Abstract
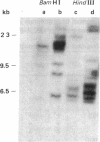
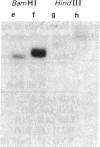
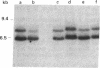
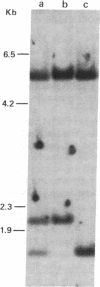
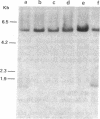
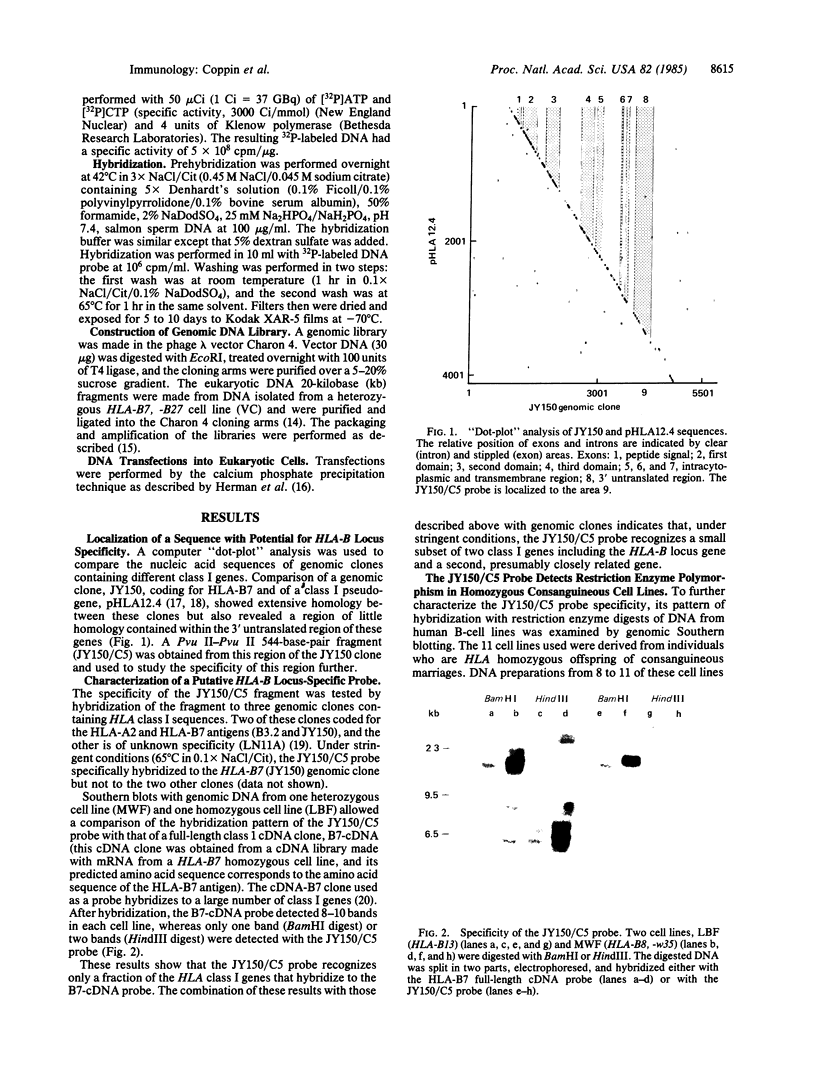
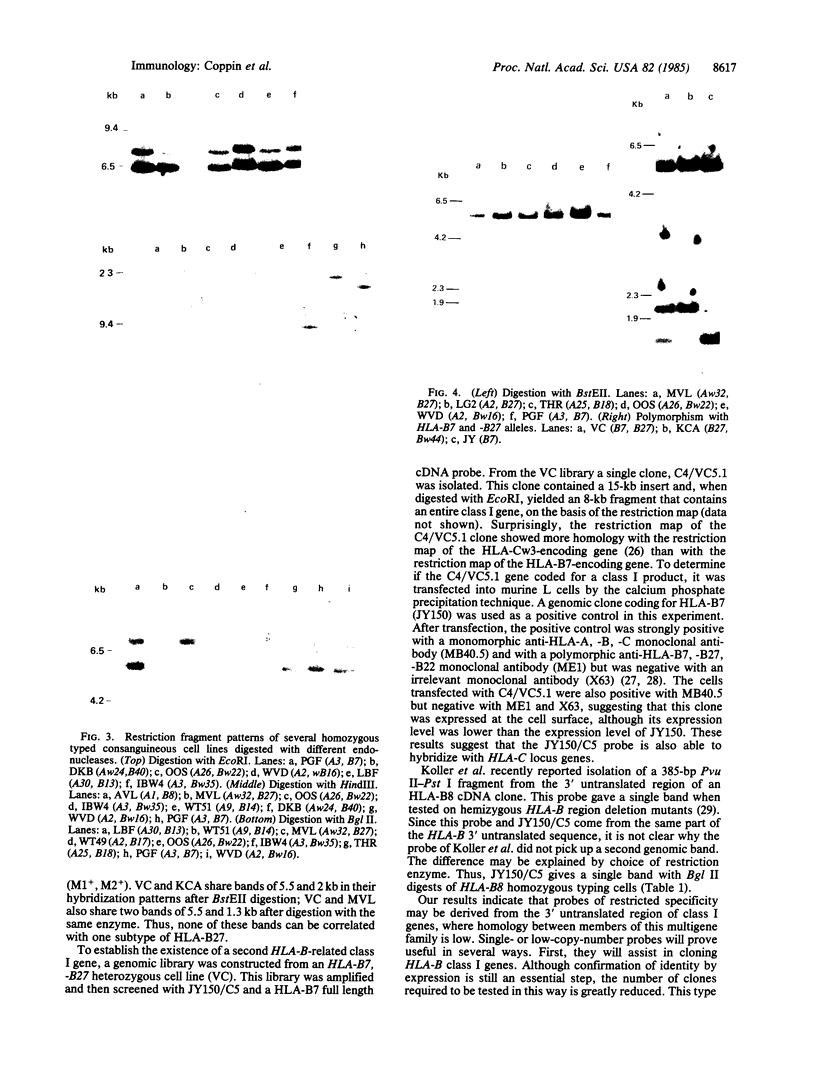
The large number of class I histocompatibility genes (HLA) and their extensive homology has made it difficult to assign bands on genomic Southern blots to known genes. Therefore, we have tried to obtain nucleic acid probes for class I genes that are locus specific or have restricted locus specificity. Computer sequence-homology analysis was used to compare the nucleic acid sequences of two genomic clones, one coding for the HLA-B7 antigen (JY150) and one containing a class I pseudogene (pHLA12.4). A sequence in the 3' untranslated region with very low homology was identified. This sequence from the HLA-B7 gene was subcloned into M13 phage. This fragment, JY150/C5, hybridized with two genomic bands in DNA from human HLA homozygotes--presumably the HLA-B locus gene and a closely related gene. The probe was used to assess restriction fragment polymorphism at the HLA-B locus in homozygous consanguineous cell lines. This analysis permitted the association of certain polymorphic restriction enzyme fragments with some alleles of this locus. However, many HLA-B alleles have identical restriction fragments produced by a number of restriction endonucleases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa J. A., Kamarck M. E., Biro P. A., Weissman S. M., Ruddle F. H. Identification of human genomic clones coding the major histocompatibility antigens HLA-a2 and HLA-B7 by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6327–6331. doi: 10.1073/pnas.79.20.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Cohen D., Paul P., Font M. P., Cohen O., Sayagh B., Marcadet A., Busson M., Mahouy G., Cann H. M., Dausset J. Analysis of HLA class I genes with restriction endonuclease fragments: implications for polymorphism of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6289–6292. doi: 10.1073/pnas.80.20.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Turner M. J., Strominger J. L. Papain-solubilized HL-A antigens from cultured human lymphocytes contain two peptide fragments. Proc Natl Acad Sci U S A. 1973 May;70(5):1603–1607. doi: 10.1073/pnas.70.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. A., Taylor C., McMichael A. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol. 1982 Aug;5(1):49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Collins F., Kole R., Stoeckert C. J., Jr, Jagadeeswaran P., Duncan C. H., Weissman S. M., Jagadeeswaran P., Pan J., Forget B. G. Sequences of human repetitive DNA, non-alpha-globin genes, and major histocompatibility locus genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1079–1086. [PubMed] [Google Scholar]
- Grumet F. C., Fendly B. M., Fish L., Foung S., Engleman E. G. Monoclonal antibody (B27M2) subdividing HLA-B27. Hum Immunol. 1982 Aug;5(1):61–72. doi: 10.1016/0198-8859(82)90031-3. [DOI] [PubMed] [Google Scholar]
- Herman A., Parham P., Weissman S. M., Engelhard V. H. Recognition by xenogeneic cytotoxic T lymphocytes of cells expressing HLA-A2 or HLA-B7 after DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5056–5060. doi: 10.1073/pnas.80.16.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersild C., Rubinstein P., Day N. K. The HLA system and inherited deficiencies of the complement system. Transplant Rev. 1976;32:43–71. doi: 10.1111/j.1600-065x.1976.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky P. Genetic exchanges between partially homologous nucleotide sequences: possible implications for multigene families. Biochimie. 1983 Feb;65(2):85–93. doi: 10.1016/s0300-9084(83)80178-3. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Mawas C., Charmot D., Mercier P. Split of HLA-D into two regions alpha and beta by a recombination between HLA-D and GLO. I. Study in a family and primed lymphocyte typing for determinants coded by the beta region. Tissue Antigens. 1980 May;15(5):458–466. doi: 10.1111/j.1399-0039.1980.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Lernmark A., Rask L., Peterson P. A., Platz P., Svejgaard A. Detection of HLA-D/DR-related DNA polymorphism in HLA-D homozygous typing cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3758–3761. doi: 10.1073/pnas.80.12.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulik M. D., Reisfeld R. A. beta2-Microglobulins. Contemp Top Mol Immunol. 1975;4:157–204. [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]