Abstract
The current study investigated the effect of education on retrospective metamemory accuracy in 143 healthy older adults and 143 early to moderate AD patients, using retrospective measures of confidence in the accuracy of retrieval responses in an episodic odor recognition memory task. Relative confidence accuracy was computed as the difference between confidence judgments for correct and incorrect responses. In both AD patients and controls, individuals reporting 17 years of education or more had significantly more accurate levels of confidence than individuals with 12 years or less. Thus, education was a significant predictor of retrospective metamemory accuracy in healthy aging and AD.
Keywords: Metamemory, Confidence, Education, Aging, Alzheimer’s disease
Declines in episodic memory are a key feature of both healthy and pathological aging processes (Gallagher & Koh, 2011). However, patterns and trajectories of these declines are heterogeneous (Reed et al., 2010).
An important factor potentially influencing the effects of healthy and pathological aging on episodic memory is metamemory, i.e., the capacity to successfully monitor one’s memory processes and the accuracy of these processes. Metamemory abilities include the use of memory strategies and knowledge of memory capacity and limitations, and are important in directing the use of memory processes in overall decision-making (Devolder, 1989; Marquie & Huet, 2000; Pannu & Kaszniak, 2005).
Evidence from imaging studies suggests that the accuracy of metamemory judgments relies on key brain areas within the frontal and medial temporal lobes, which are known to be affected in both healthy aging and AD (Do Lam et al., 2012; Kao, Davis & Gabrielli, 2005; Pannu & Kaszniak, 2005). Importantly however, reports on metamemory in healthy aging and AD have varied, with many studies reporting that metamemory is preserved in aging and AD, and other studies reporting significant metamemory declines in aging and AD. One explanation for this variation could be the different aspects of metamemory being measured across studies, particularly because different types of metamemory judgments have been shown to differ in the degree to which they rely on medial temporal vs. frontal regions.
For example, prospective metamemory, used when making predictive judgments about future memory performance, tends to rely more heavily on medial temporal structures such as the hippocampus, than frontal regions; whereas, retrospective metamemory, used when making judgments about prior memory performance, has been shown to rely more heavily on frontal regions such as the right and medial prefrontal cortex, than on medial temporal structures (Do Lam et al., 2012; Kao, Davis & Gabrielli, 2005; Pannu & Kaszniak, 2005; Chua, Schacter, & Sperling, 2009). These functional differences are particularly important in AD where degeneration occurs in medial temporal regions much earlier than in frontal regions (Braak & Braak, 1997), potentially allowing frontal-dependent retrospective metamemory processes to remain relatively preserved despite marked medial temporal-related declines in memory and prospective metamemory (Bäckman & Lapinska, 1993; Souchay, Bacon, & Danion, 2006).
Support for this theory has come from research on the accuracy of retrospective confidence judgments, which represent the level of confidence that an individual has in the accuracy of their memory performance (Chua et al., 2009; Marquie & Huet, 2000). For example, studies have reported that those with early to moderate AD report confidence judgments that are as accurate as those of healthy older controls, despite having marked deficits in memory compared to controls (Pappas et al., 1992). Moreover, studies have reported no age differences in the accuracy of retrospective confidence judgments when comparing older adults between the ages of 60–93 (Dahl, Allwood, & Hagberg, 2009), as well as when comparing older adults to younger adults (Marquie & Huet, 2000; Moulin, James, Perfect, & Jones, 2003). Notably however, contrary findings have also been reported suggesting that retrospective confidence accuracy does decline in healthy aging and AD. For example, healthy older adults have commonly been found to exhibit overall higher rates of high-confidence false recognition than younger adults, and those with AD have been shown to exhibit even higher rates of high-confidence false recognition than healthy older adults (Chua et al., 2009; Jacoby & Rhodes, 2006; Cosentino, Metcalfe, Butterfield, & Stern, 2007). Thus, the precise differences between normally aging older adults and AD patients on retrospective metamemory accuracy remain unclear.
Another potential explanation for varying reports on retrospective metamemory in healthy aging and AD might be the wide use of auditory-based memory tasks, such as word lists, to assess retrospective metamemory accuracy. Stigmas associated with age-related declines in hearing are prevalent and this may contribute to test anxiety and response bias among older adults (Wallhagen, 2009). This can be particularly important to consider when analyzing retrospective judgments of memory, as they involve a subjective component based on feelings of certainty in one’s responses (Kennedy, 2001) which can be influenced by factors such as test anxiety and response bias. Unlike auditory abilities, olfactory abilities are not typically associated with aging stigmas, and studies have shown that performance on olfactory tasks is not negatively influenced by factors like age-related stereotype priming. For example, a study by Miller et al. (2013) on stereotype activation and olfactory function found that while performance on auditory-based memory and motor tasks significantly declined in a group primed for age-related memory and motor stereotypes, no significant differences in olfactory performance occurred when the group was primed for age-related olfactory stereotypes. Moreover, this effect held across various olfactory abilities and tasks, including odor threshold detection, odor identification, hedonic ratings of odors, ratings of odor familiarity, and odor reaction times.
Another positive aspect of employing an olfactory task is that, with the exception of those in certain professions involving chemosensory-related tasks, most individuals have not been exposed to odor-based cognitive tasks. In fact, this relative lack of experience with olfactory cognitive tasks is thought to be related to findings that odor processing is resistant to the negative effects of aging stereotypes, as the strength of association between a construct and a behavior is a key factor in stereotype activation and its effects on behavior and performance (Miller et al., 2013). Therefore, the current study utilized an odor memory task to analyze the accuracy of retrospective metamemory judgments.
Although certain non-modifiable risk factors like genetics play an undeniable role in the way cognitive abilities decline with age, one important potential risk factor for age-related cognitive decline is education level, which has been reported to influence the effects of aging across various domains including processing speed, executive functioning, learning, and memory (Le Carret et al., 2011). While the specific effects of education level vary within cognitive domains, a number of studies have reported that that those with higher levels of education show significantly less cognitive decline than those with lower levels of education, whom a number of studies have reported to show the most severe cognitive declines (Albert et al., 1995; Anstey & Christensen, 2000; Wilson, et al., 2009). Moreover, higher levels of education have also been associated with delays in the onset of terminal decline, a decreased risk of conversion from MCI to dementia, and an overall reduced risk of Alzheimer’s disease (AD) and other dementias (Stern, Alexander, Prohovnik, & Mayeux, 1992; Allegri et al., 2010; Batterham, Mackinnon, & Christensen, 2011). Despite these findings on the influence of education on episodic memory, the influence of education on metamemory is largely unexplored in normally aging adults or in pathological aging.
A major theory regarding the role of education in age-related cognitive declines is the cognitive reserve hypothesis, which holds that individual neurological differences allow some individuals to cope better with brain damage and the neurophysiological effects of aging than others (Stern, 2009). Accordingly, higher levels of education are theorized to result in a higher neurological capacity for cognitive reserve which allows highly educated individuals to compensate for normal and pathological age-related neurodegeneration (Scarmeas, Albert, Manly, & Stern, 2006; Batterham, et al., 2011). Findings from imaging research suggest that this compensatory ability may be due to a greater number of healthy synapses or neurons, more efficient circuits of synaptic connectivity, or more efficient use of alternative brain networks, which are thought to provide a buffer against various forms of neurodegenerative decline (Springer, McIntosh, Winocur, & Grady, 2005).
A potential link between the effects of education level and metamemory on episodic memory in healthy older adults and AD patients is found in research demonstrating that higher levels of education are associated with fewer age-related effects on frontal-dependent activities (Plumet, Gil, & Gaonac’h, 2005). For example, evidence from fMRI research suggests that in older adults, higher levels of education are associated with increased frontal activity, which in turn, has been linked to better memory performance (Springer et al., 2005). Because retrospective metamemory abilities are frontal-dependent, it is implied that higher levels of education would also result in better retrospective metamemory accuracy. Interestingly, studies of patients with Multiple Sclerosis have found that patients with higher levels of education report more accurate retrospective metamemory ratings (Plumet, et al., 2005). However, to the authors’ knowledge, an influence of education level on metamemory has not been reported in healthy older adults or AD patients.
Thus, the main aim of the current study was to investigate the effect of education level on retrospective metamemory accuracy in healthy older individuals and those with early to moderate AD using retrospective measures of confidence in the accuracy of retrieval responses in an episodic recognition memory task. We hypothesized that regardless of diagnosis, those with higher levels of education would demonstrate more accurate metamemory than those with lower levels of education. Because retrospective metamemory abilities are frontal-dependent we did not hypothesize an education by diagnosis interaction.
Method
Participants
A retrospective study was conducted on participants (n = 286) who were volunteers in a longitudinal study at the Alzheimer’s Disease Research Center (ADRC) at the University of California, San Diego. Data were included from 143 patients who met the NINCDS/ADRDA (National Institute on Communicative Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association) criterion for probable AD (McKhann et al., 1984), and were compared with data from 143 non-demented controls. Controls were members of the community who had responded to advertisements for participation in studies at the ADRC, and included family members and spouses of participants. All participants in the present study were recruited while at the ADRC, and the test battery was administered either at the participant’s residence, the ADRC, or at the Lifespan Human Senses Lab at San Diego State University. Participants were treated in accordance with the “Ethical Principles of Psychologists and Code of Conduct” (American Psychological Association, 1992).
A diagnosis for all participants was made by two independent neurologists from the ADRC, and multiple examinations were conducted in order to rule out all other possible causes of dementia. Cognitive areas examined included attention, abstraction/problem solving, motor, verbal/language, perceptual/constructional, memory, and orientation (for tests details see Weintraub et al., 2009). EEGs, CT scans, MRIs, EKGs and tests of cerebrospinal fluid were performed when necessary to aid in making a diagnosis of Alzheimer’s disease.
Participants ranged from 60 to 92 years of age (M = 73.7, SD = 6.72). Both males (N = 136) and females (N = 150) were included. Exclusionary demographic criteria included age less than 60 years, a diagnosis other than Alzheimer’s disease (i.e. vascular dementia, dementia with Lewy Bodies, Parkinson’s disease, etc.), and a total score lower than 95 on the Dementia Rating Scale (DRS) thus limiting our AD sample to those with only mild to moderate levels of dementia (Murphy, Nordin, & Jinich, 1999). After limiting our sample based on demographic criteria, as well as excluding participants who did not complete all portions of the odor threshold and/or odor recognition memory task, there were more AD subjects than control subjects in the sample. Thus, random selection was used to reduce the number of AD subjects to match the number of controls (N = 143 per group).
Independent samples t-tests revealed that diagnostic groups differed significantly on the DRS (t = 26.16, p < .001), the MMSE (t = 20.11, p < .001), and on odor threshold (t = 4.39, p < .001), all of which are in line with previous findings (Murphy et al., 1999; Dulay & Murphy, 2002; Gilbert, Barr, & Murphy, 2004; Davidson & Murphy, 1997). No significant differences in gender, age or education level were found between diagnosis groups (p > .05).
Education level was measured in number of years of education, and ranged from four to twenty years with an average education level of 14.36 years (SD = 3.33).
Procedures
Recognition Memory Task
We used a task previously developed by Murphy, Cain, Gilmore, & Skinner (1991) to assess odor recognition memory. The olfactory stimuli included 15 common household odors presented in amber-colored glass jars (e.g., cinnamon, peppermint, etc.). Odor stimuli were embedded in a context of visual stimuli (faces of US presidents and vice presidents and abstract engineering symbols). Ten odors were randomly selected and presented one at a time, embedded in the sequence of odor, face, symbol. Visual stimuli are not a focus of this study and will be discussed elsewhere.
Familiarity Phase
From each stimulus set 10 items were randomly selected and presented one at a time, in the sequence of odor, face, symbol. For olfactory stimuli, participants were instructed to close their eyes while the jar containing the odorant was presented by the experimenter directly beneath the nose of the participant. Each stimulus was presented for 5 seconds with a 10 second interval between stimuli, so that at least 45 seconds had passed between each olfactory stimulus to prevent adaptation to the olfactory stimuli. After the presentation of each item, participants were asked to provide a retrospective rating of the item’s familiarity using a 160mm bipolar, visual-analog scale ranging from not familiar to very familiar. The rating scale chosen for this study was selected for its simplicity, in order to avoid confounding due to the process of cognitive estimation (Cosentino & Stern, 2005). Participants were informed that identification of the item was not necessary in order to rate the item as familiar, and they were not informed that a recognition memory test would follow.
Recognition Phase
The recognition phase immediately followed the initial presentation session. The participant was again presented with ten items from each stimulus set, however for this phase, five stimuli in each set were randomly selected from the stimuli presented during the familiarity phase, and the remaining five stimuli were distracters randomly selected from the stimuli not previously presented to the participant. For each item, participants were instructed to respond “yes” if the stimulus had been previously presented during the familiarity phase, and “no” if it had not, and then to rate their level of confidence in the accuracy of their response along a 160mm-bipolar visual-analog scale ranging from not confident to very confident, similar to the scale used to rate familiarity.
The number of hits (response of “yes” to a previously presented stimulus), misses (response of “no” to a previously presented stimulus), correct rejections (response of “no” to a stimulus that had not been presented), and false alarms (response of “yes” to a stimulus that had not been presented) were recorded for each participant. The proportion-correct responses (PC; Murphy et al., 1999) were calculated for each participant and stimulus modality as a performance measure of recognition memory: PC = (hits + correct rejections)/n; where n represents the sum of possible hits and correct rejections (n = 10). Thus, a PC score could range from 0 to 1, with chance level at .5.
Odor Threshold
Because our focus was on metamemory for performance in an odor recognition memory task, odor thresholds were obtained for all participants, and any participant with an average threshold score below 1 was excluded from the sample in order to exclude anosmic individuals. Odor thresholds for the left and right nostril were measured using a two alternative (odorant and blank), forced choice ascending method of limits test with butanol as the stimulus (Cain, 1989; Murphy, Gilmore, Seery, Salmon, & Lasker, 1990). On each trial, the participant was presented with a blank bottle (containing the distilled water) and an odorant bottle (containing a dilution step of butanol), and instructed to select which of the two bottles contained the odorant. A 45s inter-trial interval was used to avoid adaptation (Ekman, Berglund, Berglund, & Lindvall, 1967; Murphy et al., 1990). A threshold score was obtained for each nostril and the two were averaged to yield a single score.
Metamemory Accuracy
The accuracy of metamemory is typically measured by the level of correspondence between metamemory judgments and actual memory performance, which for this task, means that high levels of confidence should be given to correct responses and lower levels of confidence should be given to incorrect responses. Participants who reported low confidence in accurate recognition (i.e. underestimated performance) were considered just as unaware of their memory ability as those who reported high confidence in inaccurate recognition (i.e. overestimated performance).
Confidence judgments were analyzed for relative accuracy, which refers to the difference between confidence judgments for correct and incorrect responses. To calculate the relative accuracy of confidence judgments (hereby referred to as “confidence accuracy”), an average level of confidence was computed for each response type (hit, miss, correct rejection, and false alarm) and confidence level averages were calculated for correct (hits + correct rejections/2 = Confidence-correct) and incorrect (misses + false alarms/2 = Confidence-incorrect) responses. Finally, the average confidence level for incorrect responses was subtracted from the average confidence level for correct responses (Confidence-correct - Confidence-incorrect = confidence accuracy), resulting in an index of confidence accuracy. A positive score indicates that confidence judgments appropriately discriminated between correct and incorrect responses, assigning higher confidence levels to correct responses than to incorrect responses, while a negative score or a score of zero indicates that confidence judgments showed inaccurate or no discrimination between correct and incorrect responses, assigning lower confidence levels to correct responses than to incorrect responses or assigning the same confidence levels to both correct and incorrect responses.
Statistical Analysis
Consistent with education categories utilized in previous studies of healthy aging and AD that found significant differences in memory performance between low and highly educated individuals (e.g., Economou, 2009; Grabe, Kamhawi & Yegiyan, 2009; and Vermeiren et al., 2013), participants were stratified into three groups based on education level: those reporting twelve years of education or less, here referred to as the high school level, or HS, group (N = 101), those reporting between thirteen and sixteen years of education, here referred to as the Associates-Bachelor’s level, or AB, group (N = 112), and those reporting greater than sixteen years of education, here referred to as the graduate level, or Grad group (N = 73). Chi squared tests indicated that education level groups did not significantly differ on age or gender distribution (p > .05). See Table 1 for a breakdown of education levels by diagnosis, and Table 2 for Mean (+SE) for Dementia Rating Scale (DRS) scores, recognition memory accuracy scores (percent correct), and confidence accuracy scores grouped by education level and diagnosis.
Table 1.
Mean (+MSE) Years of Education for Control and AD Groups
| N | Mean Years of Education | Mean Standard Error | |
|---|---|---|---|
|
|
|||
| Control | 143 | 14.83 | .27 |
| AD | 143 | 13.90 | .28 |
| Total | 286 | 14.35 | .20 |
Table 2.
Mean (+MSE) for Dementia Rating Scale (DRS) Scores, Percent Correct on the Memory Task, and Confidence Accuracy Scores, Grouped by Education Level and Diagnosis.
| Dx Group | Education Level | N | DRS | MSE* | Recognition Memory Percent Correct | MSE | Confidence Accuracy | MSE |
|---|---|---|---|---|---|---|---|---|
| Control | HS | 45 | 136.69 | .84 | .71 | .03 | 9.25 | 6.62 |
| AB | 50 | 139.30 | .65 | .69 | .03 | 8.40 | 5.03 | |
| Grad | 48 | 140.06 | .57 | .71 | .03 | 23.39 | 7.92 | |
| Total | 143 | 138.73 | .41 | .70 | .02 | 13.13 | 3.66 | |
|
| ||||||||
| AD | HS | 56 | 112.27 | 1.20 | .61 | .03 | 2.25 | 3.90 |
| AB | 62 | 114.15 | 1.25 | .59 | .02 | 3.52 | 4.37 | |
| Grad | 25 | 120.68 | 1.90 | .65 | .03 | 14.52 | 6.64 | |
| Total | 143 | 114.55 | .83 | .61 | .01 | 5.35 | 2.74 | |
|
| ||||||||
| Total | HS | 101 | 123.15 | 1.43 | .65 | .02 | 5.34 | 3.49 |
| AB | 223 | 125.38 | 1.40 | .63 | .02 | 5.59 | 3.39 | |
| Grad | 73 | 133.42 | 1.32 | .68 | .02 | 19.41 | 5.27 | |
| Total | 286 | 126.64 | .85 | .65 | .01 | 8.94 | 2.26 | |
MSE = Mean Standard Error
Data were initially tested for violations against the assumptions of analysis of covariance (ANCOVA), and to analyze descriptive characteristics of the variables. The effects of diagnostic condition and education level on memory and confidence accuracy were examined with both ANCOVA and analysis of variance (ANOVA) models. Any significant effects of education level were then analyzed using Newman-Keuls post hoc analyses. All analyses were conducted using SPSS version 18.0.
Results
The control group performed significantly better (Mean PC = .700, SE = .02; 95% confidence interval, .67–.73) on the episodic recognition memory task than AD group (Mean PC = .615, SE = .02; 95% confidence interval, .59–.65), F(1, 203) = 14.95, MSE = .34, p < .001, η2 = .07. However, there was no significant effect of education, F(2, 203) = 1.16, MSE = .03, p >.30, or of the diagnosis by education interaction, F(2,203) = .35, MSE = .01, p >.70, on odor recognition memory accuracy.
In terms of relative confidence accuracy, 52% of the overall sample reported confidence levels that either failed to discriminate between correct and incorrect responses (i.e. had an index of confidence accuracy near zero), or assigned higher confidence levels to incorrect responses than to correct responses (N = 103).
In order to investigate the possible existence of covariate effects, an analysis of covariance (ANCOVA) was used to analyze the effects of diagnosis and education level on the relative confidence accuracy index, with age and odor threshold included as the covariates. There were no significant effects of age or odor threshold (p > .05), and the main effect of education level was significant even after inclusion of the covariates, thus we report the results of a simple ANOVA model, without age and threshold included as covariates.
Analysis of variance (ANOVA) was conducted to examine the effects of diagnosis and education level on the relative confidence accuracy index. Because Levene’s test of the homogeneity of variance was significant, Brown & Forsythe’s F test was conducted. It indicated that the ANOVA was robust to the violation of the assumption of homogeneity of variances. Results of the ANOVA revealed no significant effect of diagnosis, F (1, 191) = 2.27, MSE = 2225.70, p > .05, or the diagnosis by education level interaction, F (2, 191) = .06, MSE = .61.54, p >.05; however, there was a significant main effect of education level, F (2, 191) = 3.18, MSE = 3116.87, p < .05, η2 = .03. For the main effect of education level, Newman Keuls post hoc tests revealed that the Grad group had a significantly higher level of relative confidence accuracy (M = 18.96) than both the AB (M = 5.96) and HS (M = 5.75) groups, which did not significantly differ from each other (see Figure 1). This indicates that those with higher education levels were better able to discriminate between correct and incorrect responses with their confidence levels.
Figure 1.
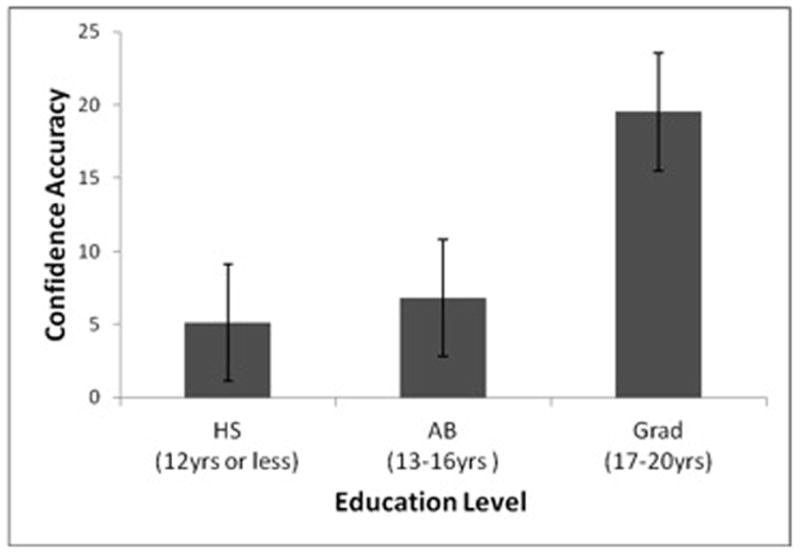
Effect of education level on relative confidence accuracy across diagnosis groups.
In order to further investigate the effects of diagnosis and education level on relative confidence accuracy, separate ANOVAs were conducted on average confidence levels for correct responses and average confidence levels for incorrect responses. For both confidence in correct and incorrect responses, Levene’s Test of Equality of Error Variances was not significant (Correct: p = .23; Incorrect: p = .24), indicating that the assumption of homogeneity of variance was met.
Results of the ANOVA revealed no significant effects of diagnosis, F (1, 198) = 2.24, MSE = 1442.05, p > .13 .05, or education level on confidence levels for correct responses, F (2, 198) = 1.03, MSE = 664.35, p >.35. On the other hand, education level emerged as a significant predictor of confidence levels for incorrect responses, F (2, 191) = 3.46, MSE = 3442.41, p < .05, η2 = .04 (Although no significant effect of diagnosis was found, F (1, 198) = .12, MSE = 119.84, p >.73). For the main effect of education on confidence levels for incorrect responses, Newman Keuls post hoc tests revealed that the HS group had significantly higher average confidence levels for incorrect responses (M = 106.41, SE = 3.85) than the Grad group (M = 90.77, SE = 4.53), but that the BA group (M = 99.89, SE = 3.57) did not significantly differ from either the HS or Grad groups. This indicates that the Grad group was more accurate at judging when they were incorrect than the HS group, as those in the Grad group assigned significantly lower levels of confidence to incorrect responses than the HS group (see Figure 2).
Figure 2.
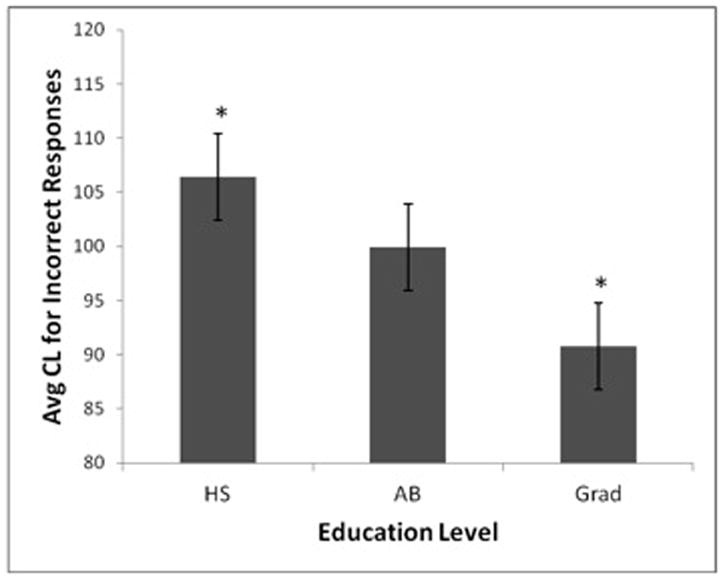
Effect of education level on confidence in incorrect responses.
Incorrect response types were also analyzed using average confidence in false alarms and average confidence in misses as the dependent variables for two separate ANOVAs. Results of the ANOVAs revealed no significant effects of diagnosis on confidence in misses, F (1,116) = .83, MSE = 1511.86, p > .36, or false alarms, F (1, 178) = 1.00, MSE = 966.34, p > .31; or of education level on confidence levels for misses, F (1,116) = 1.31, MSE = 2401.94, p > .27. However, education level significantly predicted confidence levels in false alarms, F (2, 178) = 3.33, MSE = 3203.19, p < .05, η2 = .04. Newman Keuls post hoc tests, however, were not significant (p > .05).
Finally, the relationship between the relative confidence accuracy index, education level, and percent correct on the episodic memory task was analyzed using bivariate correlation analyses (Pearson’s r). Separate correlations were conducted to analyze the continuous relationships between years of education and percent correct, and between years of education, percent correct, and the variables: relative confidence accuracy, confidence in correct responses, confidence in incorrect responses, and confidence in false alarms. The correlation between years of education and percent correct on the memory task was not significant, r = .05, p > .05. Years of education was negatively associated with confidence in incorrect responses, r = −.14, p < .05. None of the other confidence measures were significantly correlated with either education or percent correct on the recognition memory task (p > .05) (See Table 3). Partial correlations were also conducted between percent correct and the difference confidence measures controlling for education level, however, no significant correlations were found (p > .05). This indicates that no linear relationships were detected between relative confidence accuracy, education level, and performance on the recognition memory task in the present study.
Table 3.
Pearson’s r Bivariate Correlation Analyses for the Variables: Years of Education, Percent Correct
| Years of Education | Percent Correct | |
|---|---|---|
|
|
||
| Years of Education | - | .053 |
| Percent Correct | - | - |
| Confidence Correct | −.043 | .067 |
| Confidence Incorrect | −.140* | .083 |
| Confidence False Alarms | −.118 | .098 |
| Relative Confidence Accuracy | .113 | −.049 |
Note.
p < .05; other correlations were n.s.
Discussion
The primary aim of the present study was to investigate the effects of AD and education level on retrospective metamemory accuracy using an odor recognition memory task in healthy older adults and patients with early to moderate AD. As expected, AD patients performed significantly worse than healthy older adults on the recognition memory task, though no significant differences were found between diagnostic groups in terms of relative confidence accuracy. Overall, the majority of participants in both diagnostic groups reported levels of confidence that failed to accurately differentiate between correct and incorrect responses; supporting findings that retrospective confidence accuracy is impaired in both healthy aging and AD. However, in line with our main hypothesis, those with higher education levels were better able to monitor the accuracy of their responses and appropriately adjust their confidence levels according to their performance on the recognition memory task, despite the fact that education level did not significantly affect performance on the actual memory task.
In particular, across diagnostic group, participants reporting at least seventeen years of education reported more accurate relative confidence levels and more accurate levels of confidence in their incorrect responses than those with twelve years of education or less. This suggests that education beyond the number of years typically required to obtain a bachelor’s degree positively affects retrospective metamemory in both healthy aging and early to moderate AD, even when education level does not influence actual memory performance. These results are in accordance with past research showing that education level and other variables associated with cognitive reserve are positively related to frontal functioning in aging populations (Plumet et al., 2005). Because frontal functioning is more important to metacognitive judgment and monitoring abilities such as metamemory than it is to temporal-lobe dependent memory abilities, it signifies that education level would exert a more powerful influence over metamemory accuracy than it would over memory.
Many studies have examined linear effects of years of education, and several have also categorized participants into education level groups including primary education, secondary education, and post-secondary education, however, to the authors’ knowledge, no study to date has investigated differences that may exist within levels of post-secondary education.
While effect sizes for the main effects of education level are relatively small, it is important to note that the variance explained by education likely reflected the large amount of variance in confidence levels and accuracy among those with less than seventeen years of education.
A potential explanation for the positive effect of education level on relative confidence accuracy is the sustained cognitive activity that research has associated with higher levels of education. For example, numerous studies have found education level to be moderately correlated with both past and current cognitive activity levels among older adults (Wilson et al., 2007; Wilson et al., 2012). Moreover, a meta-analysis by Anstey (1999) on the importance of cognitive activity indicated that a significant amount of the positive relationship between cognitive activity and cognition can be explained by education.
“Cognitive activity” in this context generally refers to activities considered to be mentally stimulating, such as reading the newspaper, or playing cards. More frequent engagement in cognitive activity has been associated with a reduced risk for developing dementia, as well as slower trajectories of cognitive decline in normal aging, and is also associated with higher levels of cognitive reserve (Wilson et al., 2007; Reed et al., 2011). The present findings suggest that continued cognitive activity and other variables related to education level and cognitive reserve may have a positive impact on age- and AD-related decline in metamemory as well.
The current results also indicated that across diagnostic groups, individuals with at least seventeen years of education were significantly more accurate when assigning confidence levels to incorrect responses than those with twelve years of education or less, particularly when rating confidence in false alarms. These findings are particularly noteworthy considering the large number of findings reporting age- and AD- related increases in high-confidence false alarms, consistent across task type and stimulus modality (Jacoby & Rhodes, 2006; Thomas & Dubois, 2011; Royet et al., 2011). One potential explanation for this phenomenon is that when older adults lack strong cue–target associative signals, they may instead base their confidence decisions on information related to the cue, such as cue familiarity and ease of processing the cue. Using cue-related factors to assess confidence can lead to higher confidence levels that are unrelated to actual memory accuracy, causing older adults to report higher confidence levels in their incorrect responses (Busey, Tunnicliff, Loftus, & Loftus, 2000; Chua et al., 2009). The present results suggest that higher levels of education are associated with better retrospective memory monitoring, and specifically in fewer inappropriate high-confidence memory errors.
A complement to the hypothesis of cognitive reserve is the hypothesis of cognitive reserve capacity (Mortimer, 1988), which states that highly educated individuals have a greater capacity for cognitive reserve than those with lower educational levels. It is thought that due to this greater capacity for cognitive reserve, highly educated individuals are able to delay the appearance of clinical symptoms of AD by compensating for the AD-related neuropathology so that the patient’s impairment is masked in the early stages of disease. Thus, the neurodegeneration associated with AD is proposed to have later expression among highly educated individuals than those with lower levels of education.
On the other hand, evidence also suggests that individuals, particularly individuals with A, who benefit from higher levels of education are also likely to see sharper cognitive declines during older ages than those with lower levels of education when they do develop dementia (Proust-Lima et al., 2008; Borroni, Alberici, Agosti, Premi, & Padovani, 2008). Theories on this phenomenon typically hold that the delay of the clinical expression of AD causes clinical diagnoses of AD to be made during the later stages of the disease’s pathological progression. Therefore, when AD is diagnosed in highly educated individuals they may be more advanced in the disease’s pathology, resulting in the more rapid declines reported in individuals with high levels of education (Albert et al., 1995; Le Carret et al., 2005; Allegri et al., 2010; Tucker & Stern, 2011).
Despite some evidence for highly educated individuals experiencing sharper declines in older age however, no effects of age (range = 60–92 yrs) were found in the current study. Thus, exploratory analyses were conducted using only those in the graduate level group (age range = 62–89 yrs), in order to examine the potential effects of age on metamemory among highly educated older individuals. Interestingly, no correlation between age and relative confidence accuracy was found (p > .05), either across or within diagnostic groups. Moreover, average relative confidence levels were in the accurate range for the control group regardless of age, and in the AD group only those that were 75 years or older demonstrated non-discriminatory, but not inaccurate, levels of confidence. These findings further support the hypothesis that retrospective metamemory abilities may not suffer the same sharp declines that other areas of cognition have been shown to undergo among highly educated individuals in the later stages of aging and AD, and are in line with research linking higher education levels with fewer declines in frontal-dependent functioning in older adults and AD patients (Plumet et al., 2005).
In general, the finding that higher levels of education predict more accurate retrospective metamemory has significant implications and applications for multiple settings. For example, research suggests age and AD-related declines in metamemory can be improved through techniques like cognitive strategy training, highlighting the potential for therapeutic and neuropsychological intervention to aid older individuals in preserving and utilizing retrospective metamemory processes (Miller et al., 2012). Preserving and enhancing aging metamemory is important, particularly because knowledge about tasks and the ways they can be accomplished using alternative procedures can be used to select cognitive strategies helpful in surmounting the physiological effects of aging (Devolder & Pressley, 1989). Moreover, studies have shown that older adults with higher metamemory scores are more likely to report active lifestyles, have more frequent contacts in their social network, and have a higher internal locus of control, indicating that accurate metamemory is associated with a better quality of life in older age (Stevens, Kaplan, Ponds, & Jolles, 2001).
On the other hand, poor awareness of memory ability can produce serious day-to-day consequences for both the individual and those they interact with, negatively affecting judgment and decision-making abilities. This is especially true as the degree of confidence that an individual expresses in a memory plays a critical role in how an outsider evaluates the verity of that memory (Chua et al., 2009), and deficits in confidence accuracy may lead older individuals to express inflated confidence in false memories, causing them to mislead or misinform others. Concern may also arise in situations such as eyewitness testimony, where a large amount of weight is given to the confidence an individual holds in the accuracy of their own memories (Dodson, Bawa, & Kreuger, 2007). For individuals with early to mild AD in particular, unawareness of one’s deficits has great potential to hinder early identification efforts, pose obstacles to treatment compliance, and decrease the likelihood of effective therapeutic and neuropsychological intervention (Consentino and Stern, 2007). Likewise, healthy older adults may not realize a need to compensate for normal age-related cognitive declines if they cannot accurately assess their own memory abilities (Wong, Cramer, & Gallo, 2012).
A consideration to note about the present findings is that the sample was obtained from a clinic in a high SES area. Accordingly, there are more highly-educated participants in the sample than might be expected in a population sample of normally aging adults. Future studies may wish to examine the role of education and metamemory accuracy on episodic memory performance in a population with a larger number of individuals reporting fewer than twelve years of education.
It should also be noted that the current study did not measure prospective metamemory, thus these findings apply solely to retrospective metamemory, which may differ from prospective metamemory in the degree to which it is affected by AD. Future studies may which to compare retrospective and prospective metamemory among healthy older adults and older adults with mild to moderate AD, as the literature suggests that there may be an interaction between diagnosis and type of metamemory (retrospective vs. prospective).
The current study employed an odor recognition memory task in order to assess the accuracy of retrospective metamemory judgments. While other studies have investigated the accuracy of prospective metamemory judgments within the context of olfactory tasks (Jönsson & Olsson, 2003; Jönsson et al., 2005), to the authors’ knowledge this is the first study to investigate the accuracy of retrospective confidence judgments using an odor memory task.
We speculate that an odor memory task may provide an assessment of metamemory abilities in aging populations that may be freer of age-related stereotype priming than a task conducted in a modality that has been associated with age-related stigma and associated anxiety, thus contributing to our understanding of the variability seen across previous studies. However, because this study did not compare the olfactory task with one in another modality, this view is speculative and warrants additional investigation. Further, episodic memory retrieval has been shown to rely not only on memory areas, but also on areas activated during initial encoding, thus, olfactory memory processing involves different regions than memory processing in other modalities. Since such differences may potentially impact metamemory, and affect the generalizability of the current study, this should be explored in future research in multiple modalities. Finally, future studies may also wish to directly compare levels of task-related anxiety across different modalities.
Acknowledgments
Supported by NIH grants R01AG004085-26 (CM) and P50AG005131-28 (UCSD ADRC). SJ was supported by an NIH Diversity Supplement to R01AG004085-26. We gratefully acknowledge the patients and staff of the UCSD Alzheimer’s Disease Research Center (ADRC) and the SDSU Lifespan Human Senses Center for assistance.
References
- Albert M, Jones K, Savage C, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: Macarthur studies of successful aging. Psychology and Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Allegri RF, Taragano FE, Krupitzki H, Serrano CM, Dillon C, Sarasola D, Feldman M, Tufro G, Martelli M, Sanchez V. Role of cognitive reserve in progression from mild cognitive impairment to dementia. Dementia e Neuropsychologia. 2010;4(1):28–34. doi: 10.1590/S1980-57642010DN40100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Ethical principles of psychologists and code of conduct. American Psychologist. 1992;47:1597–1611. [Google Scholar]
- Anstey K. How important is mental activity in old age? The Australian Psychologist. 1999;34(2):128–131. [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein e as predictors of cognitive change in old age: A review. Gerontology. 2000;46(3):163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lapinska B. Monitoring of general knowledge: Evidence for preservation in early Alzheimer’s disease. Neuropsychologia. 1993;31:335–345. doi: 10.1016/0028-3932(93)90157-u. [DOI] [PubMed] [Google Scholar]
- Batterham PJ, Mackinnon AJ, Christensen H. The effect of education on the onset and rate of terminal decline. Psychology and Aging. 2011;26(2):339–350. doi: 10.1037/a0021845. [DOI] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Agosti C, Premi E, Padovani A. Education plays a different role in Frontotemporal Dementia and Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2008;23:796–800. doi: 10.1002/gps.1974. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiology of Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Busey T, Tunnicliff J, Loftus G, Loftus E. Accounts of the confidence-accuracy relation in recognition memory. Psychonomic Bulletin & Review. 2000;7(1):26–48. doi: 10.3758/bf03210724. [DOI] [PubMed] [Google Scholar]
- Cain WS. Comparability of two tests of olfactory functioning. Chemical Senses. 1989;14(4):479–485. [Google Scholar]
- Chan AS, Choi M, Salmon DP. The effects of age, education, and gender on the Mattis Dementia Rating Scale performance of elderly Chinese and American individuals. The Journals Of Gerontology: Series B: Psychological Sciences And Social Sciences. 2001;56B(6):356–363. doi: 10.1093/geronb/56.6.p356. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. Neural basis for recognition confidence in younger and older adults. Psychology and Aging. 2009;24(1):139–153. doi: 10.1037/a0014029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. Chronology of the United States. New York: McGraw-Hill; 1975. [Google Scholar]
- Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43(7):1004–1019. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Stern Y. Metacognitive theory and assessment in dementia: Do we recognize our areas of weakness? Journal of the International Neuropsychological Society. 2005;11(7):910–919. doi: 10.1017/s1355617705050964. [DOI] [PubMed] [Google Scholar]
- Dahl M, Allwood CM, Hagberg B. The realism in older people’s confidence judgments of answers to general knowledge questions. Psychology and Aging. 2009;24(1):234–238. doi: 10.1037/a0014048. [DOI] [PubMed] [Google Scholar]
- Davidson TM, Murphy C. Rapid clinical evaluation of anosmia: The alcohol sniff test. Archives of Otolaryngology—Head & Neck Surgery. 1997;123(6):591–594. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]
- Devolder PA, Pressley M. Metamemory across the adult lifespan. Canadian Psychology. 1989;30(3):578–587. [Google Scholar]
- Do Lam AT, Axmacher N, Fell J, Staresina BP, Gauggel S, Wagner T, Olligs J, Weis S. Monitoring the mind: The neurocognitive correlates of metamemory. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Kreuger LE. Aging, metamemory, and high-confidence errors: A misrecollection account. Psychology and Aging. 2007;22(1):122–133. doi: 10.1037/0882-7974.22.1.122. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Murphy C. Olfactory acuity and cognitive function converge in older adulthood: Support for the common cause hypothesis. Psychology and Aging. 2002;17(3):392–404. [PubMed] [Google Scholar]
- Economou A. Memory score discrepancies by healthy middle-aged and older individuals: The contributions of age and education. Journal of the International Neuropsychological Society. 2009;15:963–972. doi: 10.1017/S1355617709990580. [DOI] [PubMed] [Google Scholar]
- Ekman G, Berglund B, Berglund U, Lindvall T. Perceived intensity of odor as a function of time of adaptation. Scandinavian Journal of Psychology. 1967;8(1):177–186. doi: 10.1111/j.1467-9450.1967.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer’s disease. Current Opinion in Neurobiology. 2011;21:929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Andel R, Crowe M, Fiske A, Reynolds CA, Pedersen NL. Accounting for the relationship between low education and dementia: A twin study. Physiology & Behavior. 2007;92:232–237. doi: 10.1016/j.physbeh.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Barr PJ, Murphy C. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer’s disease and the Lewy body variant of Alzheimer’s disease. Journal of the International Neuropsychological Society. 2004;10:835–842. doi: 10.1017/s1355617704106024. [DOI] [PubMed] [Google Scholar]
- Grabe ME, Kamhawi R, Yegiyan N. Informing citizens: How people with different levels of education process television, newspaper, and web news. Journal of Broadcasting & Electronic Media. 2009;53(1):90–111. [Google Scholar]
- Herlitz A, Nilsson L, Bäckman L. Gender differences in episodic memory. Memory & Cognition. 1997;25(6):801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Heun R, Kockler M. Gender differences in the cognitive impairment in Alzheimer’s disease. Archives of Women’s Mental Health. 2002;4:129–137. doi: 10.1007/s00737-002-0144-4. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Rhodes MG. False remembering in the aged. Current Directions in Psychological Science. 2006;15(2):49–53. [Google Scholar]
- Jönsson FU, Olsson MJ. Olfactory metacognition. Chemical Senses. 2003;28(7):651–658. doi: 10.1093/chemse/bjg058. [DOI] [PubMed] [Google Scholar]
- Jönsson FU, Tchekova A, Lönner P, Olsson MJ. A metamemory perspective on odor naming and identification. Chemical Senses. 2005;30(4):353–365. doi: 10.1093/chemse/bji030. [DOI] [PubMed] [Google Scholar]
- Kao YC, Davis ES, Gabrieli JDE. Neural correlates of actual and predicted memory formation. Nature Neuroscience. 2005;8:1776–1783. doi: 10.1038/nn1595. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. Journal of Clinical and Experimental Neuropsychology. 1998;20:536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Marquie JC, Huet N. Age differences in feeling-of-knowing and confidence judgments as a function of knowledge domain. Psychology and Aging. 2000;15(3):451–461. doi: 10.1037//0882-7974.15.3.451. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Siddarth P, Gaines JM, Parrish JM, Ercoli LM, Marx K, Ronch J, Pilgram B, Burke K, Barczak N, Babcock B, Small GW. The memory fitness program: Cognitive effects of a healthy aging intervention. American Journal of Geriatric Psychiatry. 2012;20(6):514–523. doi: 10.1097/JGP.0b013e318227f821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Gordon AR, Olsson MJ, Lundström JN, Dalton P. Mind over age- Stereotype activation and olfactory function. Chemical Senses. 2013;38:167–174. doi: 10.1093/chemse/bjs086. [DOI] [PubMed] [Google Scholar]
- Moulin CJA, James N, Perfect TJ, Jones RW. Knowing what you cannot recognise: Further evidence for intact metacognition in Alzheimer’s disease. Aging, Neuropsychology, and Cognition. 2003;10(1):74–82. [Google Scholar]
- Muniz-Terrera G, Matthews F, Dening T, Huppert FA, Brayne C. Education and trajectories of cognitive decline over 9 years in very old people: Methods and risk analysis. Age and Ageingm. 2009;38:277–282. doi: 10.1093/ageing/afp004. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: Elderly vs. young persons. American Journal of Psychology. 1991;104:161–192. [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BP. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiology of Aging. 1990;11(4):465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, Jinich S. Very early decline in recognition memory for odors in Alzheimer’s disease. Aging, Neuropsychology, and Cognition. 1999;6(3):229–240. [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: A review. Neuropsychology Review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Sunderland T, Weingartner HM, Vitiello B, Martinson H, Putnam K. Alzheimer’s disease and the feeling-of-knowing for knowledge and episodic memory. Journal of Gerontology: Psycholological Sciences. 1992;47 (3):159–164. doi: 10.1093/geronj/47.3.p159. [DOI] [PubMed] [Google Scholar]
- Plumet J, Gil R, Gaonac’h D. Neuropsychological assessment of executive functions in women: Effects of age and education. Neuropsychology. 2005;19(5):566–577. doi: 10.1037/0894-4105.19.5.566. [DOI] [PubMed] [Google Scholar]
- Proust-Lima C, Amieva H, Letenneur L, Orgogozo J, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: A general cognitive factor approach. Psychology and Aging. 2008;23(3):608–620. doi: 10.1037/a0012838. [DOI] [PubMed] [Google Scholar]
- Randolph JJ, Arnett PA, Higginson CI. Metamemory and tested cognitive function in Multiple Sclerosis. The Clinical Neuropsychologist. 2001;15(3):357–368. doi: 10.1076/clin.15.3.357.10278. [DOI] [PubMed] [Google Scholar]
- Reed BR, Dowling M, Farias ST, Sonnen J, Strauss M, Schneider JA, Bennett DA, Mungas D. Cognitive activities during adulthood are more important than education in building reserve. Journal of the International Neuropsychological Society. 2011;17(4):615–624. doi: 10.1017/S1355617711000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harbey D, Beckett L, Widaman K, Hinton L, DeCarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain: A Journal of Neurology. 2010;133(8):2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J, Morin-Audebrand L, Cerf-Ducastel B, Haase L, Issanchou S, Murphy C, Fonlupt P, Sulmont C, Plailly J. True and false recognition memories of odors induce distinct neural signatures. Frontiers in Human Neuroscience. 2011;5(65):1–17. doi: 10.3389/fnhum.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scameas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(3):308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchay C, Bacon E, Danion JM. Metamemory in Schizophenia: An exploration of the feeling-of-knowing state. Journal of Clinical and Experimental Neuropsychology. 2006;28:828–840. doi: 10.1080/13803390591000846. [DOI] [PubMed] [Google Scholar]
- Souchay C, Isingrini M, Espagnet L. Aging, episodic memory feeling-of-knowing, and frontal functioning. Neuropsychology. 2000;14(2):299–309. doi: 10.1037//0894-4105.14.2.299. [DOI] [PubMed] [Google Scholar]
- Springer MV, McIntosh AR, Winocur G, Grady CL. The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology. 2005;19(2):181–192. doi: 10.1037/0894-4105.19.2.181. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of Neurology. 1992;32(3):371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Stevens FCJ, Kaplan CD, Ponds RWHM, Jolles J. The importance of active lifestyles for memory performance and memory self-knowledge. Basic and Applied Social Psychology. 2001;23(2):137–145. [Google Scholar]
- Thomas AK, Dubois SJ. Reducing the burden of stereotype threat eliminates age differences in memory distortion. Psychological Science. 2011;22(12):1515–1517. doi: 10.1177/0956797611425932. [DOI] [PubMed] [Google Scholar]
- Tucker AM, Stern Y. Cognitive reserve in aging. Current Alzheimer’s Research. 2011;8(4):354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiren APA, Bosma H, Visser P, Zeegers MP, Graff C, Ewers M, Frisoni GB, Verhey FRJ. The association between APOE e4 and Alzheimer-type dementia among memory clinic patients is confined to those with a higher education. The DESCRIPA study. Journal of Alzheimer’s Disease. 2013;35:241–246. doi: 10.3233/JAD-122182. [DOI] [PubMed] [Google Scholar]
- Wallhagen MI. The stigma of hearing loss. The Gerontologist. 2009;50(1):66–75. doi: 10.1093/geront/gnp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Marcaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings, DeCarli C, Foster NL, Galasko D, et al. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer’s Disease & Associated Disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment in cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Sullivan M, deToledo-Morrell L, Stebbins GT, Bennet DA. Association of memory and cognition in Alzheimer’s disease with volumetric estimates of temporal lobe structures. Neuropsychology. 1996;10(4):459–463. [Google Scholar]
- Wong JT, Cramer SJ, Gallo DA. Age-related reduction of the confidence-accuracy relationship in episodic memory: Effects of recollection quality and retrieval monitoring. Psychology & Aging. 2012 doi: 10.1037/a0027686. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, Manly JJ. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society. 2011;17(6):1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]


