Abstract
The brain's reward systems evolved to reinforce behaviors required for species survival, including sex, food consumption, and social interaction. Drugs of abuse co-opt these neural pathways, which can lead to addiction. Here, we use Drosophila melanogaster to investigate the relationship between natural and drug rewards. In males, mating increased Neuropeptide F (NPF) levels, whereas sexual deprivation reduced NPF. Activation or inhibition of the NPF system in turn enhanced or reduced ethanol preference. These results thus link sexual experience, NPF system activity, and ethanol consumption. Artificial activation of NPF neurons was in itself rewarding and precluded the ability of ethanol to act as a reward. We propose that activity of the NPF/NPF receptor axis represents the state of the fly reward system and modifies behavior accordingly.
Natural rewards and abused drugs affect the function of the brain's reward systems, and abnormal function of these brain regions is associated with addictive behavior (1-3). Some aspects of drug reward can be modeled in the genetically tractable fruit fly, Drosophila melanogaster. Flies exhibit complex addiction-like behaviors, including a lasting attraction for a cue that predicts ethanol intoxication (4) and a preference for consuming ethanol-containing food, even if made unpalatable (5). Here, we extend studies in the Drosophila model to incorporate the effect of social experiences, which can have long-lasting effects on behavior (6, 7).
We used the courtship-conditioning paradigm (8) to generate two cohorts of male flies. Males in the rejected-isolated cohort experienced one-hour sessions of sexual rejection by mated females, three times a day, for four days (Fig. 1A). Such conditioning suppresses future male courtship behavior, even towards receptive virgin females (9) (Fig. S1). Flies in the mated-grouped cohort experienced six-hour sessions of mating with multiple receptive virgin females (ratio 1:5) for four days. Flies from each cohort were then tested in a two-choice preference assay (10), where they voluntarily choose to consume food with or without 15% ethanol supplementation (11). Results for the two cohorts differed dramatically. The value of the ethanol preference index (which when positive signifies attraction) was consistently higher for the rejected-isolated cohort (Fig. 1B). The experiences did not alter food consumption when tested in the absence of ethanol (Fig. S2).
Fig. 1. Mating and chronic sexual deprivation have opposite effects on voluntary ethanol consumption.
(A) Schematic of the behavioral assay. Virgin wild-type males were allowed to mate with virgin females (groups of 4 males and 20 females) for 6 h daily (“mated-grouped”, green blocks) or were subjected to courtship conditioning for 1 h, 3 times daily (“rejected-isolated”; blue squares). Training was repeated for four days, after which males were placed in vials where they could choose to feed from capillaries containing food solutions with (red) or without (brown) 15% ethanol (10). Ethanol consumption was measured on days 6-8. (B) “Rejected-isolated” males exhibited higher ethanol preference than “mated-grouped” males (**P<0.005, n=12). (C) “Mated-grouped” males showed lower ethanol preference than “virgin-grouped” males (*P<0.05, n=12). (D) Males conditioned with decapitated virgins showed enhanced ethanol preference compared to “mated-grouped” males (**P<0.01, n=12). (E) Mating reversed the effects of rejection on ethanol preference. “Rejected-isolated” males that were allowed to mate after the end of the last conditioning session showed lower ethanol preference than similarly conditioned males that were left undisturbed (**P<0.001, n=8). Statistical analysis was carried out by two-way repeated-measures ANOVA with Bonferroni post-tests; comparisons are between treatment groups across all days of the assay. Data shown is mean+SEM or mean–SEM.
The rejected-isolated and mated-grouped cohorts differ in several respects in addition to sexual deprivation (lack of copulation) per se, including individual versus group housing, exposure to the social experience of rejection, and exposure to aversive chemosensory cues found on mated females. Several experiments were designed to determine which of these was the predominant contributor to the enhanced ethanol preference seen in rejected-isolated males (11). First, we compared males that differed in sexual experience but not in housing conditions – that is, mated and virgin males that were both group-housed. The virgin males showed higher ethanol preference, although in general not quite as high as rejected-isolated males. This argues that isolation is not the major explanation for the enhanced ethanol preference.
We next investigated ethanol preference in males that were sexually deprived (blocked from copulating), but not exposed to the social experience of rejection. For this purpose, males were exposed individually to decapitated virgin females on the same schedule as the rejected-isolated cohort, using a protocol that results in courtship suppression (9). These males, which experience neither rejection nor copulation, showed enhanced ethanol preference when compared to the mated-grouped cohort (Fig. 1C); the preference index was similar to that displayed by the rejected-isolated cohort. These results point to sexual deprivation per se, rather than rejection, as the major factor influencing ethanol preference.
Finally, we sought to establish whether there was a role for the repellant chemosensory cue cis-vaccenyl acetate (cVA), which is found on the cuticle of mated but not virgin females (12). We compared males trained with either mated females (rejected-isolated) or decapitated virgin females (11). Both groups endured sexual deprivation (lack of copulation), but only the former was exposed to cVA. There was no difference in ethanol preference between these two cohorts (Fig. S3A). There was also no difference between males exposed individually to biologically relevant concentrations of cVA (13) and vehicle-exposed controls (Fig. S3B)(11). Together, these experiments point to sexual deprivation per se, rather than other factors, as the major contributor to enhanced ethanol preference.
To further test the strength of this conclusion, we divided a cohort of rejected-isolated males into two subgroups, one of which was left undisturbed, and the other of which was allowed to mate with virgin females for 2.5 hours immediately before testing. Ethanol preference was markedly lower in the rejected, then mated subgroup (Fig. 1E) compared to the subgroup that had only experienced rejection. Thus, the effects of sexual deprivation can be reversed by copulation, which is consistent with sexual deprivation being the major contributor to ethanol preference.
We focused on Drosophila Neuropeptide F (NPF) as a potential mediator of the effects of sexual experience. The mammalian NPF homolog, Neuropeptide Y (NPY, (14)), regulates ethanol consumption (15), the NPF/NPF receptor (NPFR) system regulates acute ethanol sensitivity in Drosophila (16), and the C. elegans NPY receptor homolog NPR-1 regulates ethanol behaviors (17). Intriguingly, stressful experiences regulate mammalian NPY levels. These include restraint stress and early maternal separation in rodents and post-traumatic stress disorder (PTSD) in humans (18-20). However, a direct connection between social experience, NPY, and ethanol-related behaviors has not been established.
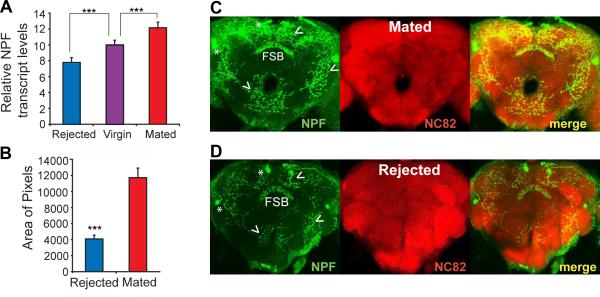
To investigate whether NPF mediates ethanol preference in Drosophila, we first compared NPF transcript levels in heads of males subjected to different sexual experiences: rejected-isolated, virgin-grouped, and mated-grouped (11). Rejected-isolated males showed the lowest transcript levels, virgin-grouped males showed higher levels, and mated-grouped males showed the highest (Fig. 2A, Fig. S4). Rejected-isolated males also showed strikingly lower NPF protein levels than mated-grouped males by immunohistochemistry (Fig. 2B-D).
Fig. 2. Sexual experience regulates levels of NPF and NPF mRNA.
(A) Total RNA extracted from heads of virgin, rejected and mated males was analyzed for NPF mRNA levels by qPCR. NPF mRNA levels were reduced by sexual rejection and increased by mating (***P<0.001 compared to virgin control, Dunnett's test, n=3 independent experiments). NPF transcript levels were normalized to rp49 mRNA. (B-D) Effect of rejection on NPF protein expression as determined by immunohistochemistry. (B) Quantitative analysis of overall NPF staining intensity in brains of rejected and mated males (***P<0.001, t-test). (C, D) Differential NPF staining in rejected and mated males was observed in all major regions of NPF expression (arrowheads). Asterisks denote the positions of NPF-expressing cell bodies (which are obscured by high levels of expression in mated males). FSB = fan-shaped body.
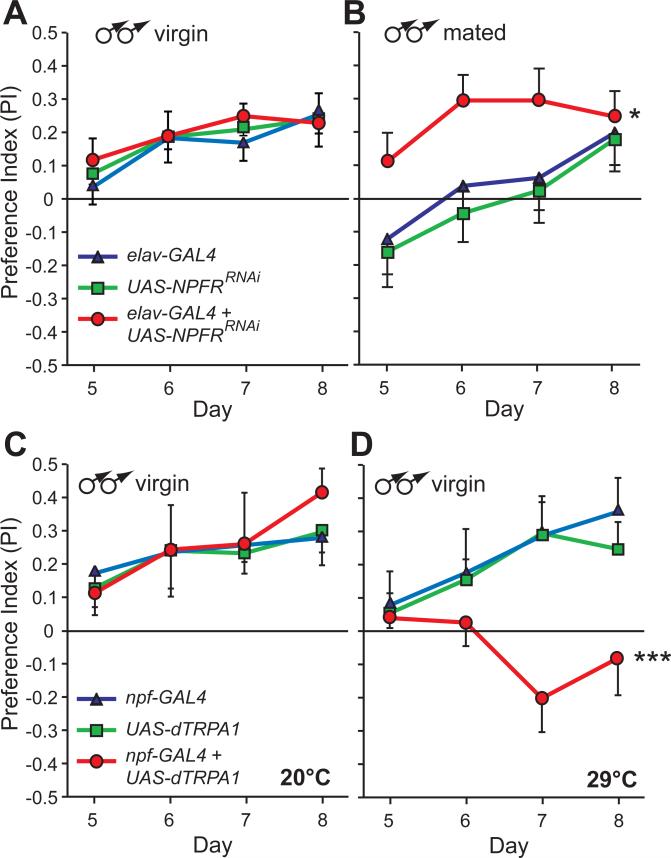
To ask whether the inverse correlation between NPF levels and ethanol preference reflects a cause and effect relationship, we manipulated the NPF/NPFR system genetically. We first tested the effect of NPFR down-regulation by expressing an NPFR-specific short interfering RNA (UAS-NPFRRNAi) pan-neuronally (using elav-GAL4). This manipulation significantly reduced ethanol preference in mated males, which have elevated NPF levels, but not in virgin males (Fig. 3A, B). Second, we tested the effect of artificial activation of NPF neurons by expressing the heat-activated cation channel dTRPA1 (21) under NPF-GAL4 control (22). There was no effect on ethanol preference if virgin males were tested at 20°C, when the channel is inactive, but there was aversion to ethanol-supplemented food at 29°C, when the channel is active (Fig. 3C, D). An intermittent dTRPA1 activation protocol that more closely mimics our conditioning protocol produced similar aversion (Fig. S5). These data suggest a causal relationship between sexual experience, NPF levels, and ethanol preference.
Fig. 3. NPF signaling regulates ethanol preference.
(A, B) Expression of an NPFR RNAi transgene (UAS-NPFRRNAi) using a pan-neuronal driver (elav-GAL4) increased ethanol preference in mated males compared to the genetic controls carrying either transgene alone (B) (*P<0.05, n=12), but not in virgin males (A) (P>0.5). (C, D) Activating NPF neurons reduced ethanol preference. Virgin males expressing dTRPA1 in NPF neurons (NPF-GAL4 + UAS-dTRPA1), and the genetic controls carrying either transgene alone, developed similar levels of ethanol preference at 20°C (C) when dTRPA1 is not active (P>0.05, n=8), but developed aversion to ethanol containing food at 29°C (D), when dTRPA1 is active (***P<0.001, n=8). Statistical analysis was carried out by two-way repeated-measures ANOVA with Bonferroni post-tests; comparisons are between treatment groups across all days of the assay. Data is mean + or – SEM (for clarity purposes).
We propose that the activity of the NPF/NPFR system may be a neural representation of the state of the Drosophila reward system. If so, experiences that change NPF/NPFR activity should promote behaviors that restore the system to its normal state. In this model, sexual deprivation would create an NPF deficit that increases reward-seeking behavior such as ethanol consumption. Conversely, successful copulation would create a NPF surfeit that reduces reward seeking. This model predicts that mating and ethanol consumption should be rewarding (4, 5), that activation of the NPF/NPFR pathway is rewarding per se, and that artificial activation of the NPF circuit will diminish ethanol reward-seeking behavior.
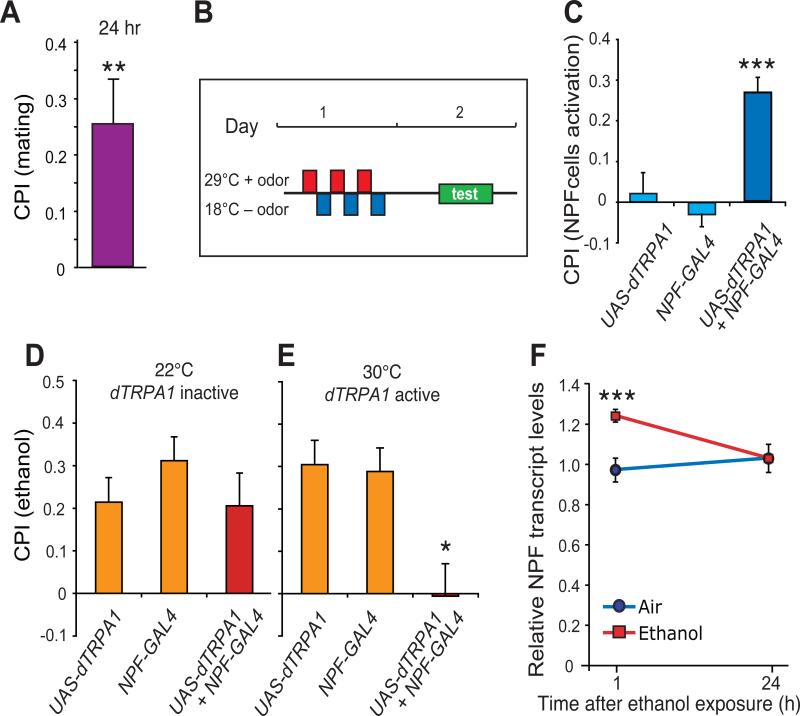
To test these predictions we used a series of conditioning assays in which male flies were trained to associate the proposed rewarding experiences (mating, ethanol exposure, or NPF circuit activation) with one of two neutral odor cues. After 24 hours, flies were tested for their odor preference; development of a preference for the odor associated with these experiences would imply that flies found the events rewarding. To test if mating is rewarding, males were exposed sequentially for 30 minutes to two odorants (ethyl acetate, EA, or iso amyl alcohol, IAA), one in the absence and the other in the presence of virgin females, and tested for odor preference 24 hours later in the absence of females. A conditioned odor preference index (CPI) for mating was calculated by averaging preference indices for reciprocally trained groups of flies. Positive CPI values indicate conditioned preference, negative values indicate aversion. Males displayed a strong preference for the mating-associated odor (Fig. 4A). We have separately shown that flies exhibit conditioned preference for an odor associated with ethanol intoxication in a similar assay (4). Together, these results indicate that both mating and ethanol intoxication, the latter of which is achieved in the two-choice consumption assay (5), are indeed rewarding experiences to male flies.
Fig. 4. Mating and NPF cell activation is rewarding and reduces ethanol reward.
(A) Mating is rewarding to male flies. Males trained to associate an odor with mating (presence of virgin females) develop preference for that odor. P values were calculated by Wilcoxon analysis against zero. Mating against zero was **P=0.001; each reciprocal group against zero was P=0.004 for one odor (IAA) plus mating and P=0.02 for the reciprocal odor (EA) plus mating. CPI=Conditioned Preference Index (calculated by averaging the odor preference indexes for reciprocally trained males). (B, C) NPF cell activation is rewarding. Males expressing dTRPA1 in NPF neurons (NPF-GAL4 + UAS-dTRPA1) and the genetic controls carrying either transgene alone, were exposed to three one-hour training sessions at 29°C in the presence of odor (red rectangles in B) that where spaced by one-hour rest periods at 18°C in the absence of odor (blue rectangles in B). Testing for odor preference was performed 24 hours after training at 21°C. Experimental males, but not the genetic controls, showed preference for the odor that was associated with dTRPA1 activation in NPF neurons. Data are averages of three independent experiments. Statistical analysis was carried out by two-way repeated-measures ANOVA with Bonferroni post-tests; comparisons are between treatment groups (**P<0.001, n=24). (D, E) Activation of NPF neurons abolishes ethanol reward. Activation of NPF neurons using dTRPA1 (NPF-GAL4 + UAS-dTRPA1) eliminated conditioned ethanol preference compared to the singly transgenic controls when tested 24 hours after training (*P<0.01, one-way ANOVA with Wilcoxon/Kruskal-Wallis post-tests, n=22). F. NPF transcript levels are induced by ethanol intoxication. Males were exposed to moderately intoxicating levels of ethanol vapor (three 10-minute ethanol exposures spaced by one hour), collected, and frozen one or 24 hours later. NPF mRNA levels, measured by qPCR, were elevated one hour after ethanol exposure and returned to basal level after 24 hours (**P<0.001 compared to air-exposed controls, Dunnett's test, n=3 independent experiments with 30 males each).
To test whether activation of the NPF/NPFR pathway is rewarding per se, we trained virgin males to associate artificial activation of NPF neurons with either EA or IAA. Males expressing dTRPA1 in NPF neurons (NPF-GAL4 + UAS-dTRPA1) and the genetic controls each carrying only one of the two transgenes were trained for three one-hour sessions at 29°C, with dTRPA1 active, interspersed with three one-hour rest periods at 18°C, with dTRPA1 inactive (Fig. 4B). When tested 24 hours later, males in the experimental group demonstrated strong preference for the odor associated with NPF neuron activation. The genetic controls, which did not undergo NPF neuron activation, but were exposed to the same training protocol, developed no odor preference (Fig. 4C). Other controls, which underwent NPF neuron activation but were not exposed to the training protocol, similarly developed no odor preference (Fig. S6C). Thus, activation of the NPF/NPFR system is in itself rewarding to flies.
We next tested whether artificial activation of the NPF/NPFR system diminishes ethanol reward seeking. Flies were trained to associate EA or IAA with a moderately intoxicating exposure of ethanol vapor (three 10-minute training sessions spaced by one hour) as described before (4). Wild-type flies normally show conditioned aversion to ethanol (negative CPI) when tested 30 min after training, and conditioned preference 24 hours later (4). We used this assay to compare virgin male flies that expressed dTRPA1 in NPF neurons (NPF-GAL4 + UAS-dTRPA1) with genetic controls that did not. Artificial activation of NPF cells, which occurs at 30°C but not 22°, had no affect on the initial aversion (Fig. S6A, B), but abolished conditioned preference for ethanol (Fig. 4D, E). Thus, NPF neuron activation, which is in itself rewarding to flies, interferes with the ability of flies to form a positive association between ethanol intoxication and an odor cue, which is reflected in lower ethanol consumption.
If the NPF/NPFR system were to function generally to signal the state of the Drosophila reward system, NPF levels should be increased by rewarding experiences other than mating, such as exposure to intoxicating levels of ethanol. To test this, we exposed virgin males to ethanol vapor using a conditioning paradigm previously shown to be rewarding (three 10-minute exposures spaced by one hour) (4). NPF transcript levels increased one hour post-exposure and returned to basal levels 24 hours later (Fig. 4F). Because the ethanol-induced changes in NPF levels are transient, whereas the memory of ethanol reward lasts for several days, it is possible that ethanol-induced changes in NPF levels set in motion a process, likely involving dopaminergic systems (4, 22), that modifies the fly reward system. In fact, the activity of the NPF circuit could remain altered long after the levels of NPF have returned to baseline. Regardless of the exact mechanism, these data suggests that activity of the NPF system is regulated by at least two rewarding experiences, mating and ethanol intoxication. NPF has been shown to influence several complex behaviors in flies, including larval intake of noxious food (23), a switch in feeding behavior (24), and responses to physical stressors (25) and ethanol (16). In addition, NPF neurons modulate the effect of satiety on sugar reward memory (22). In our paradigm, NPF appears to play a different role: its expression is regulated by sexual experience and ethanol intoxication, and activation of NPF neurons acts as a reward signal thereby abolishing ethanol reward and the enhanced ethanol consumption observed after sexual deprivation. It is likely that the effects of NPF we describe here are mediated by a different set of NPFR-expressing neurons than those mediating NPF's role in sugar reward memory.
Mammalian NPY has several distinct behavioral functions that are mediated by different brain regions, including roles in feeding (26, 27), anxiety, stress (28), sleep regulation (29), sexual motivation (30), and ethanol consumption (15, 31). Stressors also regulate NPY levels (18-20). In addition, injection of NPY into the nucleus accumbens of rats is rewarding (32) and NPY administration relieves the negative affective states of drug withdrawal and depression (33, 34).
Our findings are thus not only consistent with known functions of mammalian NPY and its mode of regulation, but they provide evidence for NPF functioning as a key molecular transducer between social experience and drug reward. Drosophila is a useful and accessible model system in which to decipher the mechanisms by which social experiences interact with reward systems.
Supplementary Material
Summary Sentence: Sexual experiences alter voluntary ethanol consumption in Drosophila, and neuropeptide F serves as a key molecular transducer.
Acknowledgements
We thank Fred Wolf, Maya Schuldiner, Oren Schuldiner, Anita Devineni, Kim McClure, Ryan Joseph and Norma Velazquez Ulloa for advice and comments on the manuscript, Joel Levine for discussions, Ping Shen for fly strains and antibodies, and the Bloomington Stock Center for fly strains. Funding was provided by the Sandler Research Fellowship (GS-O), the Program for Breakthrough Biomedical Research at UCSF (UH), and NIH/NIAAA (UH).
Footnotes
The authors declare that they have no competing interests. A detailed description of all Materials and Methods as well as Supplementary Figures are available as Supporting Online Material.
References
- 1.Kelley AE, Berridge KC. J Neurosci. 2002;22:3306. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman SE. Am J Bioeth. 2007;7:8. doi: 10.1080/15265160601063969. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. Neuropharmacology 56 Suppl. 2009;1:18. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. Nat Neurosci. 2011;14:612. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devineni AV, Heberlein U. Curr Biol. 2009;19:2126. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson GE, Fernald RD, Clayton DF. Science. 2008;322:896. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan V, Nestler EJ. Curr Top Behav Neurosci. 2011;7:121. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride SM, et al. Neuron. 1999;24:967. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 9.Mehren JE, Ejima A, Griffith LC. Curr Opin Neurobiol. 2004;14:745. doi: 10.1016/j.conb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Ja WW, et al. Proc Natl Acad Sci U S A. 2007;104:8253. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M. a. d. i. d. i. S. o. Materials.).
- 12.Ejima A, et al. Curr Biol. 2007;17:599. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Nature. 2009;461:987. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 14.Hewes RS, Taghert PH. Genome Res. 2001;11:1126. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Nature. 1998;396:366. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 16.Wen T, Parrish CA, Xu D, Wu Q, Shen P. Proc Natl Acad Sci U S A. 2005;102:2141. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Neuron. 2004;42:731. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Thorsell A, Carlsson K, Ekman R, Heilig M. Neuroreport. 1999;10:3003. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Vasquez PA, Mathe AA, Thomas JD, Riley EP, Ehlers CL. Brain Res Dev Brain Res. 2001;131:149. doi: 10.1016/s0165-3806(01)00264-4. [DOI] [PubMed] [Google Scholar]
- 20.Sah R, et al. Biol Psychiatry. 2009;66:705. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenzweig M, et al. Genes Dev. 2005;19:419. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krashes MJ, et al. Cell. 2009;139:416. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Zhao Z, Shen P. Nat Neurosci. 2005;8:1350. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, et al. Neuron. 2003;39:147. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Li M, Shen P. J Neurosci. 30:2504. doi: 10.1523/JNEUROSCI.3262-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorsell A, Rimondini R, Heilig M. Neurosci Lett. 2002;332:1. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- 27.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Nat Neurosci. 1998;1:271. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 28.Heilig M. Neuropeptides. 2004;38:213. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Dyzma M, Boudjeltia KZ, Faraut B, Kerkhofs M. Sleep Med Rev. 14:161. doi: 10.1016/j.smrv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Kalra SP, Clark JT, Sahu A, Dube MG, Kalra PS. Synapse. 1988;2:254. doi: 10.1002/syn.890020313. [DOI] [PubMed] [Google Scholar]
- 31.Thorsell A. Peptides. 2007;28:480. doi: 10.1016/j.peptides.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Josselyn SA, Beninger RJ. Pharmacol Biochem Behav. 1993;46:543. doi: 10.1016/0091-3057(93)90542-2. [DOI] [PubMed] [Google Scholar]
- 33.Redrobe JP, Dumont Y, Quirion R. Life Sci. 2002;71:2921. doi: 10.1016/s0024-3205(02)02159-8. [DOI] [PubMed] [Google Scholar]
- 34.Stogner KA, Holmes PV. Eur J Pharmacol. 2000;387:R9. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.