Abstract
Background
The ability to assess gene function is essential for understanding biological processes. Currently, RNA interference (RNAi) is the only technique available to assess gene function in planarians, in which it has been induced via injection of double-stranded RNA (dsRNA), soaking, or ingestion of bacteria expressing dsRNA.
Results
We describe a simple and robust RNAi protocol, involving in vitro synthesis of dsRNA that is fed to the planarians. Advantages of this protocol include the ability to produce dsRNA from any vector without subcloning, resolution of ambiguities in quantity and quality of input dsRNA, as well as time, and ease of application. We have evaluated the logistics of inducing RNAi in planarians using this methodology in careful detail, from the ingestion and processing of dsRNA in the intestine, to timing and efficacy of knockdown in neoblasts, germline, and soma. We also present systematic comparisons of effects of amount, frequency, and mode of dsRNA delivery.
Conclusions
This method gives robust and reproducible results and is amenable to high-throughput studies. Overall, this RNAi methodology provides a significant advance by combining the strengths of current protocols available for dsRNA delivery in planarians and has the potential to benefit RNAi methods in other systems.
Keywords: RNA-interference, RNAi, siRNA, planarian, Schmidtea mediterranea, Dugesia japonica
INTRODUCTION
Freshwater planarians have become a popular model organism for studies of stem cell biology, regeneration, germline development, and organogenesis (Newmark et al., 2008; Forsthoefel and Newmark, 2009; Shibata et al., 2010; Gentile et al., 2011; Tanaka and Reddien, 2011; Umesono et al., 2011; Elliott and Sánchez Alvarado, 2012; Rink, 2012). Regeneration and homeostasis of these organisms depend on a population of somatic stem cells whose progeny undergo terminal differentiation into cells of all tissues (Shibata et al., 2010; King and Newmark, 2012). Because of their ability to regenerate and develop every tissue as adults, planarians have been useful in studies to identify factors involved in organ formation.
In the absence of forward genetic techniques, planarian research relies upon RNA interference (RNAi) as a means to assess gene function. RNAi involves destruction of a target gene's mRNA in response to homologous double-stranded RNA (dsRNA) sequence (Fire et al., 1998). RNAi experiments have revealed the role of a growing number of planarians genes in formation and/or function of photoreceptors (Lapan and Reddien, 2011; Yamamoto and Agata, 2011; Lapan and Reddien, 2012), excretory cells (Rink et al., 2011; Scimone et al., 2011), the intestine (Forsthoefel et al., 2012), the brain (Cebria et al., 2002; Cebria et al., 2007), and the germline (Wang et al., 2007; Collins et al., 2010; Wang et al., 2010). High- and medium-throughput RNAi screens, often conducted in combination with global gene-expression analyses, have also identified functional requirements for specific genes for proper stem cell function and/or regeneration (Reddien et al., 2005a; Rouhana et al., 2010; Labbe et al., 2012; Onal et al., 2012; Solana et al., 2012; Wagner et al., 2012; Wenemoser et al., 2012).
RNAi was first induced in planarians by injection of double-stranded RNA (dsRNA) into the mesenchyme of Schmidtea mediterranea (Sanchez Alvarado and Newmark, 1999). Injection of dsRNA is highly effective and results in specific mRNA decay within 6 - 24 hours, but is also laborious and time consuming, and therefore, not practical for high-throughput screens. Furthermore, the physical damage experienced by planarians during the injection process may be undesirable when analyzing events related to wound healing and regeneration. Orii et al. (2003) reported a simpler technique for inducing RNAi in planarians, which involves soaking amputated planarian fragments in dsRNA for several hours. This approach, however, requires large volumes of concentrated dsRNA (0.1-0.5 μg/μl), and is not applicable to analysis of homeostasis in intact planarians (Orii et al., 2003). A second popular alternative to injection, developed for performing RNAi in nematodes, is the use of Escherichia coli to express and deliver dsRNA by feeding (Timmons and Fire, 1998). This approach was applied in planarians by mixing dsRNA-expressing bacteria with food (Newmark et al., 2003). This method was also applied in the first large-scale screen in planarians (Reddien et al., 2005a), and subsequently, in other screens (Wang et al., 2010; Forsthoefel et al., 2012; Wagner et al., 2012). A weakness of this approach, however, is that verifying the quantity and quality of dsRNA being expressed in the bacteria requires laborious steps; when ignored, this can add ambiguity to the assessment of the observed phenotypes, or lack thereof.
Here, we describe an improved protocol for synthesis and delivery of dsRNA to planarians. This protocol combines the flexibility and precision of injection and soaking protocols, with the ease of bacterial feeding, by directly mixing in vitro-synthesized dsRNA with planarian food. We present our observations of uptake, processing, activity, and perdurance of dsRNA in planarians using this method. Additionally, we compare parameters to achieve successful inhibition of different genes, genes expressed in different tissues, and in animals of different sizes. The RNAi methodology described here provides simplicity and robustness, while minimizing the time and effort involved in analysis of planarian gene function. This protocol should serve as a valuable asset for the planarian community and beyond, as modifications based upon it could be applicable to studies in other organisms.
RESULTS AND DISCUSSION
The synthesis of dsRNA in vitro and direct delivery by feeding mixed with planarian food as a carrier has proven successful in assaying gene function in a few recent studies (Collins et al., 2010; Pellettieri et al., 2010; Rouhana et al., 2010; Nakagawa et al., 2012; Rouhana et al., 2012; Sakurai et al., 2012). We exploited one advantage that in vitro dsRNA synthesis has over bacterial expression of dsRNA: it does not require subcloning genes or gene-fragments into a particular vector. Templates for dsRNA synthesis are generated by Polymerase Chain Reaction (PCR) using primers that contain a specific RNA polymerase promoter sequence on the 5′-end and sequence corresponding to the gene of interest on the 3′end (Fig. 1A). RNA molecules are synthesized simultaneously from the resulting templates in sense and antisense orientations, and are annealed during progression of the transcription reaction (See Experimental Procedures). The quality of dsRNA can be assessed by non-denaturing agarose gel electrophoresis. Double-stranded RNA appears as one or two intense bands prolonged as an upward smear that is not seen in DNA or single-stranded RNA (ssRNA) (Fig. 1B-C). DsRNA migrates approximately (although not exactly) as predicted for DNA, and slower than ssRNA (Fig. 1C). It is not necessary to apply DNAse to the dsRNA transcription reaction, denature and anneal the RNA molecules after transcription, or purify the synthesized product. The transcription reaction can be directly fed to planarians to induce RNAi, or stored at -20°C for long periods of time. We have observed that dsRNA is extremely stable and functional after being stored at -20°C for over two years (Fig. 1D; below).
Figure 1. Double-stranded RNA synthesis for RNA-interference.
(A) Schematic representation of PCR products that can be used as templates for dsRNA synthesis. Oligonucleotide primers with sequences homologous to the multiple cloning site (MCS) can be used to amplify template for various gene constructs on the same vector backbone. Template for gene-specific or fragment-specific dsRNA synthesis can be synthesized using primers specific to the ends or specific regions withn the cDNA sequence, respectively. RNA polymerase promoter sequences present in the 5′ end of all primers (T7 is used as an example here) allow for dsRNA transcription using the PCR products as templates. (B) Argonaute-2 dsRNA prepared from a single transcription reaction to induce RNAi in planarians. DsRNA analyzed by non-denaturing 1% agarose gel electrophoresis with (+) or without (-) undergoing annealing, DNase I, or ethanol precipitation (EtOH ppt) treatments after transcription. (C) Single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) analyzed by nondenaturing 1% agarose gel electrophoresis. (D) Freshly and previously (2.5 years ago) synthesized dsRNA analyzed by non-denaturing 1% agarose gel electrophoresis. The position of ssRNA (*), dsRNA (**), and higher complexes (***) during electrophoresis is indicated, as are the positions of DNA size markers (left on (B)).
The approach described for synthesis of dsRNA is also amenable if one desires to target different ends of a transcript or specific exons, as primers can be designed to target any particular region of a gene. Alternatively, template for dsRNA synthesis to target an array of different genes can be generated from a single primer pair that anneals to the vector sequence flanking the multiple cloning site of specific vectors (Fig. 1A). We have taken this approach to target sequences inserted in a common vector (pBluescript SK+) from a cDNA library (Zayas et al., 2005), in 96-well plate format.
Dynamics of double-stranded RNA processing and RNAi in planarians
Processing of dsRNA occurs in the intestine
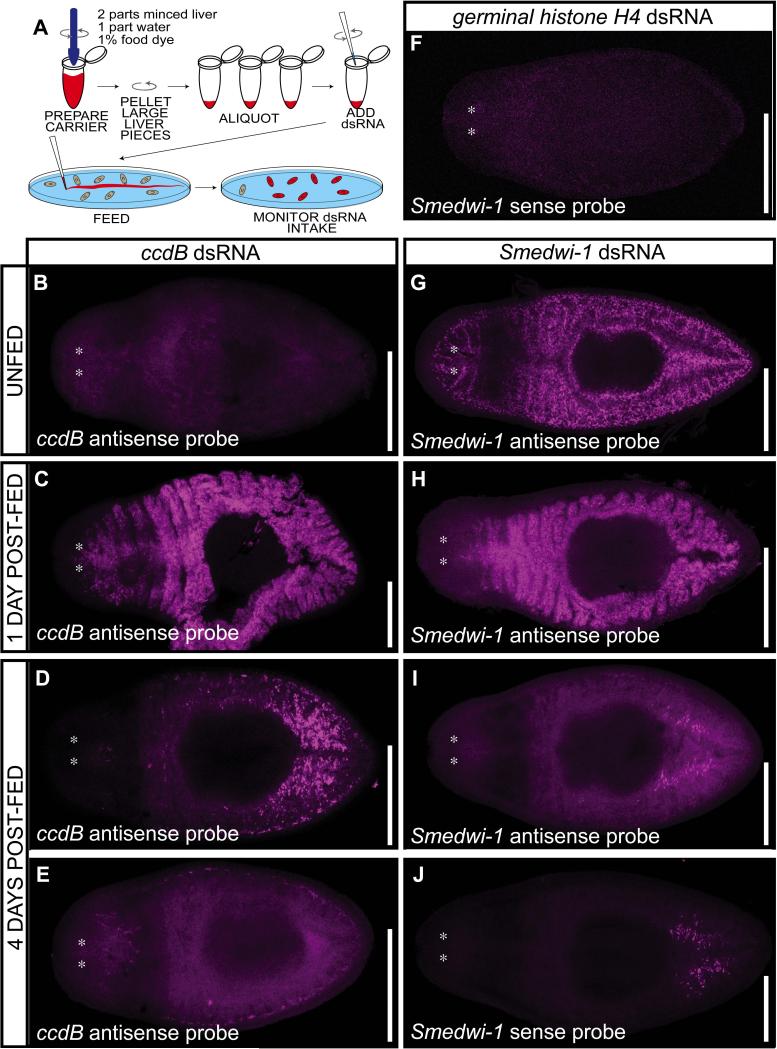
Systemic RNAi, the phenomenon by which dsRNA-mediated inhibition of gene expression is transmitted between cells and tissues, has been observed in planarians and nematodes fed with bacteria expressing dsRNA with homology to target genes (Timmons and Fire, 1998; Newmark et al., 2003). To analyze where and when processing occurs during feeding-induced RNAi in planarians using our method, we monitored the stability of in vitro-synthesized dsRNA after feeding. Control dsRNA homologous to approximately one kilobase of the ccdB bacterial gene sequence (not present in the planarian genome) was subjected to DNase treatment, purified, and fed to planarians using liver puree as carrier (Fig. 2A; see Experimental Procedures). We monitored the presence of dsRNA by fluorescent in situ hybridization (FISH) one and four days after ingestion (Fig. 2B-E). Whereas no control dsRNA was detected in unfed planarians (Fig. 2B), intense signal was detected throughout the planarian intestine 24 hours after control dsRNA feeding (Fig. 2C). Ingested dsRNA was mostly undetectable four days after feeding (Fig. 2D-E). These results show that dsRNA ingested under these conditions is only detectable in the intestine and processed within four days after feeding.
Figure 2. Ingestion and detection of dsRNA in planarians.
(A) Illustration of in vitro synthesized dsRNA feeding. Liver puree carrier colored with food dye is mixed with a pestle. Centrifugation then isolates large liver matter to bottom of eppendorf tube and supernatant is aliquoted. DsRNA is added to aliquots by swirling with pipette tip. Food containing dsRNA is smeared into the bottom of the container housing planarians, which are then monitored for ingestion of dsRNA by coloration. (B-J) Ingested dsRNA is detected in the intestine and active in neoblasts within four days. Detection by fluorescent in situ hybridization (FISH) shows no ccdB control RNA (bacterial sequence) detected in unfed planarians (B), but present throughout the intestine of planarians fed ccdB dsRNA one day post-feeding (C). Detection of ccdB dsRNA is lost mostly (D) or entirely (E) four days post-feeding. (F) Smedwi-1 sense probe failed to detect antisense RNA in planarians fed germinal histone H4 dsRNA. Detection of Smedwi-1 expression in neoblasts by FISH (G) is reduced after dsRNA ingestion (H-I). Smedwi-1 dsRNA is detected in the intestine one day (H), but to a much lesser extent four days (I-J) post-ingestion. Asterisks represent planarian eye location. Scale bars = 0.5 mm.
Next we analyzed RNAi dynamics using dsRNA homologous to Smedwi-1, a PIWI homolog expressed in neoblasts of S. mediterranea (Sanchez Alvarado et al., 2002; Reddien et al., 2005b). Smedwi-1 RNAi does not affect neoblast maintenance (Reddien et al., 2005b; Palakodeti et al., 2008), which allows us to analyze loss of Smedwi-1 RNA, without loss of neoblasts per se. Sense and antisense fluorescent in situ hybridization probes were used to distinguish between exogenous and endogenous Smedwi-1 RNA, respectively. Double-stranded RNA homologous to Smedwi-1 is not detectable in planarians unless specifically introduced exogenously (Fig. 2F). Smedwi-1 mRNA is present exclusively in neoblasts throughout the planarian mesenchyme (Fig. 2G). As in control experiments (Fig. 2B-E), dsRNA homologous to Smedwi-1 was detected throughout the intestine one day after RNAi feeding (Fig. 2H), but was processed by the fourth day post-feeding (Fig. 2I-J). Interestingly, Smedwi-1 expression in neoblasts was unnoticeable on the fourth day post-feeding, demonstrating that RNAi had taken place (Fig. 2I).
Although the precise physiological details remain to be elucidated, these results suggest that when taken up by feeding, dsRNA processing into small interfering RNA (siRNA) occurs in the intestine. After long molecules of dsRNA are processed, RNAi likely spreads to other tissues via uptake of siRNA originating in the intestine, and its effect on neoblast target mRNAs can be observed within the first four days of dsRNA exposure.
Penetrance and persistence of RNAi-mediated silencing in planarian cell types
The timing for establishment and maintenance of RNAi is important for planning experiments and interpreting knockdown phenotypes. Experiments inducing RNAi in planarians by dsRNA injection or bacterial feeding have demonstrated continuous inhibition of gene expression for several weeks after feeding (Pineda et al., 2000; Newmark et al., 2003; Rink et al., 2011). We analyzed the longevity of RNAi after a single feeding of in vitro-synthesized Smedwi-1 dsRNA and observed loss of target mRNA for at least 20 days post-feeding (Fig. 3A-E). As expected from our previous experiments (Fig. 2I-J), Smedwi-1 mRNA was undetectable by the fifth day after dsRNA feeding (Fig. 3B). RNAi-mediated silencing of Smedwi-1 expression was robustly maintained in neoblasts for 10 and 15 days after feeding (Fig. 3C-D), with very modest detection of mRNA observed on the 20th day post-feeding (Fig. 3E).
Figure 3. Dynamics of RNAi activity after dsRNA ingestion in planarians.
(A-R) Whole-mount in situ hybridization used to monitor Smedwi-1 (A-E), Smed-bruno-like (F-J), Bicaudal C (K-N), and germinal histone H4 (O-R) mRNA expression in asexual (A-J) or sexual (K-R) planarians subjected to a single dsRNA feeding. Times after feeding are indicated at the top of each panel. Fraction of animals representative of imaged sample is shown in parenthesis. Scale bars = 0.5 mm (A-J) and 1 mm (K-R).
Next, we tested whether similar RNAi dynamics could be observed for genes also expressed in somatic tissues. To do this, we monitored knockdown of Smed-bruno-like, a gene expressed in neoblasts and the central nervous system (CNS) (Fig. 3F; Guo et al., 2006). As with Smedwi-1, robust RNAi-mediated silencing was observed in neoblasts within five days of dsRNA feeding, and modest recovery of expression was observed by the 20th day after exposure to dsRNA (Fig. 3G-J). Surprisingly, Smed-bruno-like expression in the brain was detectable throughout 20 days after dsRNA exposure (Fig. 3F-J). The observations with Smed-bruno-like RNAi suggest that disruption of gene expression via dsRNA feeding is not equally efficient in all tissues.
We decided to further investigate the dynamics of RNAi penetrance in different tissues. Thus far, our analyses of RNAi dynamics were performed using an exclusively asexual strain of S. mediterranea, which lacks developed gonads and accessory reproductive organs. We analyzed the effect of dsRNA feeding on expression of Bicaudal C and germinal histone H4 in sexually mature planarians. Bicaudal C is a post-transcriptional regulator required for spermatogenesis in planarians (Wang et al., 2010). Bicaudal C mRNA is expressed in testes lobes and the copulatory apparatus region in control animals (Fig. 3K). Testes expression of Bicaudal C was gradually lost by the eighth day after dsRNA feeding (Fig. 3L-M), whereas expression around the copulatory apparatus was not lost until eleven days post-feeding (Fig. 3M-N). germinal histone H4 is expressed in neoblasts, testes and ovaries in control sexual animals (Fig. 3O; Wang et al., 2007; Rouhana et al., 2012). After dsRNA feeding, neoblast expression of germinal histone H4 was undetected in neoblasts within five days (Fig. 3P), whereas expression persisted in testes for more than eight days (Fig. 3Q) and in ovaries for more than eleven days post-feeding (Fig. 3R). Altogether, these results show robust RNAi-mediated silencing in neoblasts and differentiated tissues long after the initial dsRNA is processed in the intestine. However, RNAi-mediated silencing is temporary, for accumulation of target mRNAs could be observed 20 days post-feeding for Smedwi-1 and Smed-bruno-like (Fig. 3E and 3J). Furthermore, the timing for establishing RNAi-mediated silencing is variable between tissues. Thus, multiple feedings may be required to target genes expressed in differentiated tissues, or genes whose protein stability or phenotypic manifestations outlive the period of active RNAi.
Expression of the basic RNAi machinery (Dicer and Argonaute homologs) has been readily detected in planarian stem cells, the central nervous system, and at lower levels in the gut (Rouhana et al., 2010; Li et al., 2011). Thus cellular presence of the basic RNAi machinery alone cannot explain why Smed-bruno-like RNAi is less efficient in the brain than it is in neoblasts. In C. elegans, post-mitotic neurons are less susceptible to exogenous RNAi (Timmons et al., 2001; Kamath et al., 2003). However, neuronal susceptibility to RNAi by feeding in nematodes can be achieved by enhanced neuronal expression of SID-1 (Calixto et al., 2010), a transmembrane protein required for systemic RNAi (Winston et al., 2002). Transport of RNAi signals between some tissues is also possible in a SID-1-independent manner (Jose et al., 2009). Vesicle transport has been shown to mediate dsRNA cell entry (Saleh et al., 2006), and has been proposed as a means of RNAi signal transport from the gut (Jose et al., 2009). Modest homologs of human and nematode SID-1 proteins are present and expressed in planarians (Hermaphroditic Schmidtea mediterranea EST Database (Zayas et al., 2005), Contig ID PL06019B2E12 [e-value = 5e-57] and PL08008A1F03 [e-value = 8e-19], respectively). However, cell type-specific expression of these homologs in specific planarian tissues is not known. It is likely that systemic RNAi in planarians involves transport of siRNAs from the intestine via vesicles as well as SID-1 mediated uptake, thus tissues in closer proximity to the intestine and those with higher levels of SID-1 expression would be more susceptible to RNAi. Nevertheless, efficient knockdown of neuronal transcripts has been obtained by subjecting planarians to multiple feedings of in vitro-synthesized dsRNA (Collins et al., 2010).
Functional assessment of variables during RNAi-mediated silencing by feeding in vitro synthesized dsRNA
We wished to verify the robustness of RNAi-mediated silencing on gene function using our protocol. To do this, we targeted Argonaute-2 (Ago-2), a gene with conserved functions in micro-RNA and siRNA regulation of gene expression (reviewed by Kim et al., 2009). Ago-2 is broadly expressed in planarians, but previous studies have shown that RNAi-mediated silencing of this gene leads to gradual loss of neoblasts and ultimately death (Rouhana et al., 2010; Li et al., 2011). Thus, using Ago-2 RNAi-induced death as a readout for RNAi penetrance, we tested the effects of using different degrees of dsRNA purity, age, concentration, and inclusion of agarose in the carrier (Fig. 4A-C). We also tested variations in animal size, dsRNA concentration, and application of multiple dsRNA feedings, on RNAi-mediated gene silencing (Fig. 4D-H).
Figure 4. Functional assessment of variations in dsRNA feeding methodology.
(A-G) RNAi penetrance measured by monitoring survival of planarians subjected to a single feeding of liver paste containing 100 ng/μl of purified Ago-2 dsRNA (except panel E). Planarians fed with control dsRNA were unaffected (not shown). Daily time points after feeding are depicted along the x-axis. (A) Unpurified dsRNA transcription reaction (gray) compared to dsRNA treated with DNase, annealed and ethanol precipitated (black). (B) dsRNA stored frozen for over 2.5 years (gray) compared to recently synthesized dsRNA (black). (C) RNAi activity of liver paste preparation including agarose (gray) compared to dsRNA without agarose (black). (D) Ago-2 RNAi penetrance compared between larger (7-9 mm long; black) and smaller planarians (1-3 mm; gray). (E) Analysis of the effects of Ago-2 dsRNA concentration on RNAi penetrance. Death of planarians fed Ago-2 dsRNA at a concentration of 10 ng/μl (gray circles) compared to 100 ng/μl (black squares) and 1000 ng/μl (gray triangles) concentration. (F) Effects of multiple dsRNA feedings on Ago-2 RNAi phenotype. Planarians fed once with Ago-2 dsRNA (gray squares) compared to those fed twice (black circles) or three times (gray triangles). (G) Effects of multiple feedings compared to multiple Ago-2 dsRNA feedings on appearance of phenotype. Death of planarians fed Ago-2 dsRNA once (gray squares), were compared to those fed Ago-2 dsRNA twice (gray circles), and to planarians fed once with Ago-2 dsRNA and once with control dsRNA (black circle). (H) Analysis of correlation between P2X-A dsRNA concentration and increased fission induced by P2X-A RNAi in D. japonica. Number of fission events per group (x-axis) after last of three feedings with increasing concentrations (see figure) of control (left) or P2X-A (right) dsRNA. Timing of fission events after the third of three feedings is shown on y-axis. n ≥ 10 for each category.
First we tested whether RNAi by feeding planarians with purified dsRNA is more effective than feeding with unprocessed dsRNA transcription reaction (See Experimental Procedures). We did not find a noticeable difference in penetrance of Ago-2 RNAi by feeding pure Ago-2 dsRNA or unprocessed dsRNA transcription reaction (Fig. 4A). Next, we tested whether storage of dsRNA at -20°C for long periods of time affects RNAi activity. We found that feeding planarians dsRNA that had been frozen for 2.5 years (Fig. 1D) was just as effective in disrupting Ago-2 function as freshly synthesized dsRNA (Fig. 4B). Finally, we tested whether addition of agarose to the dsRNA/liver mixture, which facilitates handling during the feeding procedure, had an effect on RNAi penetrance. Previous experiments using bacterially expressed dsRNA observed an improvement in RNAi penetrance by using dsRNA/liver paste (a.k.a. “SOFT SERVE”) over inclusion of agarose (Gurley et al., 2008). We failed to observe an effect on RNAi penetrance by inclusion of agarose, when using in vitro-synthesized dsRNA (Fig. 4C). However, planarians do not seem to retain as much food when agarose is present.
One caveat of feeding bacterially expressed dsRNA, as compared to dsRNA delivery by injection, is the uncertainty in amount of delivered nucleic acid. Feeding dsRNA synthesized in vitro allows for controlled concentration of nucleic acid in the food carrier. The amount of food that is ultimately ingested by planarians may vary. However, we assumed that the amount of food ingested would correlate with planarian size, and therefore keeping the concentration of dsRNA in the food constant would suffice to see similar RNAi penetrance in animals of various sizes. To test this, we subjected small (1-3 mm) and large (7-9 mm) planarians to a single feeding with excess amount of liver paste containing a constant concentration of Ago-2 dsRNA (100 ng/μl). Contrary to our expectations, we observed 75% survival of large animals subjected to Ago-2 RNAi days after all small animals had died (Fig. 4D). Next, we tested whether exposing small planarians to different concentrations of dsRNA could result in various degrees of Ago-2 RNAi penetrance. Small planarians were subjected to a single feeding containing 10 ng, 100 ng, or 1000 ng of Ago-2 dsRNA per microliter of liver paste, and scored for survival. Animals presented with the smallest dose of dsRNA were mostly unaffected by the treatment up to 32 days post-feeding (Fig. 4E). Animals presented with the largest dose of dsRNA showed similar Ago-2 RNAi penetrance as those treated with the usual (100ng/μl) dose of dsRNA (Fig. 4E). These results demonstrate that full manifestation of the Ago-2 RNAi phenotype is dose dependent and is achieved at approximately 100 ng/μl. We currently use this concentration for disrupting gene function by RNAi in individual experiments and screens. However, weekly feedings are routinely applied to induce robust RNAi in larger animals, including sexually mature animals (Collins et al., 2010; Chong et al., in revision) and/or to analyze phenotypes that require prolonged knockdown schemes (Rouhana et al., 2012; Roberts-Galbraith and Newmark, 2013).
Whether using dsRNA injection or feeding of bacterial or in vitro synthesized dsRNA, researchers normally utilize multiple rounds of delivery to induce RNAi in planarians. To directly test whether multiple rounds of dsRNA feeding affect RNAi penetrance, planarians were fed Ago-2 dsRNA once, twice or three times, with two days separating each round of feeding. We observed that in fact, planarians subjected to two or three rounds of Ago-2 dsRNA feedings died about twice as fast as those subjected to only one round of feeding (Fig. 4F). Planarians subjected to one round of Ago-2 dsRNA feeding, died 20 to 28 days after the initial feeding (Fig. 4F). Whereas, planarians subjected to two rounds of dsRNA feeding died between 14 and 17 days after the initial feeding, and those subjected to three rounds died between 13 and 15 days after the initial feeding (Fig. 4F). These results suggested that multiple ingestions of dsRNA accelerate full manifestation of RNAi phenotype. However, it remained unclear whether multiple rounds of dsRNA delivery accelerated the manifestation of the Ago-2 RNAi phenotype due to more effective destruction of Ago-2 transcripts, or due to the demands of cell proliferation induced in response to feeding (Baguñà, 1976). To distinguish between these two scenarios, we first subjected planarians to an initial round of Ago-2 dsRNA feeding and a second feeding three days later with either control dsRNA or Ago-2 dsRNA. Planarians subjected to one round of Ago-2 dsRNA died 15 to 27 days after the initial feeding (Fig. 4G). Planarians fed Ago-2 dsRNA first and control dsRNA second, died between 14 and 20 days after the initial feeding (Fig. 4G). Animals subjected to two rounds of Ago-2 dsRNA died between 11 and 15 days after the initial feeding (Fig. 4F). These results show that multiple rounds of dsRNA feeding can enhance RNAi penetrance, although feeding alone causes a modest acceleration of the Ago-2 RNAi phenotype, likely due to a higher demand for neoblasts capable of proliferation after feeding.
Altogether, these results demonstrate the importance of controlling the amount of dsRNA delivered to planarians. Multiple rounds of dsRNA feeding can be used to enhance RNAi penetrance or accelerate phenotype manifestation. Within limits, increasing amounts of dsRNA can also improve phenotype manifestation. Experiments analyzing a neoblast receptor required to regulate nutritional influence on proliferation, P2X-A, support this observation (Fig. 4H). P2X-A RNAi leads to increased fission events in D. japonica (Sakurai et al., 2012). We found that increasing the concentration of P2X-A dsRNA had a limited correlation with the number of fission events observed after RNAi (Fig. 4H).
The concentration of dsRNA that most efficiently revealed Ago-2 or P2X-A phenotypes in our experiments, ranged between 10 – 100 ng/μl (Fig. 4E and 4H), but how do these amounts compare to RNAi protocols using bacterial expression of dsRNA? To directly analyze this we silenced Smed-nkx2.2 expression by feeding planarians dsRNA produced in vitro or in bacteria. Smed-nkx2.2 is an intestinally expressed homeobox transcription factor required for neoblast proliferation (Forsthoefel et al., 2012). Knockdown of nkx2.2 initially causes lesioning on the dorsal surface of planarians in a pattern over the intestinal branches, which is followed by complete lysis and death (Fig. 5A). In animals fed in vitro-synthesized dsRNA, lysis begins sooner and progresses more rapidly than those fed E. coli-expressing nkx2.2 dsRNA (Fig. 5B). Planarians fed liver paste with concentrations of in vitro-synthesized nkx2.2 dsRNA of 20, 40 or 80 ng/μl showed lysis 8 – 10 days post-feeding, whereas planarians fed with E. coli showed lysis 9 – 13 days post-feeding (Fig. 5B). Death of planarians subjected to nkx2.2 dsRNA synthesized in vitro occurred 9 – 12 days post-feeding, whereas bacteria-fed counterparts died between 11 – 16 days post-feeding (Fig. 5B). Although it is possible that in some situations RNAi by bacterial feeding could be as effective as this protocol, these results demonstrate that RNAi using dsRNA synthesized in vitro leads to an earlier manifestation of nkx2.2 phenotypes than bacterially expressed dsRNA. Theoretically, such delays in phenotypic manifestation could make a difference between erroneous “false-negative” classification and functional revelation in direct experiments or screens.
Figure 5. Phenotypes manifest more rapidly in animals fed dsRNA synthesized in vitro compared to E. coli-expressed dsRNA.
(A) Range of lysis phenotypes observed after nkx2.2 knockdown, increasing in severity from left (early/no phenotype) to right (late/complete lysis and death). Phenotypes manifested earlier and progressed more quickly for animals fed in vitro-synthesized nkx2.2 dsRNA. (B) Plot of the percentage of animals (Y-axis) that lyse (dashed lines) or survive (solid lines) for the number of days indicated (X-axis) after a single feeding of either E. coli expressing nkx2.2 dsRNA (black diamonds) or in vitro-synthesized nkx2.2 dsRNA at 20, 40, and 80 ng/μl (gray, yellow and red circles, respectively). n ≥ 24 for each feeding category. Scale bars = 1 mm.
Summary
In conclusion, feeding of in vitro synthesized dsRNA is an effective alternative to more commonly used approaches for inducing RNAi in planarians. This simple approach is robust, non-invasive, and allows for direct assessment of dsRNA quantity and quality. Additional advantages of our methodology, which make it amenable for high-throughput screening, include low cost, labor and time requirements (Table 1). The feeding of in vitro synthesized dsRNA is the most convenient way to inhibit gene function in planarians. Application of this protocol will accelerate efforts of researchers using the planarian as a model system in molecular, regenerative, and developmental biology research. Additionally, researchers trying to establish protocols for RNAi by dsRNA feeding of other organisms can use our parameters as a starting point for their efforts.
Table 1. Comparison of RNAi methodologies used in planarian research.
Evaluation of the different techniques used for synthesis and delivery of dsRNA in planarian research according to robustness (penetrance and reliability), ease of use (time and difficulty involved in methodology), and quality control (ease of qualitative and quantitative assessment of dsRNA synthesis).
| RNAi Methodology | Robustness | Ease of Use | Quality Control |
|---|---|---|---|
| in vitro feeding | + | + | + |
| Bacteria feeding | + | + | - |
| Injection | + | - | + |
| Soaking | - | + | + |
EXPERIMENTAL PROCEDURES
RNA Interference (RNAi) Methodology
First Step: Template Preparation
-
1
Amplify the desired target sequence from a cDNA clone using PCR with primers including T7 bacteriophage promoter sequence (or RNA polymerase of preference).
PCR reaction components
1 μl plasmid DNA template* (final concentration 0.4 – 4 ng/μl)
1 μl sense primer** (10 μM stock)
1 μl antisense primer (10 μM stock)
2.5 μl hot-start mix (420 mM Tris-HCl (pH 9.5), 160 mM (NH4)2SO4, 0.84%
Tween-20, 0.4% ortho-phosphoric acid; modified from (Barnes and Rowlyk, 2002)) or preferred PCR buffer.
2.5 μl 35 mM MgCl2
0.5 μl 10 mM (each) dNTPs
0.5 μl Taq polymerase
Bring up to a final volume of 25 μl using ultrapure water.
PCR reaction conditions
| Denature: | 95°C for 1 minute. |
| Amplify (40 cycles): | 95°C for 30 seconds. |
| 55°C for 30 seconds. | |
| 72°C for 1 minute per kb of sequence. | |
| Complete and end: | 72°C for 5 min. |
| 4°C (short-term) or -20°C (long-term) storage |
NOTES: *We routinely use templates that include cDNA sequence flanked by T7 promoter sequences in opposite orientation (See Fig. 1A).
**Alternatively, use 2 μl of RNA Polymerase promoter sequence primer if desired cDNA has been cloned in a vector with flanking promoters for dsRNA synthesis (e.g. T7 promoters in pJC53.2 (Collins et al., 2010)).
-
2Precipitate the resulting DNA and re-suspend in 20 μl of RNase-free water.
- - Add 1/10 volume of 3 M NaOAc (pH 5.2) to PCR solution and mix.
- - Add two volumes of cold 100% ethanol, mix, and store at -20°C for 1 hr.
- - Centrifuge at a speed > 15,000 × g in a 4°C microcentrifuge.
- - Decant supernatant and wash pellet with cold 70% RNase-free ethanol.
- - Centrifuge briefly, decant all supernatant, and dry at room temperature.
- - Re-suspend DNA in 20 μl of RNase-free water.
NOTES: Alternatively, one may use DNA Clean & Concentrator spin columns and reagents (Zymo Research, Irvine, CA).
RNase-free template DNA can be used directly for dsRNA transcription reaction with appropriate RNA polymerase. The resulting DNA product serves as template for simultaneous sense and antisense RNA synthesis. Yields range between 50-150 ng/μl.
Second Step: Double-stranded RNA preparation
-
1
Double-stranded RNA can be synthesized in a single reaction with the appropriate template. Prepare transcription reaction as follows: Modified from (Milligan et al., 1987; Milligan and Uhlenbeck, 1989).
Reaction components and conditions
1 μg of DNA template
2 μl of 10X Buffer (0.1 M MgCl2; 0.4 M Tris, pH 8.0; 0.1M DTT; 20 mM spermidine)
5 μl of 25 mM (each) rNTPs
1 μl of T7 RNA polymerase
1 μl of Thermostable Inorganic Pyrophosphatase (TIPP) [2000 U/ml] (New England Biolabs, Ipwich, MA).
0.5 μl of recombinant RNAse inhibitor (RNasin) [2500 U/ml] (Promega, Madison, WI).
Bring up to a final volume of 20 μl using ultrapure water.
-
2
Incubate reaction at 37°C for 4-5 hours. Reaction can also be incubated overnight.
NOTES: Yields of 1-5 mg/ml of RNA are expected under these conditions. For most applications, the integrity of dsRNA obtained from this transcription reaction can be checked by agarose gel electrophoresis (below) and fed directly to induce RNAi (below).
DNase Treatment
-
3
Add 1 μl of RQ1 RNase-free DNase [2500 U/ml] (Promega, Madison, WI), directly into the transcription reaction and incubate at room temperature for 20 minutes.
DsRNA Clean Up By Ammonium Acetate Precipitation
-
4
Bring volume of dsRNA solution to 100 μl with RNAse-free water.
-
5
Add equal volume of 5 M ammonium acetate (2.5 M final concentration). The solution may become opaque.
-
6
Add two volumes of 100% ethanol (optional; this step helps precipitate RNA molecules smaller than 800 nts).
-
7
Incubate at -20°C for one hour.
-
8
Centrifuge at over 18,000 RCF for 15 minutes at 4°C. A white pellet is usually visible after centrifugation.
-
9
Remove supernatant and wash pellet with cold 70% ethanol (centrifuge again for 5 minutes if necessary to remove all supernatant).
-
10
Resuspend RNA pellet in 50-100 μl of RNAse-free water.
Annealing
Denature RNA at 95°C for 3 minutes.
Incubate at 75°C for 3 minutes.
Incubate at 50°C for 3 minutes
Incubate at room temperature for 5 minutes.
NOTE: The annealing step is optional if sense and antisense RNA were synthesized simultaneously, but required if separate sense and antisense transcription reactions are combined.
Analysis of dsRNA integrity
Load 1 μl of dsRNA solution mixed with 1 μl of formaldehyde load dye (Ambion, Austin, TX) in a non-denaturing 1% agarose gel containing ethidium bromide.
Resolve bands with a 80-120 Volts constant until blue dye has migrated halfway through the gel.
Visualize bands under UV light.
NOTE: Double-stranded RNA maintains secondary structure during electrophoresis under non-denaturing conditions, and is manifested as band doublets, slower mobility, and/or smearing material between the main band and well (Fig. 1B). If in doubt, dsRNA integrity can be verified by insensitivity to DNase and Ribonuclease A (cleaves single-stranded RNA).
Measuring RNA Concentration
NOTE: DNase treatment and ammonium acetate precipitation are recommended if calculating RNA concentration using UV-spectrometry. We routinely obtain good approximation of dsRNA concentration using NanoDrop UV-Vis spectrometers (NanoDrop products, Wilmington, DE) with the Sample Type Constant set at “Other-45”. The recommended range for NanoDrop measurements is between 50 ng/μl and 3700 ng/μl, so a 1:10 dilution in water may be necessary for accurate quantification of dsRNA concentration. The use of UV-spectrometry is not suited for measuring concentration of RNA directly from transcription reactions, as single nucleotides interfere with absorbance measurements. However, concentration of dsRNA in transcription reactions can be approximated from ethidium bromide uptake during agarose gel electrophoresis. To do this, simply include a range of reference samples with known dsRNA concentration, or one of a number of commercial ladders available with concentration and size standards, during electrophoresis.
Third Step: Feeding
The following steps for dsRNA feeding are illustrated in Figure 2A.
Deposit liver puree and ultrapure water in a 2:1 ratio inside a 1.5 ml microcentrifuge tube (600 μl of liver and 300 μl of ultrapure water). A P1000 pipette with a cut at the end of the tip works well for pipetting minced liver.
Add 1% food coloring dye (McCormick, Sparks, MD) to monitor intake by planarians. Blue, green and red dyes work best.
Mix the liver, water and dye into a feeding paste. To do this, use a Kontes plastic pestle (Kimble Chase, Vineland, NJ) with a rotating motion up and down the inside of the microcentrifuge tube, then centrifuge for 10 to 30 seconds using a mini centrifuge (approximate RCF of 2000 × g) to pellet larger liver pieces to the bottom of the tube.
Avoiding bubbles, distribute paste into aliquots of 50-150 μl using a P200 pipette. A 50 μl aliquot is enough to treat one group of ten asexual planarians, whereas 150 μl works for a group of ten large sexual animals.
Add 1 μg of dsRNA per 10 μl of paste using a P10 pipette, and mix by swirling paste with pipette tip (Fig. 2A). Do not mix by pipetting up and down, since this will create problematic bubbles.
Serve dsRNA-containing paste by pipetting on a 45° angle in relation to the bottom of the plate containing planarians, and with a sideways motion. This will keep liver paste from floating in solution.
Allow planarians to eat the dsRNA-containing paste in the dark for over one hour. Double-stranded RNA intake can be monitored by coloration of the planarian intestine by the dye included in the liver paste, which decorates the gut anatomy and can be readily observed for a couple of days post-feeding. It is known that liver persists in cells of the planarian intestine for at least 24 hours post-feeding (Bowen et al., 1974).
Remove majority of liver, thoroughly clean or replace the container, and change the media. This must be done after every feeding or on a weekly basis to avoid contamination.
NOTES:
Pureed beef liver is routinely used to feed and maintain S. mediterranea, whereas other species, such as D. japonica are commonly fed chicken liver. Either carrier is compatible with this protocol, but it is important to prepare the meat puree as finely as possible and remove chunks of fat or connective tissue.
Liver puree can be prepared in large quantities and aliquots stored at -80°C for several months in 1.5 ml microcentrifuge tubes or plates. Liver puree should be thawed on the day of RNAi treatment.
To ensure homogeneous distribution of dsRNA in liver paste, mix dsRNA with colored dye first, and then apply to liver paste. The distribution of the dye should reflect the distribution of the dsRNA.
Agarose (0.6% final concentration) can be added during paste preparation to further solidify liver droplets. This eases handling and cleaning during feeding procedure.
Multiple dsRNA treatments are recommended large animals (Fig. 4G) and for gene knockdowns with late phenotypic manifestations.
Maintenance of planarian culture
The asexual strain of Schmidtea mediterranea (Sanchez Alvarado et al., 2002) was used in all experiments, except where specifically noted. Asexual S. mediterranea cultures were maintained in 0.05 % Instant Ocean salts (Spectrum Brands Inc., Madison, WI) in ultrapure water at 21°C. Cultures of the sexual strain of S. mediterranea (Zayas et al., 2005) were maintained as described by Wang et al., 2007). A clonal strain (GI) of Dugesia japonica was maintained in autoclaved tap water from Kyoto, Japan at 22-24°C. Animals were starved for at least one week prior to RNAi treatments.
Additional RNAi procedures
RNA-interference induced by feeding in vitro synthesized dsRNA was performed as described above (RNA interference (RNAi) Methodology). Asexual planarians were used and dsRNA concentration was kept at 100 ng/μl of liver paste in all experiments, unless otherwise specifically noted.
For nkx2.2, induction of dsRNA-expressing E. coli was conducted as in (Forsthoefel et al., 2012). For feeding, 6 ml cultures were pelleted and mixed with 60 μl liver homogenate. For in vitro synthesis of nkx2.2 dsRNA, pBluescript-specific primers (below) were utilized to synthesize template DNA from clone PL06017A1E06 (Zayas et al., 2005). Animals were fed only once.
In situ hybridization
Preparation of digoxigenin-containing riboprobes (Tautz and Pfeifle, 1989) and whole-mount in situ hybridization were performed as previously described by (Umesono et al., 1997), with modifications as per (Pearson et al., 2009) and King and Newmark (in revision). Briefly, animals were killed in 5% N-acetyl-L-cysteine (Sigma, St. Louis, MO) dissolved in 1× PBS for approximately five minutes, and fixed in 4% Formalin in PBS for 30 minutes to two hours. Samples were then washed in PBS, and dehydrated in sequential dilutions of PBS in methanol. Animals were stored for at least 24 hours in methanol at -20°C, rehydrated, washed in SSC and bleached for two hours in bleaching solution composed of 5% formamide and 1.2% hydrogen peroxide in 0.5X SSC. Bleached samples were washed once with 1× SSC and twice with PBSTx (PBS supplemented with 0.3% Triton-X), treated with Proteinase K [10 μg/ml] for 30 minutes at room temperature, fixed with 4% Formalin in PBSTx for 10 minutes, washed twice in PBSTx, and subjected to riboprobe hybridization as per Pearson et al. (2009). Fluorescent in situ hybridization signal was developed using Cy3-tyramide (Perkin-Elmer) per the manufacturer's protocol.
Imaging and microscopy
Fluorescent in situ hybridization images were processed using a Zeiss 710 confocal microscope running Zen software. Colorimetric signals from whole-mount in situ hybridization samples were imaged and recorded using a Leica M205A running LAS 3.6.0.
DNA Constructs and Primers
Smedwi-1 (Guo et al., 2006), Smed-Bruno-like (Guo et al., 2006), and Smed-germinal histone H4 (Wang et al., 2007) constructs contained cDNA sequences inserted into pBluescript II SK+ backbone. D. japonica P2X-A cDNA sequence inserted into pBluescript SK- (Nishimura et al., 2012; Sakurai et al., 2012; Shibata et al., 2012); DNA Data Bank of Japan accession no. FY959221) was used to amplify template for P2X-A dsRNA synthesis. To amplify template for probe synthesis with T3 RNA polymerase, and dsRNA synthesis with T7 RNA polymerase, cDNA sequences from these constructs were amplified using the primer AAAGGGGGATGTGCTGCAAGGCGATTAAGTTGG, which anneals upstream of pBluescript II SK+ endogenous T7 promoter, and the primer TAATACGACTCACTATAGGGAGACGCGCAATTAACCCTCACTA, which anneals to the T3 promoter in pBluescript and adds a T7 promoter. Smed-Argonaute-2 (Li et al., 2011) partial cDNA sequence was amplified from asexual planarian cDNA and ligated into the pJC53.2 vector (Collins et al., 2010) and sequenced. ccdB bacterial sequence within the pJC53.2 multiple cloning site (MCS) was used as a control. A modified T7 primer with the sequence GGATCCTAATACGACTCACTATAGGG (Collins et al., 2010) was used for dsRNA template amplification from pJC53.2 for Argonaute-2 and ccdB.
Key Findings.
- in vitro synthesized dsRNA is fed to planarians to induce robust RNAi.
- Presentation of an improved protocol for RNAi in planarians amenable to high-throughput studies.
- Analysis of dsRNA processing dynamics and spatiotemporal RNAi activity.
- Systematic comparisons of effects of variations in amount, frequency and mode of dsRNA delivery on RNAi efficiency.
ACKNOWLEDGMENTS
We thank members of the Newmark and Agata laboratories for support and advice. This work was supported by postdoctoral fellowships from the NSF (0804021) and the Ford Foundation to L.R. and NIH R01 HD043403 to P.A.N. P.A.N. is an investigator of the Howard Hughes Medical Institute.
Grants supporting this work: NSF (0804021) and Ford Foundation Postdoctoral fellowships to L.R. and NIH R01 HD043403 to P.A.N.
REFERENCES
- Baguñà J. Mitosis in the intact and regenerating planarian Dugesia mediterranea n.sp. I. Mitotic studies during growth, feeding and starvation. J. Exp. Zool. 1976;195:53–64. [Google Scholar]
- Barnes WM, Rowlyk KR. Magnesium precipitate hot start method for PCR. Mol Cell Probes. 2002;16:167–171. doi: 10.1006/mcpr.2002.0407. [DOI] [PubMed] [Google Scholar]
- Bowen ID, Ryder TA, Thompson JA. The fine structure of the planarian Polycelis tenuis Iijima. II. The intestine and gastrodermal phagocytosis. Protoplasma. 1974;79:1–17. doi: 10.1007/BF02055779. [DOI] [PubMed] [Google Scholar]
- Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010;7:554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebria F, Guo T, Jopek J, Newmark PA. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307:394–406. doi: 10.1016/j.ydbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebria F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sanchez Alvarado A, Agata K. FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature. 2002;419:620–624. doi: 10.1038/nature01042. [DOI] [PubMed] [Google Scholar]
- Collins JJ, 3rd, Hou X, Romanova EV, Lambrus BG, Miller CM, Saberi A, Sweedler JV, Newmark PA. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 2010;8:e1000509. doi: 10.1371/journal.pbio.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SA, Sánchez Alvarado A. The history and enduring contributions of planarians to the study of animal regeneration. WIREs Dev Biol. 2012 doi: 10.1002/wdev.82. doi: 10.1002/wdev.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Forsthoefel DJ, James NP, Escobar DJ, Stary JM, Vieira AP, Waters FA, Newmark PA. An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Dev Cell. 2012;23:691–704. doi: 10.1016/j.devcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel DJ, Newmark PA. Emerging patterns in planarian regeneration. Curr Opin Genet Dev. 2009;19:412–420. doi: 10.1016/j.gde.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile L, Cebria F, Bartscherer K. The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Dis Model Mech. 2011;4:12–19. doi: 10.1242/dmm.006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Peters AH, Newmark PA. A Bruno-like gene is required for stem cell maintenance in planarians. Dev Cell. 2006;11:159–169. doi: 10.1016/j.devcel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Sanchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose AM, Smith JJ, Hunter CP. Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proc Natl Acad Sci U S A. 2009;106:2283–2288. doi: 10.1073/pnas.0809760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- King RS, Newmark PA. The cell biology of regeneration. J Cell Biol. 2012;196:553–562. doi: 10.1083/jcb.201105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe RM, Irimia M, Currie KW, Lin A, Zhu SJ, Brown DD, Ross EJ, Voisin V, Bader GD, Blencowe BJ, Pearson BJ. A comparative transcriptomic analysis reveals conserved features of stem cell pluripotency in planarians and mammals. Stem Cells. 2012;30:1734–1745. doi: 10.1002/stem.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan SW, Reddien PW. dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet. 2011;7:e1002226. doi: 10.1371/journal.pgen.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan SW, Reddien PW. Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2012;2:294–307. doi: 10.1016/j.celrep.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Zeng A, Han XS, Wang C, Li G, Zhang ZC, Wang JY, Qin YW, Jing Q. Argonaute-2 regulates the proliferation of adult stem cells in planarian. Cell Res. 2011;21:1750–1754. doi: 10.1038/cr.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ishizu H, Chinone A, Kobayashi K, Matsumoto M. The Dr-nanos gene is essential for germ cell specification in the planarian Dugesia ryukyuensis. Int J Dev Biol. 2012;56:165–171. doi: 10.1387/ijdb.113433hn. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Reddien PW, Cebria F, Sanchez Alvarado A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark PA, Wang Y, Chong T. Germ cell specification and regeneration in planarians. Cold Spring Harb Symp Quant Biol. 2008;73:573–581. doi: 10.1101/sqb.2008.73.022. [DOI] [PubMed] [Google Scholar]
- Nishimura O, Hirao Y, Tarui H, Agata K. Comparative transcriptome analysis between planarian Dugesia japonica and other platyhelminth species. BMC Genomics. 2012;13:289. doi: 10.1186/1471-2164-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal P, Grun D, Adamidi C, Rybak A, Solana J, Mastrobuoni G, Wang Y, Rahn HP, Chen W, Kempa S, Ziebold U, Rajewsky N. Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. Embo J. 2012;31:2755–2769. doi: 10.1038/emboj.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii H, Mochii M, Watanabe K. A simple “soaking method” for RNA interference in the planarian Dugesia japonica. Dev Genes Evol. 2003;213:138–141. doi: 10.1007/s00427-003-0310-3. [DOI] [PubMed] [Google Scholar]
- Palakodeti D, Smielewska M, Lu YC, Yeo GW, Graveley BR. The PIWI proteins SMEDWI-2 and SMEDWI-3 are required for stem cell function and piRNA expression in planarians. Rna. 2008;14:1174–1186. doi: 10.1261/rna.1085008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BJ, Eisenhoffer GT, Gurley KA, Rink JC, Miller DE, Sanchez Alvarado A. Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn. 2009;238:443–450. doi: 10.1002/dvdy.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, Fitzgerald P, Watanabe S, Mancuso J, Green DR, Sanchez Alvarado A. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2010;338:76–85. doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda D, Gonzalez J, Callaerts P, Ikeo K, Gehring WJ, Salo E. Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. Proc Natl Acad Sci U S A. 2000;97:4525–4529. doi: 10.1073/pnas.97.9.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sanchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell. 2005a;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sanchez Alvarado A. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science. 2005b;310:1327–1330. doi: 10.1126/science.1116110. [DOI] [PubMed] [Google Scholar]
- Rink JC. Stem cell systems and regeneration in planaria. Dev Genes Evol. 2012 doi: 10.1007/s00427-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JC, Vu HT, Sanchez Alvarado A. The maintenance and regeneration of the planarian excretory system are regulated by EGFR signaling. Development. 2011;138:3769–3780. doi: 10.1242/dev.066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith RH, Newmark PA. Follistatin antagonizes Activin signaling and acts with Notum to direct planarian head regeneration. Proc Natl Acad Sci U S A. 2013;110:1363–1368. doi: 10.1073/pnas.1214053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Shibata N, Nishimura O, Agata K. Different requirements for conserved post-transcriptional regulators in planarian regeneration and stem cell maintenance. Dev Biol. 2010;341:429–443. doi: 10.1016/j.ydbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- Rouhana L, Vieira AP, Roberts-Galbraith RH, Newmark PA. PRMT5 and the role of symmetrical dimethylarginine in chromatoid bodies of planarian stem cells. Development. 2012;139:1083–1094. doi: 10.1242/dev.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Lee H, Kashima M, Saito Y, Hayashi T, Kudome-Takamatsu T, Nishimura O, Agata K, Shibata N. The planarian P2X homolog in the regulation of asexual reproduction. Int J Dev Biol. 2012;56:173–182. doi: 10.1387/ijdb.113439ts. [DOI] [PubMed] [Google Scholar]
- Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O'Farrell PH, Andino R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc Natl Acad Sci U S A. 1999;96:5049–5054. doi: 10.1073/pnas.96.9.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Newmark PA, Robb SM, Juste R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development. 2002;129:5659–5665. doi: 10.1242/dev.00167. [DOI] [PubMed] [Google Scholar]
- Scimone ML, Srivastava M, Bell GW, Reddien PW. A regulatory program for excretory system regeneration in planarians. Development. 2011;138:4387–4398. doi: 10.1242/dev.068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N, Hayashi T, Fukumura R, Fujii J, Kudome-Takamatsu T, Nishimura O, Sano S, Son F, Suzuki N, Araki R, Abe M, Agata K. Comprehensive gene expression analyses in pluripotent stem cells of a planarian, Dugesia japonica. Int J Dev Biol. 2012;56:93–102. doi: 10.1387/ijdb.113434ns. [DOI] [PubMed] [Google Scholar]
- Shibata N, Rouhana L, Agata K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Dev Growth Differ. 2010;52:27–41. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- Solana J, Kao D, Mihaylova Y, Jaber-Hijazi F, Malla S, Wilson R, Aboobaker A. Defining the molecular profile of planarian pluripotent stem cells using a combinatorial RNAseq, RNA interference and irradiation approach. Genome Biol. 2012;13:R19. doi: 10.1186/gb-2012-13-3-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21:172–185. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Tasaki J, Nishimura K, Inoue T, Agata K. Regeneration in an evolutionarily primitive brain--the planarian Dugesia japonica model. Eur J Neurosci. 2011;34:863–869. doi: 10.1111/j.1460-9568.2011.07819.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Watanabe K, Agata K. A planarian orthopedia homolog is specifically expressed in the branch region of both the mature and regenerating brain. Dev Growth Differ. 1997;39:723–727. doi: 10.1046/j.1440-169x.1997.t01-5-00008.x. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Ho JJ, Reddien PW. Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell. 2012;10:299–311. doi: 10.1016/j.stem.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Stary JM, Wilhelm JE, Newmark PA. A functional genomic screen in planarians identifies novel regulators of germ cell development. Genes Dev. 2010;24:2081–2092. doi: 10.1101/gad.1951010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zayas RM, Guo T, Newmark PA. nanos function is essential for development and regeneration of planarian germ cells. Proc Natl Acad Sci U S A. 2007;104:5901–5906. doi: 10.1073/pnas.0609708104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D, Lapan SW, Wilkinson AW, Bell GW, Reddien PW. A molecular wound response program associated with regeneration initiation in planarians. Genes Dev. 2012;26:988–1002. doi: 10.1101/gad.187377.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Agata K. Optic chiasm formation in planarian I: Cooperative netrin- and robo-mediated signals are required for the early stage of optic chiasm formation. Dev Growth Differ. 2011;53:300–311. doi: 10.1111/j.1440-169X.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- Zayas RM, Hernandez A, Habermann B, Wang Y, Stary JM, Newmark PA. The planarian Schmidtea mediterranea as a model for epigenetic germ cell specification: analysis of ESTs from the hermaphroditic strain. Proc Natl Acad Sci U S A. 2005;102:18491–18496. doi: 10.1073/pnas.0509507102. [DOI] [PMC free article] [PubMed] [Google Scholar]