Summary
Among 127 HIV-infected women, the magnitude of HDLc increases after HAART initiation predicted the magnitude of concurrent decreases in inflammation biomarkers. After HAART initiation, changes in LDLc and inflammation were unrelated. In the same population, predicted risk of coronary heart disease based upon levels of standard clinical risk factors was similar before and after HAART treatment. Thus, it remains unknown whether short-term treatment-related changes in standard risk factors may appreciably change risk of CVD.
Keywords: lipids, HAART, HIV infection, inflammation
A recent article in AIDS by Piconi et al [1] reported that among HIV-infected individuals, pro-thrombotic and inflammation factors were lower and metabolic factors (i.e. serum cholesterol and lipoproteins) were higher in persons on antiretroviral therapy (ART) compared to untreated persons. The authors concluded that HIV replication and inflammatory/thrombotic factors may be an important pathway to atherosclerosis in untreated HIV-infected individuals, while changes in metabolic factors may be important atherosclerosis risk factors in those using ART. While an important contribution, the Piconi study lacked data on women, was limited by a cross-sectional study design, and made no conclusions about changes after ART initiation in widely-used clinical measures of future cardiovascular disease (CVD) risk.
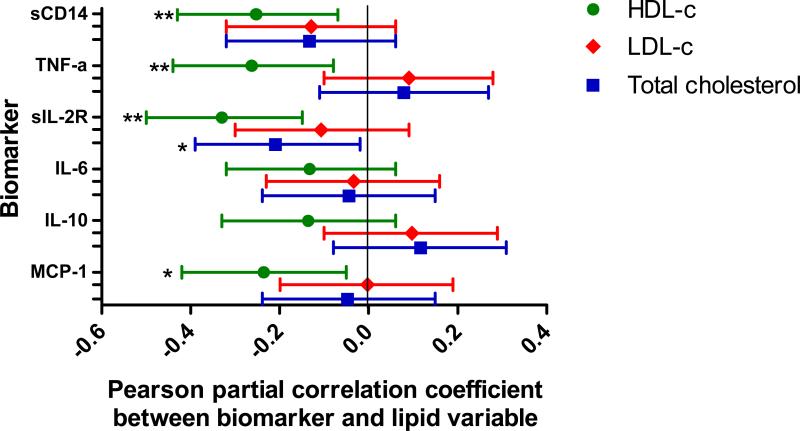
We confirmed and extended the key conclusions of Piconi et al using longitudinal data from the Women's Interagency HIV Study (WIHS). Using data from a WIHS substudy of 127 HIV-infected women who initiated highly active ART (HAART) while enrolled in the WIHS [2], we measured levels of lipids and inflammation factors at three semi-annual visits prior to first use of HAART and again at three semi-annual visits after first use of HAART. These data were used to examine the association between changes in serum lipids, and concurrent changes in levels of inflammation-related biomarkers. Levels of high-density lipoprotein cholesterol (HDLc) increased after initiation of HAART (from 48 mg/dL to 54 mg/dL), while low-density lipoprotein cholesterol (LDLc) increased only among the 67 women who initiated protease inhibitor (PI)-based HAART regimens (in PI-HAART users, 92 mg/dL to 109 mg/dL, and in non-PI based HAART users, 101 mg/dL to 103 mg/dL). Regardless of the type of HAART regimen used, the magnitude of increase in HDLc was correlated with the magnitude of decrease in soluble CD14 (sCD14), tumor necrosis factor alpha (TNF-a), soluble interleukin 2 receptors (sIL-2R), interleukin 6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) (Figure). Change in LDLc level was not associated with changes in inflammation biomarker levels, suggesting a largely inflammation-independent mechanism for increased LDLc with PIHAART.
Figure. Correlations of post-HAART changes in high density lipoprotein cholesterol (HDLc), low density lipoprotein cholesterol (LDLc) and total cholesterol (TC) with post-HAART changes in inflammation-related biomarkers among HIV-infected women.
Pearson partial correlations were computed between change variables, adjusting for change in HIV RNA and change in CD4+ T cell count.
Among the same patient population, we also investigated the net effect of changes in lipid profile and other vascular risk factors after ART initiation on predicted risk of clinical CVD events. Hence, we calculated predicted coronary heart disease risk in HIV-infected women at visits shortly before initiating HAART, and again 18 months after beginning HAART. The Framingham risk score for estimating the 10-year risk of total coronary heart disease (including angina and fatal and non-fatal acute coronary events) [4] was calculated based upon age, TC, HDLc, diastolic and systolic blood pressures, diabetes and current smoking status. We grouped women into low (10-year risk <15%), moderate (15%-25%) or high risk (≥25%) categories, while placing all diabetics into the high risk category [5,6]. We then determined the proportion of women who were reclassified after HAART initiation. Among all HIV-infected women prior to treatment, 84% had low predicted risk of coronary heart disease, 1% had moderate risk and 15% had high risk. After HAART initiation, 97% remained in the same risk category, 1% moved into a lower risk category, and 2% moved into a higher risk category.
In summary, consistent with the findings from Piconi et al [1], our data demonstrate the reciprocal relationship of inflammation and lipid perturbation in HIV-infected patients, while also suggesting that LDLc and inflammation are biologically discrete pathways that may alter atherosclerosis risk. We [2,3] and Piconi et al [1], among others, have demonstrated that in HIV-infected patients, inflammation and lipid levels are associated with common carotid artery intima-media thickness, a measure of subclinical atherosclerosis. However, it can be difficult to translate findings from this subclinical atherosclerosis measure into clinically meaningful information on risk of CVD. At least among middle-aged HIV-infected women, we find little evidence that the net balance of short-term metabolic alterations related to HAART initiation would appreciably change future risk of CVD as measured by standard clinical risk factors. Therefore, as research into novel CVD biomarkers and long-term treated HIV natural history continues to mature, it will become increasingly important to evaluate the clinical relevance of changes in intermediate biomarkers.
Acknowledgements
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). Additional co-funding is provided by the National Heart, Lung and Blood Institute (1R01HL095140, 1R01HL083760 to R.C.K.). Partial funding for laboratory and work as well as assistance with general study coordination was provided by the University of Washington's CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center (5R01HL095126).
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, et al. Atherosclerosis is Associated with Multiple Pathogenic Mechanisms in HIV-Infected Antiretroviral-Naïve or -Treated Individuals. AIDS. 2012 doi: 10.1097/QAD.0b013e32835abcc9. in press. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–68. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrinello CM, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Association of subclinical atherosclerosis with lipid levels amongst antiretroviral-treated and untreated HIV-infected women in the Women's Interagency HIV study. Atherosclerosis. 2012;225:408–11. doi: 10.1016/j.atherosclerosis.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 6.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–81. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]