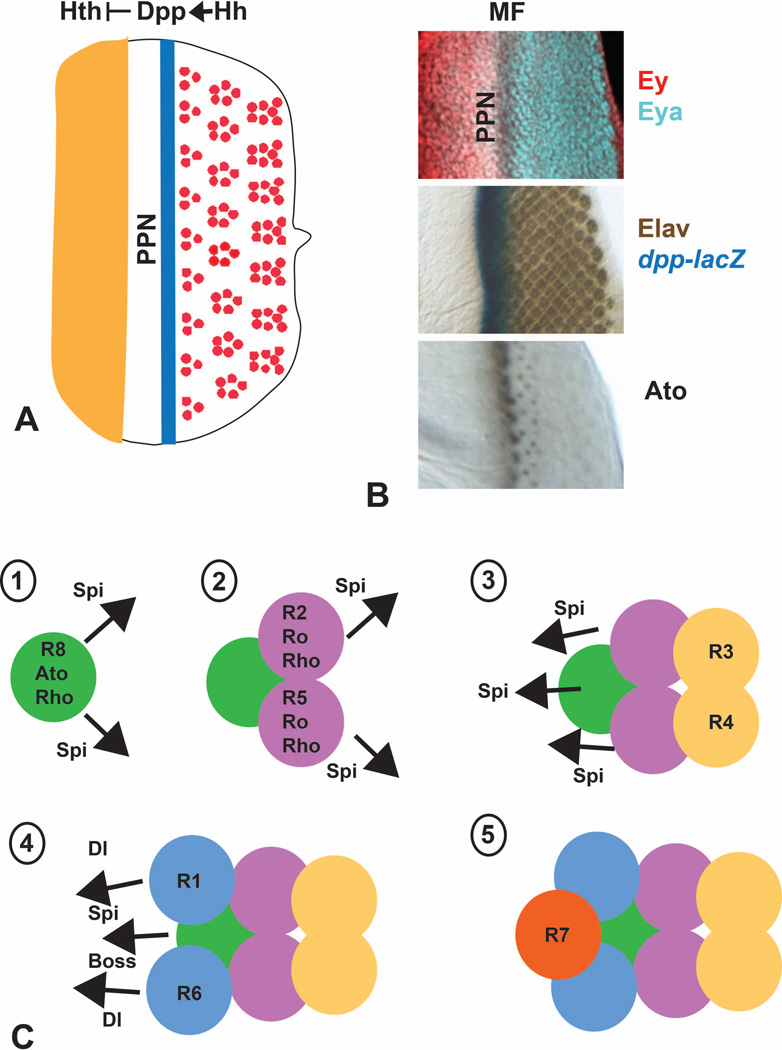
Figure 4. Progression of the morphogenetic furrow.
(A) is a diagram showing that Hh expressed in developing photoreceptors activates a stripe of dpp expression in the morphogenetic furrow. Dpp then acts at a long range to repress hth, limiting it to the anterior of the eye disc and establishing a preproneural zone (PPN) in which cells can respond to Hh. (B) shows regions of third instar eye discs stained for the indicated markers. Elav is a neuronal-specific protein used to mark differentiating photoreceptors. (C) is a diagram of the five signaling steps involved in recruitment of each photoreceptor or photoreceptor pair to the forming ommatidium. Rho proteins expressed in R8, R2 and R5 allow these cells to produce Spi, which is instrumental in recruiting all photoreceptors other than R8. Dl produced by R1 and R6 and Boss produced by R8 are also necessary to recruit R7.
Another critical target gene that is activated redundantly by Hh and Dpp signaling is atonal (ato), which encodes a proneural basic helix-loop-helix transcription factor 66, 67. ato is first expressed in all cells in a stripe just anterior to the morphogenetic furrow, but it rapidly resolves more posteriorly into regularly spaced groups of cells and then into single cells, the future R8 photoreceptors (Fig. 4B) 68. Its initial expression is driven by a 3’ enhancer region that contains essential binding sites for Ey and So 69–71, suggesting that the input from Hh and Dpp signaling may be mediated by these eye determination factors 72. A 5’ enhancer that responds to Ato autoregulation and to the zinc finger transcription factor Roughened eye mediates its later expression 69, 73. This expression is restricted to R8 precursors by lateral inhibition, which is mediated by the Notch pathway through the transcription factors Enhancer of split and Daughterless (Da) 74, 75. Notch signaling and the spacing of ato-expressing groups of cells are also regulated by the secreted protein Scabrous 76–78. Downstream of Ato, which is only transiently expressed, permanent expression of the zinc finger transcription factor Senseless (Sens) seals the fate of the R8 cell 79. Ato is nevertheless predicted to directly regulate numerous downstream genes, only a subset of which are shared with Sens 80.
Photoreceptor differentiation must be precisely coordinated in space and time. Anterior to the morphogenetic furrow, two repressors and antagonists of Ato, Extramacrochaetae (Emc) and Hairy, prevent it from functioning prematurely 81. Emc acts primarily by inhibiting the expression of Da, an obligate partner for the Ato protein 82. These antagonists are themselves induced by Hh and Dpp, and are subsequently repressed by Dl, a very short-range signal also emanating from the morphogenetic furrow 17, 67, 82, 83. Posterior to the morphogenetic furrow, Ato expression in undifferentiated cells is terminated by the homeodomain proteins BarH1 and BarH2 84.
The primary role of Hh signaling is to activate Ato and thus promote the specification of the R8 precursor in each ommatidium. This is sufficient to set in motion the construction of the entire ommatidium, not because the remaining cells are descendants of R8, but because they are recruited by R8 from the surrounding pool of undifferentiated cells (Fig. 4C) 3. Under the control of Ato, R8 expresses Rhomboid (Rho) and Rhomboid-3/Roughoid, two proteases that cleave and activate the transmembrane precursor form of Spitz (Spi), a ligand for the Epidermal growth factor receptor (EGFR) 85–87. Spi signaling promotes the stepwise differentiation of R2 and R5, R3 and R4, R1 and R6, R7, and the cone and primary pigment cells 88–90. Rho is also expressed in R2 and R5, and Spi produced by these cells contributes to the differentiation of other photoreceptors 88, 90, 91. The short range of Spi action 92 and the geometry of the initial arc may explain why R3 and R4, at the tips of the arc, differentiate later than R2 and R5, which are immediately adjacent to R8. The precursors of R1, R6 and R7 divide in the second mitotic wave, which may delay the response to Spi signaling in these cells. Activation of the EGFR pathway in more distant cells is also prevented by Argos, a secreted feedback inhibitor that prevents Spi from binding to the EGFR 93–95. Later differentiating cells require input from the Notch ligand Dl in addition to Spi. Dl is itself transcribed in response to Spi signaling, creating a feedforward loop 96. Dl produced by R1-R6 helps to recruit R7 and cone cells 96–98, while Dl produced by cone cells in response to EGFR signaling can recruit primary pigment cells 99.
In addition to promoting the differentiation of R2 and R5, Spi signaling also induces these cells to express Hh. An eye-specific enhancer of the hh gene integrates input from Pointed P2 (PntP2), the Ets transcription factor responsive to EGFR signaling, and the retinal determination transcription factor So 100. Hh secreted by more posterior photoreceptors thus promotes Ato expression, R8 differentiation, Spi-dependent recruitment of R2 and R5, and eventually its own expression in response to EGFR signaling in these cells. This indirect autoregulatory loop is the basis for the gradual anterior progression of differentiation.
Combinatorial signals control the differentiation of specific cell types
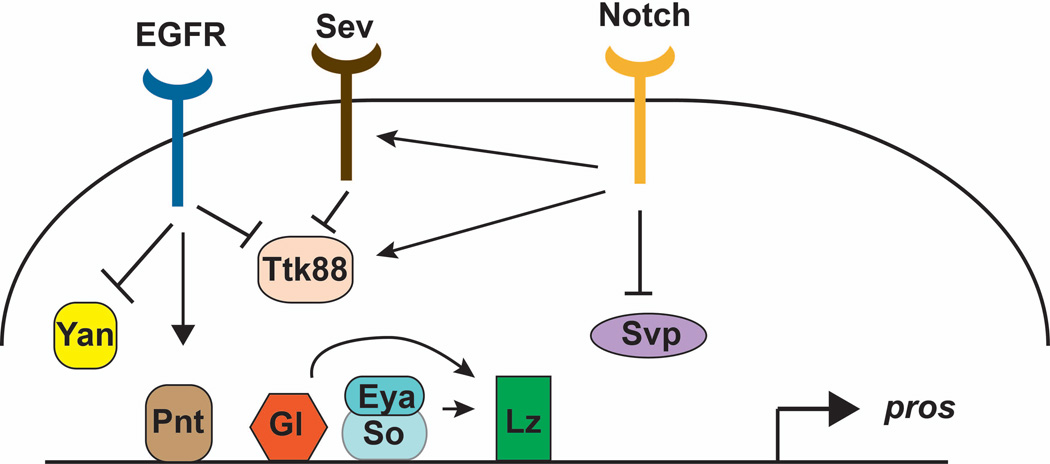
All the photoreceptors other than R8 require Spi for their induction. Nevertheless, they differ in other properties, such as their position, timing of differentiation, and gene expression. Mutations that transform one subtype into another have given some insight into how individual subtypes are specified. A classic example is the sevenless (sev) mutation, in which R7 photoreceptors are absent and the R7 precursor instead differentiates into a non-neuronal cone cell 101. A second mutation, bride of sevenless (boss) was later shown to have the same phenotype 102. Since only R7 cells express rhodopsins that detect ultraviolet light, both mutations were initially isolated based on the failure of the adult flies to choose ultraviolet light over visible light 102, 103. sev was subsequently shown to encode a receptor tyrosine kinase required in the R7 cell 104–106, while boss encodes a transmembrane ligand required in the R8 cell 102, 107. The pathway downstream of Sev was elucidated using a genetic screen for dominant modifiers of a temperature-sensitive sev allele 108. Interestingly, Sev signals through the Ras/Mitogen-activated protein kinase (MAPK) cassette, the same pathway that is downstream of the EGFR and other receptor tyrosine kinases 109, 110. Analysis of the promoter of prospero (pros), a gene expressed strongly in R7 and weakly in cone cells that encodes a transcription factor important for their differentiation, suggested that combined signaling through both receptors is necessary for high-level pros activation 111 (Fig. 5). Degradation of Tramtrack88 (Ttk88), a transcription factor that represses neuronal differentiation and pros expression, may integrate the two pathways. Ttk88 degradation is mediated by a ubiquitin ligase complex that contains both the adaptor protein Phyllopod, which is regulated by Sev, and the F-box protein Ebi, which is regulated by EGFR, as well as the RING domain protein Seven in absentia 112–114.
Figure 5. Diagram of the factors involved in regulating prospero, a gene expressed in R7.
The activator PntP2 and repressors Yan and Ttk88 are regulated by EGFR signaling, and Ttk88 also responds to Sev and Notch signaling. In addition, Notch positively regulates sev expression and negatively regulates svp, which encodes a repressor of pros. The eye-specific transcription factors Gl, Eya/So, and Lz also contribute to pros activation. lz is itself a target of Gl, Eya and So. This diagram only indicates the factors that bind to the Pros enhancer, and not the correct number or placement of their binding sites.
R7 differentiation also requires input from R1 and R6; these cells express Dl, which activates the Notch pathway in the R7 precursor 97, 115. Notch signaling exerts antagonistic activities. It appears to increase Ttk88 levels to oppose photoreceptor differentiation. However, it also induces expression of sev, allowing R7 to receive the Boss signal that overrides the effect of Ttk88 116. In addition, Notch signaling represses seven-up (svp), which encodes a direct repressor of pros expression 117. Differentiation of R7 and transcription of pros also require input from the Runt domain transcription factor Lozenge (Lz) 118 111. Lz is expressed in undifferentiated cells posterior to the furrow and is maintained in the descendants of cells that divide in the second mitotic wave 119. Its expression is activated by So and another retinal-specific transcription factor, Glass 120, 121, both of which also directly regulate pros and other cell type-specific genes 80, 117. The intricacy of pros regulation illustrates the complex combinatorial mechanisms necessary to render a single cell distinct from its immediate neighbors (Fig. 5).
While the R7 precursor becomes a cone cell if it does not receive the appropriate signals, the R7 fate can itself be a default choice for earlier differentiating cells. Svp is an orphan nuclear receptor required in R1, R3, R4 and R6; in its absence all these cells were initially thought to become R7 cells 122. However, it was recently shown that svp mutant cells are equally likely to differentiate as either R7 or R8 cells. Interestingly, in this case the cells choose one of these two fates only quite late in development, and Notch signaling promotes the R7 fate by repressing the R8-specific transcription factor Sens 123. Additional transcription factors specify the other cell fates within the ommatidium. Sens and the homeodomain protein Rough (Ro), which is expressed in the R2 and R5 cells, mutually repress each other’s expression to prevent R8, R2 and R5 from switching their fates 124, 125. In rough mutants, Sens is misexpressed in the R2 and R5 precursors, and these cells differentiate as R8 cells 125. The transcription factors Spalt, expressed in R3 and R4, and Bar, expressed in R1 and R6, differentiate these photoreceptor pairs from each other 126, 127. However, the upstream regulators of these factors are largely unknown. Finally, R3 and R4 are differently specified under the influence of planar polarity signaling, a topic covered by another review in this series (Singh and Mlodzik, 2012).
Terminal differentiation is regulated independently from cell type specification
Photoreceptors establish specific patterns of gene expression during the larval and early pupal stages, but do not take on their characteristic morphologies until later in pupal development. Analysis of mutants lacking the two adjacent spalt genes revealed that morphogenesis and cell fate are under separate control. In these mutants, the adult R7 and R8 cells resemble outer photoreceptors in that they form large rhabdomeres and express the rhodopsin Rh1 128. However, during larval development R8 differentiates normally, and although R7 fails to express genes such as pros and runt, it does not take on an outer photoreceptor fate at this stage 129. Thus Spalt transcription factors are specifically required for late steps in inner photoreceptor differentiation.
Terminal differentiation of photoreceptors involves modification of the apical membrane to create the rhabdomere, a stack of membranes packed with rhodopsin that functions as a photon detector. The apical membrane rotates 90° to face the center of the ommatidium rather than the apical surface of the eye, becomes folded into numerous microvilli, and is connected to the cell body by a proximal stalk region (Fig. 6A) 130. The transmembrane protein Crumbs and other protein complexes that regulate apical-basal polarity carry out this reorganization of the apical surface 131, 132. In addition, rhodopsin and proteins that transport it into the rhabdomere are essential for rhabdomere morphogenesis 133, 134. At the transcriptional level, rhabdomere formation is redundantly controlled by Orthodenticle (Otd) and Pph13, two homeodomain proteins 135. Together with the steroid hormone ecdysone, Otd directs the timing of photoreceptor maturation 136.
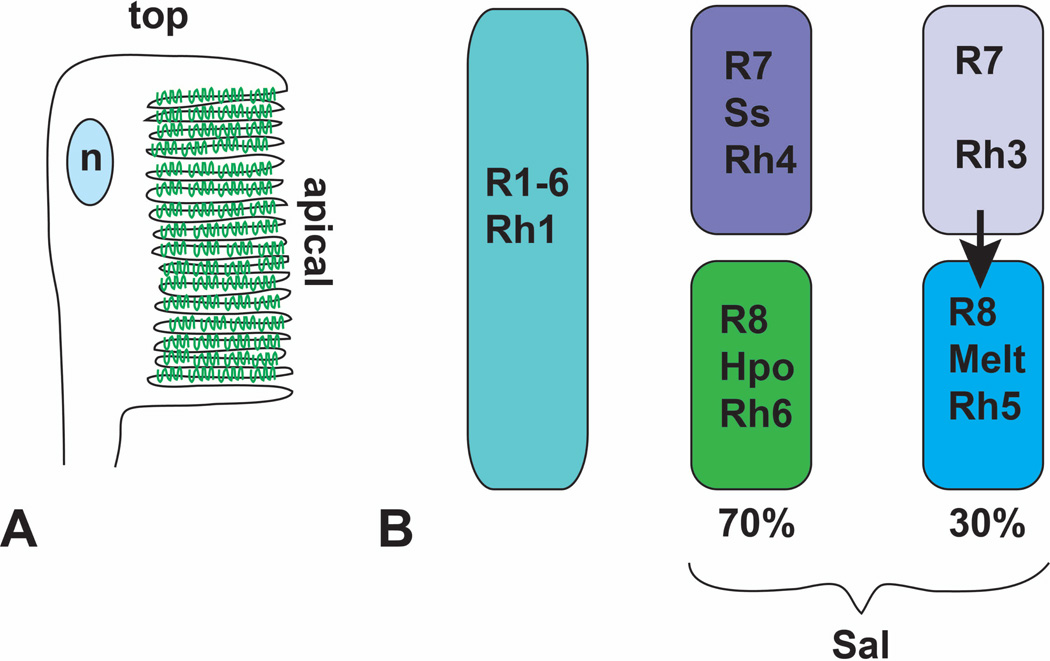
Figure 6. Terminal differentiation involves Rhodopsin expression and localization.
(A) is a diagram of the adult rhabdomere, indicating the rotation and folding of the apical surface. Rhodopsin molecules are represented in green. n, nucleus. (B) shows the distribution of the five different rhodopsins between the eight photoreceptors. All R1–6 cells express Rh1. Approximately 70% of R7 cells express Ss and Rh4. The R8 cells in the same ommatidia activate the Hpo pathway and express Rh6. In the absence of Ss, R7 cells express Rh3 and signal to the R8 cells in their ommatidia to express Melt and Rh5
During pupal development, each photoreceptor must also choose only one specific Rhodopsin (Rh) molecule to express; Rh1 and Rh5 detect blue light, while Rh6 detects green light and Rh3 and Rh4 detect ultraviolet light 137. All R1-R6 cells express Rh1, but inner photoreceptors are divided into several distinct groups. In a dorsal region of the eye specialized for polarized light detection, R7 and R8 both express Rh3, a fate determined by the transcription factor Hth 138. In the remainder of the retina, about 30% of ommatidia have R7 cells that express Rh3 and R8 cells that express Rh5, and 70% have R7 cells that express Rh4 and R8 cells that express Rh6 1. This distinction is controlled by transient stochastic expression of the transcription factor Spineless (Ss) in the subset of R7 cells that will later express Rh4 139. The Rhodopsin expressed by an R8 cell depends on signaling from the R7 cell within the same ommatidium. Rh3-expressing R7 cells induce neighboring R8 cells to express Rh5; in the absence of this signal, R8 cells express Rh6. Although the nature of the signal is still unknown, it is transduced by molecules better known for their role in growth control in proliferating tissues: components of the Hippo (Hpo) pathway and the pleckstrin homology domain protein Melted (Melt) (Fig. 6B) 140, 141.
During the pupal period, the non-photoreceptor cells also take on their final positions and morphologies. The second mitotic wave generates an excess of undifferentiated cells, and some of these must be eliminated by cell death to create a hexagonal lattice with only a single secondary pigment cell at each edge and a single tertiary pigment cell or bristle group at each vertex 142. Reorganization of these lattice cells is driven by differential adhesion between two proteins of the immunoglobulin superfamily: Roughest (Rst) on the membrane of secondary and tertiary pigment cells binds to Hibris on the membrane of primary pigment cells. Those cells with the highest levels of Rst form the largest contact surfaces with primary pigment cells, enabling them to survive and adopt an extended morphology, while other cells lose contact and ultimately die 143 144. Survival appears to depend on Spi produced by cone and primary pigment cells, which activates the EGFR in lattice cells to downregulate Head involution defective, an inducer of apoptosis 145. Computational modeling suggests that apical expansion of the cone and primary pigment cell profiles also plays an important role in generating the normal pattern of secondary and tertiary pigment cells 143.
Conclusions
Clearly, the repetitive structure and accessibility of the eye and the power of Drosophila genetics have led to a wealth of knowledge about how this organ develops. The identification of numerous genes required for normal development of the eye and the characterization of enhancer elements that control the expression of many of these genes have been particularly informative. Autoregulation and feed-forward loops play a role at several developmental stages. However, we still do not fully understand how a combination of external signals and intrinsic factors leads to the production of specific cell types in a precise temporal and spatial pattern. Further study of eye development will doubtless provide us with additional insight into fate specification, combinatorial signaling networks that generate complex patterns, and cell biological aspects of differentiation.
Acknowledgements
Work on eye development in the author’s lab is supported by the National Institutes of Health (grant EY013777). The manuscript was improved by the critical comments of Kevin Legent and Justine Oyallon.
References
- 1.Morante J, Desplan C, Celik A. Generating patterned arrays of photoreceptors. Curr Opin Genet Dev. 2007;17:314–319. doi: 10.1016/j.gde.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Vol. II. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1316. [Google Scholar]
- 3.Ready DF, Hanson TE, Benzer S. Development of the Drosophil a retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 4.Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson A, Ready DF. Neuronal differentiation in the Drosophila ommatidium. Dev. Biol. 1987;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 6.Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139:3432–3441. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez M, Casares F. Organ specification-growth control connection: new in-sights from the Drosophila eye-antennal disc. Dev Dyn. 2005;232:673–684. doi: 10.1002/dvdy.20311. [DOI] [PubMed] [Google Scholar]
- 9.Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M. twin of eyeless a second Pax-6 gene of Drosophila acts upstream of eyeless in the control of eye development. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- 10.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 11.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 12.Kronhamn J, Frei E, Daube M, Jiao R, Shi Y, Noll M, Rasmuson-Lestander A. Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development. 2002;129:1015–1026. doi: 10.1242/dev.129.4.1015. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Halder G, Zhang Z, Mardon G. Signaling by the TGF-beta homolog Decapentaplegic functions reiteratively within the network of genes controlling retinal cell fate determination in Drosophila. Development. 1999;126:935–943. doi: 10.1242/dev.126.5.935. [DOI] [PubMed] [Google Scholar]
- 15.Niwa N, Hiromi Y, Okabe M. A conserved developmental program for sensory organ formation in Drosophila melanogaster. Nat Genet. 2004;36:293–297. doi: 10.1038/ng1308. [DOI] [PubMed] [Google Scholar]
- 16.Kango-Singh M, Singh A, Henry Sun Y. Eyeless collaborates with Hedgehog and Decapentaplegic signaling in Drosophila eye induction. Dev Biol. 2003;256:49–60. doi: 10.1016/s0012-1606(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 17.Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS. Combinatorial control of Drosophila eye development by eyeless, homothorax and teashirt. Genes Dev. 2002;16:2415–2427. doi: 10.1101/gad.1009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappu KS, Chen R, Middlebrooks BW, Woo C, Heberlein U, Mardon G. Mechanism of Hedgehog signaling during Drosophila eye development. Development. 2003;130:3053–3062. doi: 10.1242/dev.00534. [DOI] [PubMed] [Google Scholar]
- 19.Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 20.Kenyon KL, Ranade SS, Curtiss J, Mlodzik M, Pignoni F. Coordinating proliferation and tissue specification to promote regional identity in the Drosophila head. Dev Cell. 2003;5:403–414. doi: 10.1016/s1534-5807(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang CW, Sun YH. Segregation of eye and antenna fates maintained by mutual antagonism in Drosophila. Development. 2012;139:3413–3421. doi: 10.1242/dev.078857. [DOI] [PubMed] [Google Scholar]
- 22.Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: Homothorax and Yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009;23:2307–2319. doi: 10.1101/gad.1820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrin EJ, Li Y, Hoffman K, Liu J, Wang K, Zhang L, Mardon G, Chen R. Genome-wide identification of direct targets of the Drosophila retinal determination protein Eyeless. Genome Res. 2006;16:466–476. doi: 10.1101/gr.4673006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niimi T, Seimiya M, Kloter U, Flister S, Gehring WJ. Direct regulatory interaction of the Eyeless protein with an eye-specific enhancer in the sine oculis gene during eye induction in Drosophila. Development. 1999;126:2253–2260. doi: 10.1242/dev.126.10.2253. [DOI] [PubMed] [Google Scholar]
- 25.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 26.Pauli T, Seimiya M, Blanco J, Gehring WJ. Identification of functional Sine oculis motifs in the autoregulatory element of its own gene, in the eyeless enhancer and in the signalling gene hedgehog. Development. 2005;132:2771–2782. doi: 10.1242/dev.01841. [DOI] [PubMed] [Google Scholar]
- 27.Pappu KS, Ostrin EJ, Middlebrooks BW, Sili BT, Chen R, Atkins MR, Gibbs R, Mardon G. Dual regulation and redundant function of two eye-specific enhancers of the Drosophila retinal determination gene dachshund. Development. 2005;132:2895–2905. doi: 10.1242/dev.01869. [DOI] [PubMed] [Google Scholar]
- 28.Kim SS, Zhang RG, Braunstein SE, Joachimiak A, Cvekl A, Hegde RS. Structure of the retinal determination protein Dachshund reveals a DNA binding motif. Structure. 2002;10:787–795. doi: 10.1016/s0969-2126(02)00769-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and Eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 30.Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 31.Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 32.Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 33.Serikaku MA, O'Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonini NM, Bui QT, Gray-Board GL, Warrick JM. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- 35.Weasner B, Salzer C, Kumar JP. Sine oculis, a member of the SIX family of transcription factors, directs eye formation. Dev Biol. 2007;303:756–771. doi: 10.1016/j.ydbio.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong W, Dabbouseh NM, Rebay I. Interactions with the Abelson tyrosine kinase reveal compartmentalization of Eyes absent function between nucleus and cytoplasm. Dev Cell. 2009;16:271–279. doi: 10.1016/j.devcel.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 38.Tootle TL, Silver SJ, Davies EL, Newman V, Latek RR, Mills IA, Selengut JD, Parlikar BE, Rebay I. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature. 2003;426:299–302. doi: 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- 39.Curtiss J, Mlodzik M. Morphogenetic furrow initiation and progression during eye development in Drosophila : the roles of decapentaplegic, hedgehog and eyes absent. Development. 2000;127:1325–1336. doi: 10.1242/dev.127.6.1325. [DOI] [PubMed] [Google Scholar]
- 40.Firth LC, Baker NE. Retinal determination genes as targets and possible effectors of extracellular signals. Dev Biol. 2009;327:366–375. doi: 10.1016/j.ydbio.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker NE. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. doi: 10.1242/dev.102.3.489. [DOI] [PubMed] [Google Scholar]
- 42.Ma C, Moses K. Wingless and Patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- 43.Treisman JE, Rubin GM. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- 44.Heslip TR, Theisen H, Walker H, Marsh JL. Shaggy and Dishevelled exert opposite effects on Wingless and Decapentaplegic expression and on positional identity in imaginal discs. Development. 1997;124:1069–1078. doi: 10.1242/dev.124.5.1069. [DOI] [PubMed] [Google Scholar]
- 45.Legent K, Treisman JE. Wingless signaling in Drosophila eye development. Methods Mol Biol. 2008;469:141–161. doi: 10.1007/978-1-60327-469-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho KO, Choi KW. Fringe is essential for mirror symmetry and morphogenesis in the Drosophila eye. Nature. 1998;396:272–276. doi: 10.1038/24394. [DOI] [PubMed] [Google Scholar]
- 47.Papayannopoulos V, Tomlinson A, Panin VM, Rauskolb C, Irvine KD. Dorsal-ventral signaling in the Drosophila eye. Science. 1998;281:2031–2034. doi: 10.1126/science.281.5385.2031. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez M, de Celis JF. A dorsal/ventral boundary established by Notch controls growth and polarity in the Drosophila eye. Nature. 1998;396:276–278. doi: 10.1038/24402. [DOI] [PubMed] [Google Scholar]
- 49.Cavodeassi F, Diez Del Corral R, Campuzano S, Dominguez M. Compartments and organising boundaries in the Drosophila eye: the role of the homeodomain Iroquois proteins. Development. 1999;126:4933–4942. doi: 10.1242/dev.126.22.4933. [DOI] [PubMed] [Google Scholar]
- 50.Sato A, Tomlinson A. Dorsal-ventral midline signaling in the developing Drosophila eye. Development. 2007;134:659–667. doi: 10.1242/dev.02786. [DOI] [PubMed] [Google Scholar]
- 51.Maurel-Zaffran C, Treisman JE. pannier acts upstream of wingless to direct dorsal eye disc development in Drosophila. Development. 2000;127:1007–1016. doi: 10.1242/dev.127.5.1007. [DOI] [PubMed] [Google Scholar]
- 52.Pereira PS, Pinho S, Johnson K, Couso JP, Casares F. A 3' cis-regulatory region controls wingless expression in the Drosophila eye and leg primordia. Dev Dyn. 2006;235:225–234. doi: 10.1002/dvdy.20606. [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and Four-jointed. EMBO Rep. 2009;10:1051–1058. doi: 10.1038/embor.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekas LA, Baeg GH, Flaherty MS, Ayala-Camargo A, Bach EA. JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development. 2006;133:4721–4729. doi: 10.1242/dev.02675. [DOI] [PubMed] [Google Scholar]
- 55.Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131:3839–3847. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- 56.Bach EA, Vincent S, Zeidler MP, Perrimon N. A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics. 2003;165:1149–1166. doi: 10.1093/genetics/165.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai YC, Sun YH. Long-range effect of Upd, a ligand for Jak/STAT pathway, on cell cycle in Drosophila eye development. Genesis. 2004;39:141–153. doi: 10.1002/gene.20035. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol. 2005;285:38–48. doi: 10.1016/j.ydbio.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 59.Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- 60.Heberlein U, Wolff T, Rubin GM. The TGF beta homolog Dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- 61.Borod ER, Heberlein U. Mutual regulation of decapentaplegic and hedgehog during the initiation of differentiation in the Drosophila retina. Dev Biol. 1998;197:187–197. doi: 10.1006/dbio.1998.8888. [DOI] [PubMed] [Google Scholar]
- 62.Bras-Pereira C, Bessa J, Casares F. odd-skipped genes specify the signaling center that triggers retinogenesis in Drosophila. Development. 2006;133:4145–4149. doi: 10.1242/dev.02593. [DOI] [PubMed] [Google Scholar]
- 63.Fu W, Baker NE. Deciphering synergistic and redundant roles of Hedgehog, Decapentaplegic and Delta that drive the wave of differentiation in Drosophila eye development. Development. 2003;130:5229–5239. doi: 10.1242/dev.00764. [DOI] [PubMed] [Google Scholar]
- 64.Ou CY, Lin YF, Chen YJ, Chien CT. Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 2002;16:2403–2414. doi: 10.1101/gad.1011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker NE, Bhattacharya A, Firth LC. Regulation of Hh signal transduction as Drosophila eye differentiation progresses. Dev Biol. 2009;335:356–366. doi: 10.1016/j.ydbio.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- 67.Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- 68.Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Jan LY, Jan YN. Transcriptional regulation of atonal during development of the Drosophila peripheral nervous system. Development. 1998;125:3731–3740. doi: 10.1242/dev.125.18.3731. [DOI] [PubMed] [Google Scholar]
- 70.Zhang T, Ranade S, Cai CQ, Clouser C, Pignoni F. Direct control of neurogenesis by selector factors in the fly eye: regulation of atonal by Ey and So. Development. 2006;133:4881–4889. doi: 10.1242/dev.02669. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka-Matakatsu M, Du W. Direct control of the proneural gene atonal by retinal determination factors during Drosophila eye development. Dev Biol. 2008;313:787–801. doi: 10.1016/j.ydbio.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker NE, Firth LC. Retinal determination genes function along with cell-cell signals to regulate Drosophila eye development: examples of multi-layered regulation by master regulators. BioEssays. 2011;33:538–546. doi: 10.1002/bies.201000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melicharek D, Shah A, DiStefano G, Gangemi AJ, Orapallo A, Vrailas-Mortimer AD, Marenda DR. Identification of novel regulators of atonal expression in the developing Drosophila retina. Genetics. 2008;180:2095–2110. doi: 10.1534/genetics.108.093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker NE, Zitron AE. Drosophila eye development: Notch and Delta amplify a neurogenic pattern conferred on the morphogenetic furrow by Scabrous. Mech Dev. 1995;49:173–189. doi: 10.1016/0925-4773(94)00314-d. [DOI] [PubMed] [Google Scholar]
- 75.Lim J, Jafar-Nejad H, Hsu YC, Choi KW. Novel function of the class I bHLH protein Daughterless in the negative regulation of proneural gene expression in the Drosophila eye. EMBO Rep. 2008;9:1128–1133. doi: 10.1038/embor.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee EC, Hu X, Yu SY, Baker NE. The scabrous gene encodes a secreted glycoprotein dimer and regulates proneural development in Drosophila eyes. Mol Cell Biol. 1996;16:1179–1188. doi: 10.1128/mcb.16.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powell PA, Wesley C, Spencer S, Cagan RL. Scabrous complexes with Notch to mediate boundary formation. Nature. 2001;409:626–630. doi: 10.1038/35054566. [DOI] [PubMed] [Google Scholar]
- 78.Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–1301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- 79.Frankfort BJ, Nolo R, Zhang Z, Bellen H, Mardon G. Senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron. 2001;32:403–414. doi: 10.1016/s0896-6273(01)00480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aerts S, Quan XJ, Claeys A, Naval Sanchez M, Tate P, Yan J, Hassan BA. Robust target gene discovery through transcriptome perturbations and genome-wide enhancer predictions in Drosophila uncovers a regulatory basis for sensory specification. PLoS Biol. 2010;8:e1000435. doi: 10.1371/journal.pbio.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown NL, Sattler CA, Paddock SW, Carroll SB. Hairy and Emc negatively regulate morphogenetic furrow progression in the Drosophila eye. Cell. 1995;80:879–887. doi: 10.1016/0092-8674(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 82.Bhattacharya A, Baker NE. A network of broadly expressed HLH genes regulates tissue-specific cell fates. Cell. 2011;147:881–892. doi: 10.1016/j.cell.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baonza A, Freeman M. Notch signalling and the initiation of neural development in the Drosophila eye. Development. 2001;128:3889–3898. doi: 10.1242/dev.128.20.3889. [DOI] [PubMed] [Google Scholar]
- 84.Lim J, Choi KW. Bar homeodomain proteins are anti-proneural in the Drosophila eye: transcriptional repression of atonal by Bar prevents ectopic retinal neurogenesis. Development. 2003;130:5965–5974. doi: 10.1242/dev.00818. [DOI] [PubMed] [Google Scholar]
- 85.Wasserman JD, Urban S, Freeman M. A family of rhomboid -like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 2000;14:1651–1663. [PMC free article] [PubMed] [Google Scholar]
- 86.Urban S, Lee JR, Freeman M. Drosophila Rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- 87.Baonza A, Casci T, Freeman M. A primary role for the epidermal growth factor receptor in ommatidial spacing in the Drosophila eye. Curr Biol. 2001;11:396–404. doi: 10.1016/s0960-9822(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 88.Freeman M. The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech Dev. 1994;48:25–33. doi: 10.1016/0925-4773(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 89.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 90.Tio M, Ma C, Moses K. spitz a Drosophila homolog of transforming growth factor-alpha is required in the founding photoreceptor cells of the compound eye facets. Mech Dev. 1994;48:13–23. doi: 10.1016/0925-4773(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 91.Freeman M, Kimmel BE, Rubin GM. Identifying targets of the Rough homeobox gene of Drosophila : evidence that rhomboid functions in eye development. Development. 1992;116:335–346. doi: 10.1242/dev.116.2.335. [DOI] [PubMed] [Google Scholar]
- 92.Miura GI, Buglino J, Alvarado D, Lemmon MA, Resh MD, Treisman JE. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev Cell. 2006;10:167–176. doi: 10.1016/j.devcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 93.Golembo M, Schweitzer R, Freeman M, Shilo BZ. argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- 94.Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- 95.Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature. 2004;430:1040–1044. doi: 10.1038/nature02840. [DOI] [PubMed] [Google Scholar]
- 96.Tsuda L, Nagaraj R, Zipursky SL, Banerjee U. An EGFR/Ebi/Sno pathway promotes Delta expression by inactivating Su(H)/SMRTER repression during inductive Notch signaling. Cell. 2002;110:625–637. doi: 10.1016/s0092-8674(02)00875-9. [DOI] [PubMed] [Google Scholar]
- 97.Tomlinson A, Struhl G. Delta/Notch and Boss/Sevenless signals act combinatorially to specify the Drosophila R7 photoreceptor. Mol Cell. 2001;7:487–495. doi: 10.1016/s1097-2765(01)00196-4. [DOI] [PubMed] [Google Scholar]
- 98.Flores GV, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U. Combinatorial signaling in the specification of unique cell fates. Cell. 2000;103:75–85. doi: 10.1016/s0092-8674(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 99.Nagaraj R, Banerjee U. Combinatorial signaling in the specification of primary pigment cells in the Drosophila eye. Development. 2007;134:825–831. doi: 10.1242/dev.02788. [DOI] [PubMed] [Google Scholar]
- 100.Rogers EM, Brennan CA, Mortimer NT, Cook S, Morris AR, Moses K. Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development. 2005;132:4833–4843. doi: 10.1242/dev.02061. [DOI] [PubMed] [Google Scholar]
- 101.Tomlinson A, Ready DF. Sevenless: a cell-specific homeotic mutation of the Drosophila eye. Science. 1986;231:400–402. doi: 10.1126/science.231.4736.400. [DOI] [PubMed] [Google Scholar]
- 102.Reinke R, Zipursky SL. Cell-cell interaction in the Drosophila retina: the bride of sevenless gene is required in photoreceptor cell R8 for R7 cell development. Cell. 1988;55:321–330. doi: 10.1016/0092-8674(88)90055-4. [DOI] [PubMed] [Google Scholar]
- 103.Harris WA, Stark WS, Walker JA. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976;256:415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banerjee U, Renfranz PJ, Pollock JA, Benzer S. Molecular characterization and expression of sevenless a gene involved in neuronal pattern formation in the Drosophila eye. Cell. 1987;49:281–291. doi: 10.1016/0092-8674(87)90569-1. [DOI] [PubMed] [Google Scholar]
- 105.Hafen E, Basler K, Edstroem JE, Rubin GM. sevenless a cell-specific homeotic gene of Drosophila encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987;236:55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- 106.Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- 107.Kramer H, Cagan RL, Zipursky SL. Interaction of Bride of sevenless membrane-bound ligand and the Sevenless tyrosine-kinase receptor. Nature. 1991;352:207–212. doi: 10.1038/352207a0. [DOI] [PubMed] [Google Scholar]
- 108.Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the Sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 109.Simon MA, Carthew RW, Fortini ME, Gaul U, Mardon G, Rubin GM. Signal transduction pathway initiated by activation of the Sevenless tyrosine kinase receptor. Cold Spring Harbor Symp Quant Biol. 1992;57:375–380. doi: 10.1101/sqb.1992.057.01.042. [DOI] [PubMed] [Google Scholar]
- 110.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 111.Xu C, Kauffmann RC, Zhang J, Kladny S, Carthew RW. Overlapping activators and repressors delimit transcriptional response to receptor tyrosine kinase signals in the Drosophila eye. Cell. 2000;103:87–97. doi: 10.1016/s0092-8674(00)00107-0. [DOI] [PubMed] [Google Scholar]
- 112.Tang AH, Neufeld TP, Kwan E, Rubin GM. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 113.Li S, Li Y, Carthew RW, Lai ZC. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- 114.Dong X, Tsuda L, Zavitz KH, Lin M, Li S, Carthew RW, Zipursky SL. ebi regulates epidermal growth factor receptor signaling pathways in Drosophila. Genes Dev. 1999;13:954–965. doi: 10.1101/gad.13.8.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cooper MT, Bray SJ. R7 photoreceptor specification requires Notch activity. Curr Biol. 2000;10:1507–1510. doi: 10.1016/s0960-9822(00)00826-5. [DOI] [PubMed] [Google Scholar]
- 116.Tomlinson A, Mavromatakis YE, Struhl G. Three distinct roles for Notch in Drosophila R7 photoreceptor specification. PLoS Biol. 2011;9:e1001132. doi: 10.1371/journal.pbio.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi T, Xu C, Carthew RW. Cell-type-specific transcription of prospero is controlled by combinatorial signaling in the Drosophila eye. Development. 2008;135:2787–2796. doi: 10.1242/dev.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daga A, Karlovich CA, Dumstrei K, Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- 119.Flores GV, Daga A, Kalhor HR, Banerjee U. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development. 1998;125:3681–3687. doi: 10.1242/dev.125.18.3681. [DOI] [PubMed] [Google Scholar]
- 120.Yan H, Canon J, Banerjee U. A transcriptional chain linking eye specification to terminal determination of cone cells in the Drosophila eye. Dev Biol. 2003;263:323–329. doi: 10.1016/j.ydbio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 121.Moses K, Rubin GM. glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 1991;5:583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- 122.Mlodzik M, Hiromi Y, Weber U, Goodman CS, Rubin GM. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990;60:211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- 123.Miller AC, Seymour H, King C, Herman TG. Loss of seven-up from Drosophila R1/R6 photoreceptors reveals a stochastic fate choice that is normally biased by Notch. Development. 2008;135:707–715. doi: 10.1242/dev.016386. [DOI] [PubMed] [Google Scholar]
- 124.Dokucu ME, Zipursky SL, Cagan RL. Atonal, Rough and the resolution of proneural clusters in the developing Drosophila retina. Development. 1996;122:4139–4147. doi: 10.1242/dev.122.12.4139. [DOI] [PubMed] [Google Scholar]
- 125.Pepple KL, Atkins M, Venken K, Wellnitz K, Harding M, Frankfort B, Mardon G. Two-step selection of a single R8 photoreceptor: a bistable loop between Senseless and Rough locks in R8 fate. Development. 2008;135:4071–4079. doi: 10.1242/dev.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Domingos PM, Mlodzik M, Mendes CS, Brown S, Steller H, Mollereau B. Spalt transcription factors are required for R3/R4 specification and establishment of planar cell polarity in the Drosophila eye. Development. 2004;131:5695–5702. doi: 10.1242/dev.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Higashijima S, Kojima T, Michiue T, Ishimaru S, Emori Y, Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992;6:50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- 128.Mollereau B, Dominguez M, Webel R, Colley NJ, Keung B, de Celis JF, Desplan C. Two-step process for photoreceptor formation in Drosophila. Nature. 2001;412:911–913. doi: 10.1038/35091076. [DOI] [PubMed] [Google Scholar]
- 129.Domingos PM, Brown S, Barrio R, Ratnakumar K, Frankfort BJ, Mardon G, Steller H, Mollereau B. Regulation of R7 and R8 differentiation by the spalt genes. Dev Biol. 2004;273:121–133. doi: 10.1016/j.ydbio.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 130.Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 131.Hong Y, Ackerman L, Jan LY, Jan YN. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci USA. 2003;100:12712–12717. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 133.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 134.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mishra M, Oke A, Lebel C, McDonald EC, Plummer Z, Cook TA, Zelhof AC. Pph13 and Orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development. 2010;137:2895–2904. doi: 10.1242/dev.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fichelson P, Brigui A, Pichaud F. Orthodenticle and Kruppel homolog 1 regulate Drosophila photoreceptor maturation. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1120276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci USA. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 139.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators Warts/Lats and Melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 141.Jukam D, Desplan C. Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev Cell. 2011;21:874–887. doi: 10.1016/j.devcel.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 143.Larson DE, Johnson RI, Swat M, Cordero JB, Glazier JA, Cagan RL. Computer simulation of cellular patterning within the Drosophila pupal eye. PLoS Comp Biol. 2010;6:e1000841. doi: 10.1371/journal.pcbi.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 145.Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–96. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]